Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Genitourinary system: Composed of internal and external organs of the urinary system and genital/reproductive system. Both systems are contained in the abdomen and pelvic region.
Urinary system: Composed of kidneys, ureters, bladder, and urethra
Reproductive system: Male (penis, testicles), female (ovaries, Fallopian tubes, uterus, vagina, vulva)
Female genitourinary system: Situated within the pelvis, except for the vulva, which is external ( Fig. 32.1 )
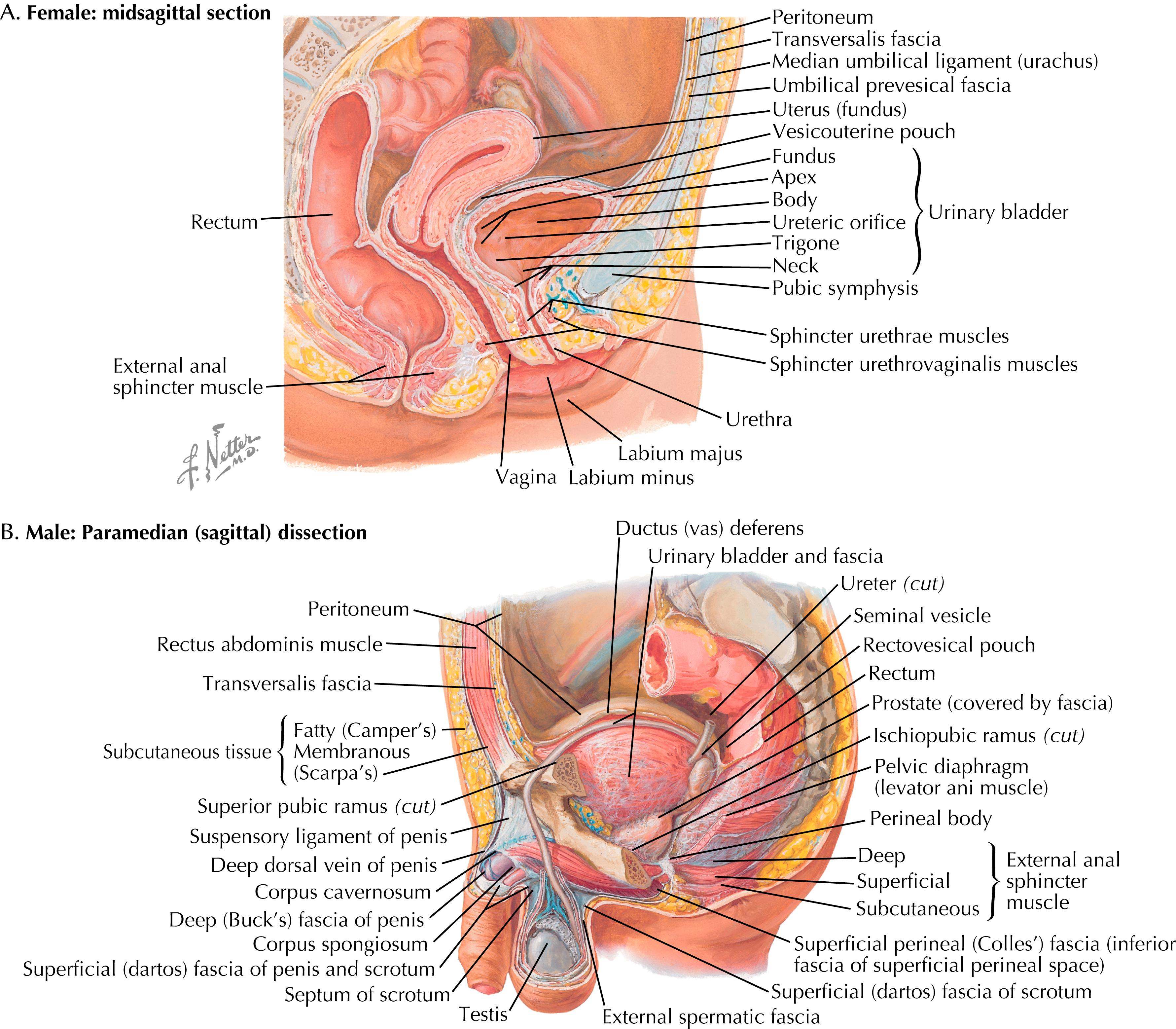
Male genitourinary system: Prostate and internal portion of male urethra located within the pelvis. Penis, scrotum, and testes are located externally and are most vulnerable in men (see Fig. 32.1 ).
