Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aldosterone represents the primary mineralocorticoid produced by the adrenal gland and specifically within the outer adrenocortical cells of the glomerulosa. The need for the structural zonation of the adrenal cortex as a mechanism to control aldosterone production is apparent when one considers that the amount of aldosterone needed to control salt balance is 100- to 1000-fold less than the amount of cortisol needed to control carbohydrate metabolism. The unique histology of the three zones of the mammalian adrenal cortex led to the names zona glomerulosa, zona fasciculata, and zona reticularis. It is now accepted that these zones have functionally distinct roles that allow the production of three major classes of steroid hormone, namely, zona glomerulosa-derived mineralocorticoids, zona fasciculata-derived glucocorticoids, and zona reticularis-derived adrenal androgens. The mechanisms leading to adrenal zonation remain poorly defined but the gland’s unique centripetal blood flow is believed to play a key role. Arterial blood enters the outer adrenal cortex, flows through fenestrated capillaries that start in the glomerulosa layer, progresses between the cords of fasciculata cells, and drains inwardly into venules in the medulla. The directional flow of blood ensures that the outer zona glomerulosa is unable to utilize fasciculata cell-produced precursor for the synthesis of aldosterone.
Aldosterone is synthesized in the glomerulosa from cholesterol through the successive actions of four enzymes ( Fig. 24.1 ). Cholesterol side-chain cleavage (CYP11A1), 21-hydroxylase (CYP21), and aldosterone synthase (CYP11B2) are members of the cytochrome P450 family of enzymes. CYP11A1 and CYP11B2 are localized to the inner mitochondrial membrane, while CYP21 is found in the endoplasmic reticulum. The fourth enzyme, type 2 3β-hydroxysteroid dehydrogenase (HSD3B2), is a member of the short-chain dehydrogenase family and is localized to endoplasmic reticulum. In the first reaction, cholesterol is converted to pregnenolone by mitochondrial CYP11A1. This represents the rate-limiting reaction for all steroid-producing tissues and requires the transport of cholesterol from the cytoplasm to the mitochondrial outer membrane, followed by movement from the outer to the inner mitochondrial membrane where CYP11A1 is located. This step is acutely regulated through the expression and phosphorylation of steroidogenic acute regulatory protein (StAR). The product of this reaction, pregnenolone, can move via passive diffusion to the endoplasmic reticulum for conversion to progesterone by HSD3B2 (and likely HSD3B1, type 1 3β-hydroxysteroid dehydrogenase). Progesterone is hydroxylated to deoxycorticosterone by CYP21. Deoxycorticosterone can be converted to aldosterone by three successive oxidation reactions (11β- and 18-hydroxylation, followed by 18-oxidation), which in humans can be mediated by a single enzyme, aldosterone synthase CYP11B2. It should be noted that cortisol biosynthesis also requires 11β-hydroxylation to produce cortisol from 11-deoxycortisol, and this is accomplished by 11β-hydroxylase (CYP11B1). However, this isozyme poorly catalyzes 18-hydroxylation and it does not catalyze 18-oxidation, thus preventing synthesis of aldosterone by the zona fasciculata.
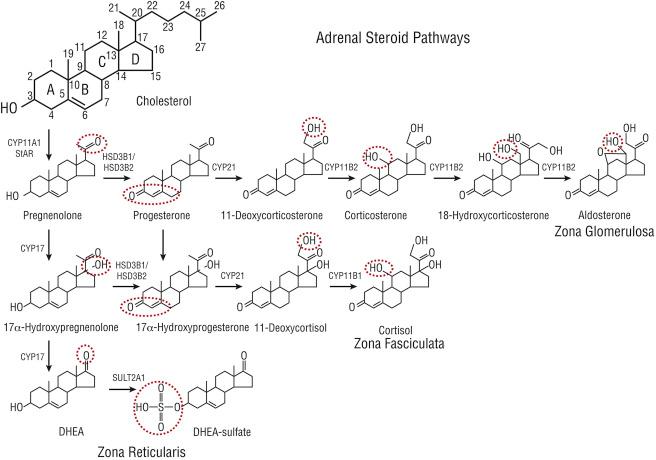
In humans, functional zonation relies in part on the localized expression of two cytochrome P450 enzymes, specifically CYP11B