Kidneys: Located in the retroperitoneal region from T12 through L3 vertebral level ( Fig. 32.2 ). The right kidney lies slightly lower than the left because of the liver. The kidneys are contained in a cushion of pericapsular fat and protected by the posterior abdominal wall musculature (erector spinae muscles, latissimus dorsi).
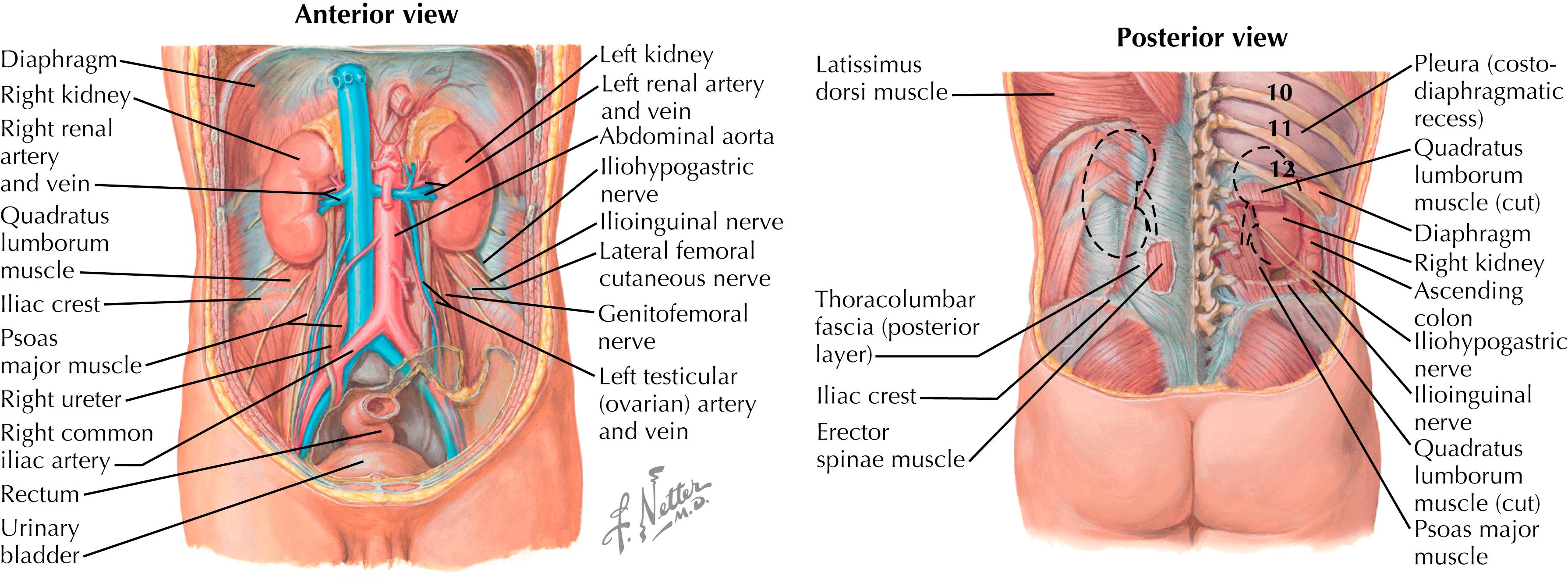
Ureters: Run along the posterior peritoneal wall from the kidneys to bladder. Also protected by the muscles of posterior abdominal wall. Most vulnerable where they cross the brim of the pelvis.
Bladder: When empty, lies completely within the pelvis and protected anteriorly by the pubic rami. Therefore, it is most vulnerable to trauma when full.
The major function of the kidneys is to keep the composition of the blood stable via regulation of fluid and electrolytes
Renal blood flow at rest is approximately 1100 mL per minute and is approximately 20% of cardiac output
Oxygen consumption of kidneys at rest is 26 mL per minute and is approximately 10% of resting metabolism
Daily urine volume may vary from 500 mL to 15 L
Volume of urine determined primarily by antidiuretic hormone (ADH)
ADH regulates water reabsorption by increasing permeability of distal tubule of the nephron and collecting duct
ADH is released from posterior pituitary in response to signals from hypothalamus
The main stimuli for release of ADH:
Increased plasma osmolality
Decreased blood volume
Decreased blood pressure
ADH release may increase threefold in heavy exercise, helping to prevent free water loss and dehydration
At rest, 15%–20% of renal plasma flow is continuously filtered by glomeruli
Results in about 170 L of filtrate per day
Ninety-nine percent is reabsorbed in tubular system
Exercise results in a reduction of renal blood flow proportional to the intensity of the activity due to shunting of blood to exercising muscle
Mechanism is via constriction of afferent and efferent arterioles because of increased circulating levels of epinephrine and norepinephrine
Moderate exercise (50%
max) results in a 30% reduction; strenuous exercise leads to a 40%–50% decrease in both renal blood flow and glomerular filtration rate (GFR)
During maximal exercise (65%
max), there is a 75% reduction in renal blood flow, which is approximately 1% of the cardiac exercise output
Renal blood flow decreases even more if the individual is dehydrated
Renal blood flow usually returns to pre-exercise levels within 60 minutes
Can be gross or microscopic ( Fig. 32.3 )
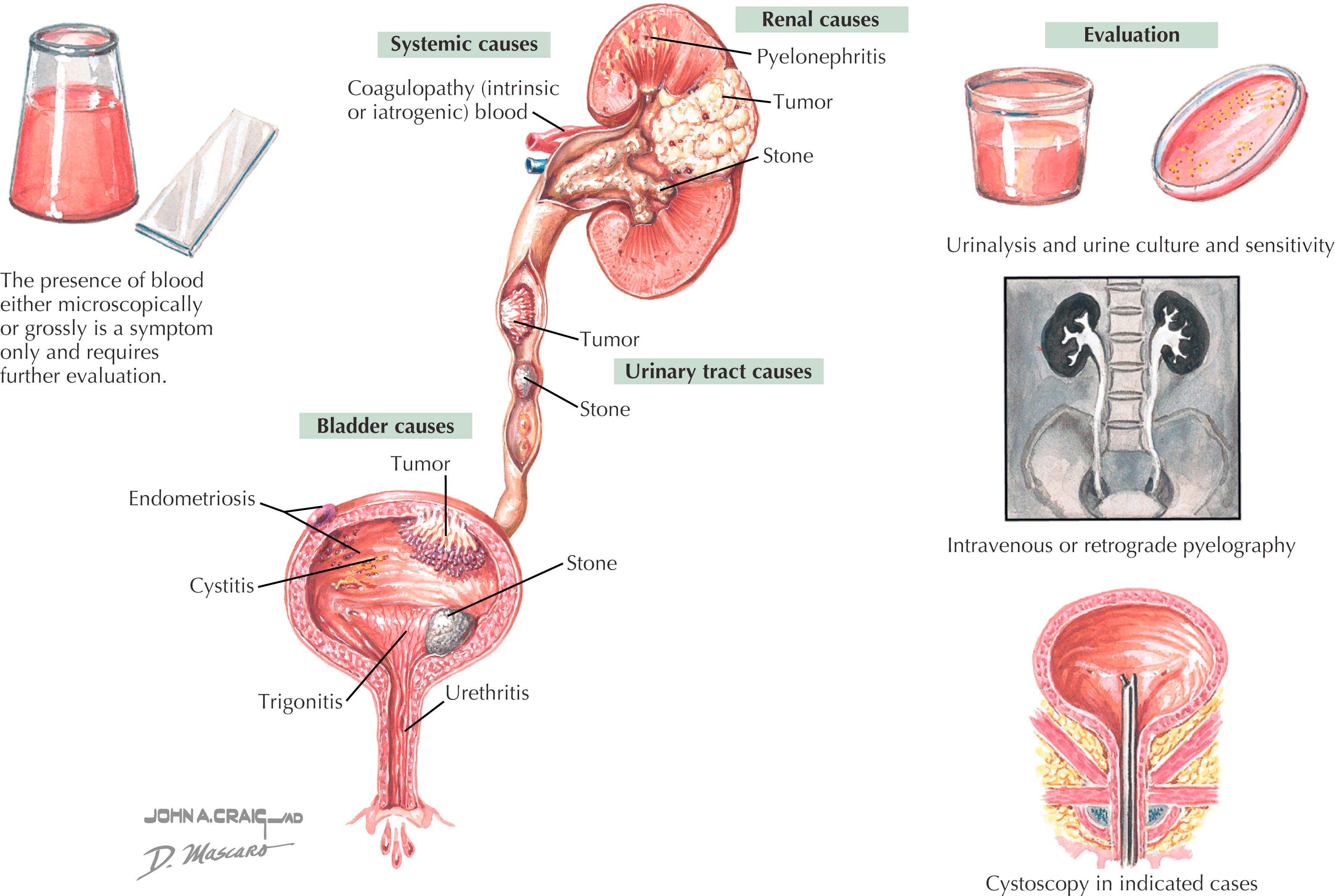
Microscopic hematuria generally defined as more than three red blood cells (RBCs) per high-power field
In athletes, rates of microscopic hematuria can be as high as 75%–80% and often asymptomatic; can occur in both contact and noncontact sports
Although hematuria typically resolves within 48–72 hours, in ultraendurance athletes resolution may take up to 7 days
Overview: Etiologic factors can be categorized by location (kidney vs. bladder).
Nontraumatic renal: Decreased renal blood flow (700 mL per minute to 200 mL per minute, with decrease proportional to intensity) leads to ischemia in the nephron, increased permeability, and subsequent passage of RBCs. Also increased glomerular filtration pressure, secondary to efferent vasoconstriction, leads to passage of RBCs at the glomerulus. Clots are usually not renal in etiology; dysmorphic cells are suggestive of renal source.
Traumatic renal: Direct contact (helmet, ski pole, balance beam, etc.) vs. indirect trauma (“jarring” during running, jumping, etc.).
Bladder: Sports hematuria of bladder origin is almost always traumatic. This can be the result of single, large blunt trauma or multiple lesser forces. Repetitive contact of flaccid posterior bladder wall against anterior wall (trigone) is known as “bladder slap.” It can occur in athletes participating in intense physical activity such as long-distance running, track, swimming, lacrosse, and football. Cystoscopy shows damage of the superficial urothelium or contusion. Incidence can be decreased if bladder is partially filled and adequate hydration maintained.
Prostate/urethra: Usually traumatic, most often seen in cyclists because of repetitive jarring. May be the result of improper seat height and pitch.
Other causes of hematuria:
Nephrolithiasis
Urinary tract infection
Sickle cell anemia or other blood disorder
Malignancy
Drug or medication use (including penicillin, cephalexin, thiazides, allopurinol, nonsteroidal anti-inflammatory drugs [NSAIDs], aspirin, furosemide, and oral contraceptives)
“Foot strike hemolysis”: possible phenomenon where trauma occurs to RBCs circulating in the feet during running, leading to hemolysis. As a result, haptoglobin binds free hemoglobin, and if haptoglobin binding becomes saturated, then hemoglobin is excreted in urine.
Rhabdomyolysis (hematuria on urinalysis [UA] with absent RBCs on microscopy).
Red-colored (without hematuria or RBCs on microscopy) urine attributed to beets, berries, food coloring, phenazopyridine, phenytoin, ibuprofen, nitrofurantoin, sulfamethoxazole, and rifampin.
Diagnosis made by urine dipstick and confirmed with microscopy
Timing of hematuria during urination is important to consider:
Initial hematuria (beginning of urination) often urethral in origin
Terminal hematuria (end of urination) may originate in bladder or prostate (men)
Continuous hematuria suggests renal origin
Some have advocated using mean corpuscular volume (MCV) and RBC morphology to help establish the origin of hematuria:
MCV less than 72 fL considered to be of glomerular origin
MCV more than 72 fL considered to be of nonglomerular origin
Casts or dysmorphic appearance of cells consistent with glomerular origin
If no concerning history, physical examination, or diagnosis, stop exercise and/or suspected medications and repeat UA after 24–72 hours.
If urine clears after 24–72 hours and the patient is under 40 years old, can diagnose exercise-induced hematuria (benign)
If gross or microscopic hematuria persists or patient is over 40 years old, consider further workup:
Urine: culture, cytology
Blood: creatinine, prothrombin time/partial thromboplastin time (PT/PTT), blood urea nitrogen (BUN), complete blood count (CBC), sickle cell (in high-risk populations)
Imaging: renal ultrasound, computed tomography (CT) urography, and/or direct visualization with cystoscopy
If testing remains normal and hematuria persists, must consider intrinsic renal disease:
Creatinine clearance and protein excretion should be measured
Renal ultrasound, retrograde pyelography, and CT scan may be useful
Indications for renal angiography and renal biopsy are controversial
Athletes with benign hematuria secondary to exercise may return to activity once hematuria resolves
Return to play for other causes of hematuria is diagnosis-dependent
Athletes, especially those with prior episodes of hematuria, should be encouraged to drink fluids before and during exercise to avoid dehydration
Keeping a slightly distended bladder during exercise can help prevent “bladder slap”
May be present in up to 70% of athletes after exertion and usually asymptomatic
Exercise-induced proteinuria is a function of the intensity of exercise
Strenuous exercise can cause protein excretion to reach 1.5 mg/min, but usually does not rise beyond 1–2 g/day (normal: 150–200 mg/day)
Usually occurs within 30 minutes of exercise and clears in 24–48 hours
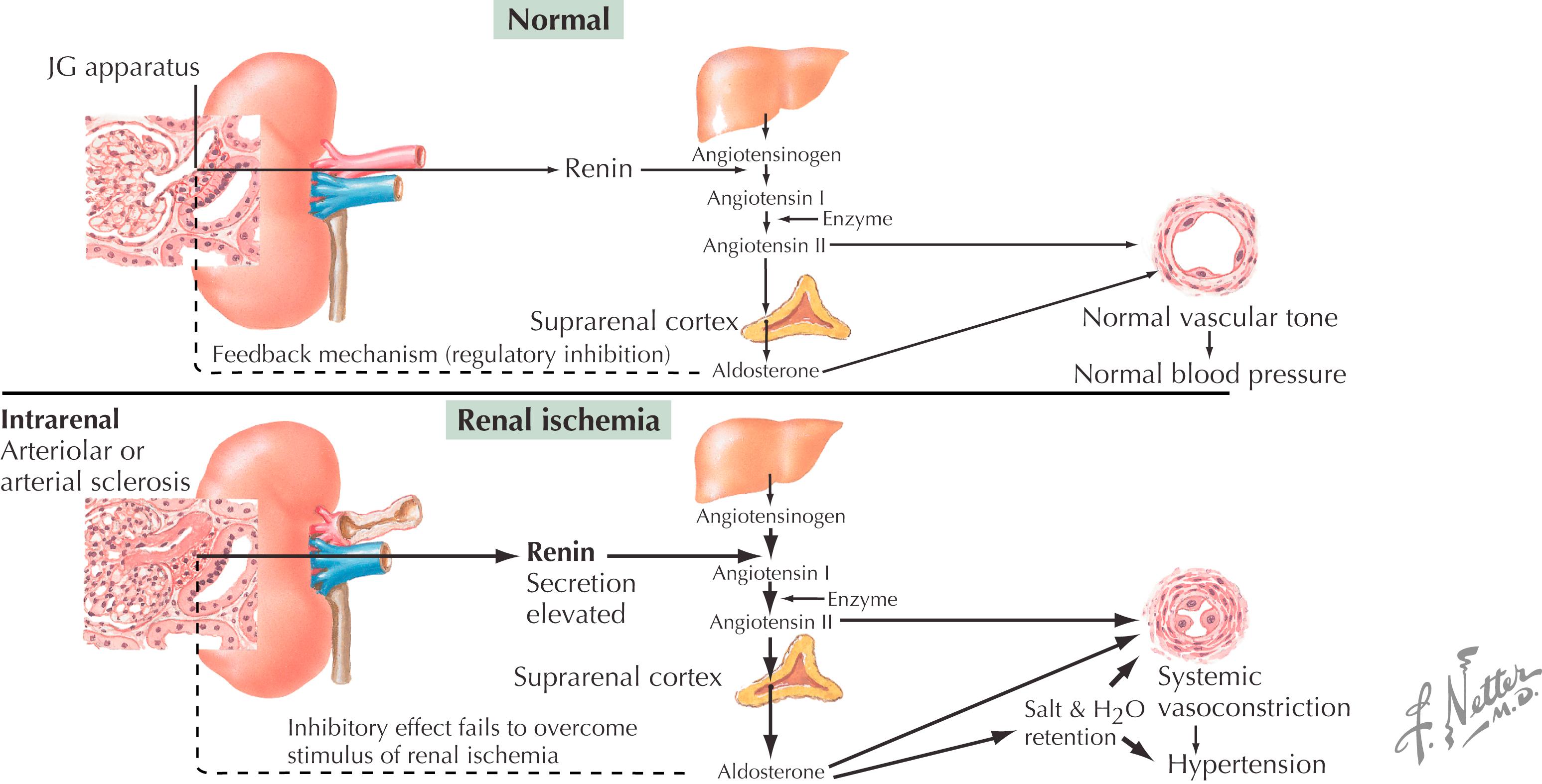
Root cause unknown, but renin–angiotensin system (RAS) and prostaglandins play a major role:
Plasma concentration of angiotensin II increases during exercise, resulting in increased protein filtration through the glomerular membrane
Strenuous exercise also activates the sympathetic nervous system, which releases catecholamines, also increasing glomerular membrane permeability
Creatine supplements do not increase proteinuria
In patients with underlying chronic kidney disease (CKD), low-intensity exercise does not increase proteinuria or lead to rapid progression of CKD
Urine dipstick is generally a good tool for initial evaluation
Screening for proteinuria at sports preparticipation examinations is not recommended, as the diagnostic utility is low
If a routine UA shows protein and was collected within 24 hours of intense exercise, repeat testing in absence of prior exercise to determine transient vs. persistent proteinuria
Other causes for false-positive proteinuria on UA:
Highly concentrated urine (specific gravity >1.030)
Contamination with antiseptics
Pyridium (phenazopyridine HCl) use
Highly alkaline urine (pH >8)
Thorough history should be taken, including personal and family history of:
Renal disease
Anemia
Hypertension—make sure to get accurate, manual blood pressure reading
Diabetes
Medication use (e.g., NSAIDs, antibiotics)
Further workup may include:
Serum tests for renal function (BUN, creatinine)
Twenty-four-hour urine for total protein, creatinine, and creatinine clearance
Fasting blood glucose or hemoglobin A1c
CBC or other tests as medically indicated
Imaging: renal ultrasound, CT
Renal biopsy
If proteinuria is transient and exercise-induced, no treatment is indicated
If proteinuria is persistent and medically related, treatment depends on underlying cause
If proteinuria clears within 24–72 hours, OK for return to play
If proteinuria persists, further workup is needed before clearance
As exercise-induced proteinuria is likely a normal physiologic process during exercise, there are no known prevention strategies
Exercise-induced proteinuria does not decrease with regular physical training, even in high-level athletes
Adequate hydration with exercise can help avoid overly concentrated urine and subsequent false-positive proteinuria on urinalysis
Acute kidney injury (AKI) is uncommon in sports, but occurrence is well documented
Criteria for meeting AKI is becoming more prevalent in athletes with rise of ultraendurance events
Combined effects of exercise to exhaustion, dehydration, hyperpyrexia, and rhabdomyolysis culminate in renal dysfunction by release of muscle enzymes and myoglobin that precipitates in renal tubules ( Fig. 32.4 )
Over 25% of participants in ultraendurance races have evidence of AKI (at least 1.5–2× normal creatinine)
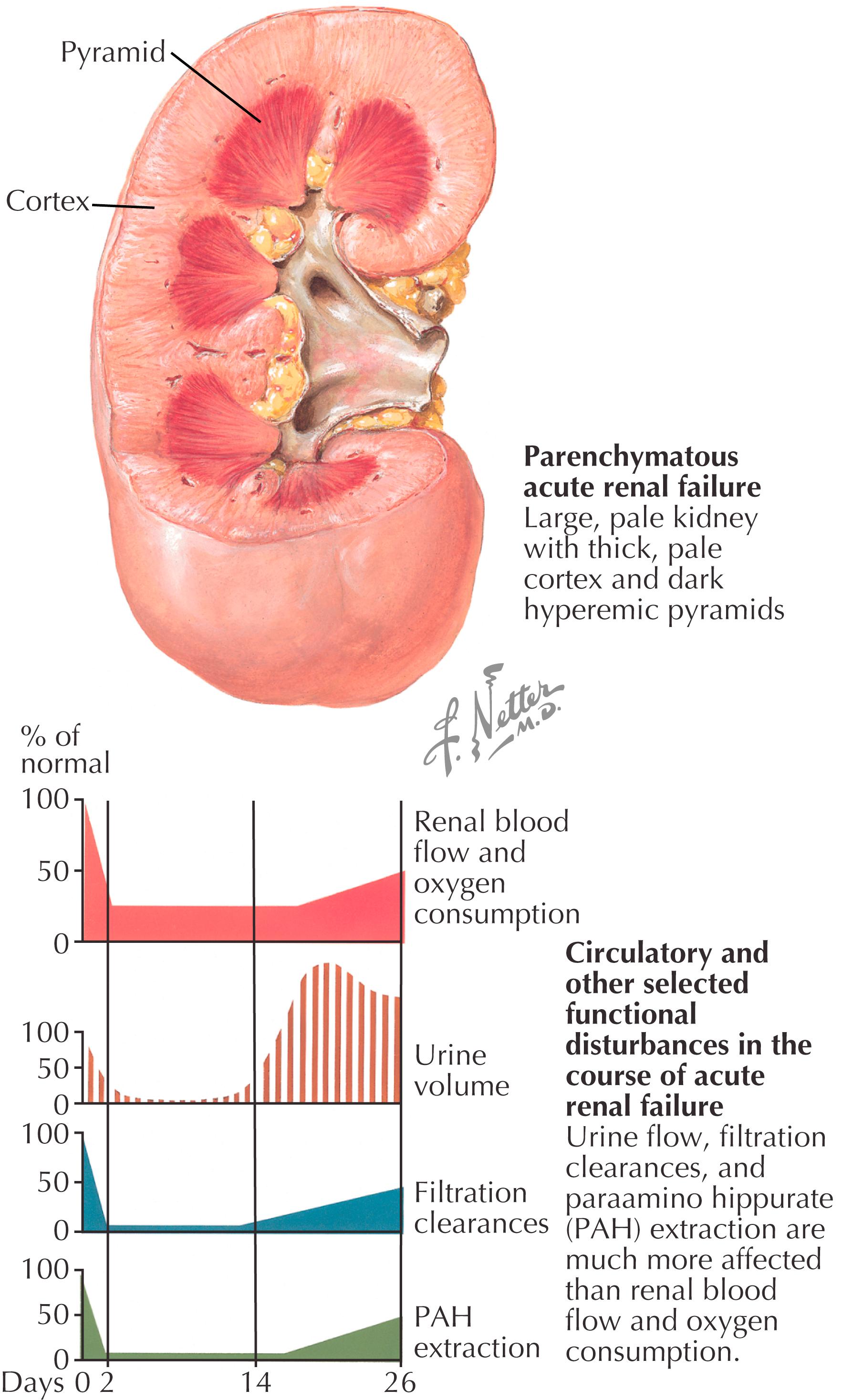
Underlying renal disease, dehydration, heat stress, genetic predisposition (i.e., sickle cell trait), and NSAID use are risk factors
AKI defined as an increase in serum creatinine by ≥0.3 mg/dL or an increase to ≥1.5 times the baseline value
Creatine kinase (CK) levels correlate well with myoglobin concentrations in the blood and can be associated with AKI in rhabdomyolysis
Although CK levels of 20,000 IU per liter is the typical threshold for treatment and hospitalization, levels over 100,000 have been reported in asymptomatic individuals during and after exercise
AKI with rhabdomyolysis may also be associated with:
Disseminated intravascular coagulation (DIC)
Hyponatremia, hyperkalemia, hyperphosphatemia, hyperuricemia
Hypocalcemia—caused by precipitation of calcium phosphate in muscle from hyperphosphatemia
An athlete with AKI should be treated according to standard treatment guidelines—crystalloid solution, electrolyte replacement, etc.
Hypertonic saline should be used in the case of acute neurologic deterioration with concurrent exercise-associated hyponatremia
Serum creatinine, CK, and electrolyte levels should return to baseline before return to play initiated
There is only theoretical risk, but no proven evidence, that repetitive AKI in athletes leads to development of CKD
Prevention is primarily related to proper hydration before and during exercise
Adequate training, heat acclimatization, and avoidance of NSAIDs may help as well
NSAIDs may have deleterious effects because of their ability to inhibit cyclooxygenase within the kidney ( Fig. 32.5 )
Cyclooxygenase is the rate-limiting enzyme for synthesis of prostaglandins (PGs)
Vasodilatory PGE 2 and PGI 2 play a protective role in the kidney by modulating renal vasoconstriction caused by:
Increased renal sympathetic activity
Renin-angiotensin II
Circulating catecholamines
Under stress conditions (salt restriction, dehydration, and heat), ibuprofen decreases GFR after 45 minutes of exercise at 65%
max, compared with placebo or acetaminophen
Both indomethacin and celecoxib showed decreases in free water clearance, which in certain environments or conditions can predispose to hyponatremia
Creatine monohydrate is widely used as an ergogenic aid for muscle performance
Increases muscle stores of creatine, leading to greater adenosine triphosphate (ATP) synthesis
In healthy individuals, there is no known link between creatine ingestion and renal dysfunction if used over the short term in typical doses (loading dose 20 g/day × 5 days and then 3–5 g/day maintenance dose)
There have been case reports of acute renal failure in individuals who were long-term, high-dose users
Those with established renal disease should avoid supplementation
End product of creatine is creatinine, which is filtered in the glomeruli and excreted; this can be a false indicator of renal dysfunction
In those using creatine, renal function can be evaluating by measuring, under resting conditions, the albumin excretion rate (normal: <20 mcg/min)
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here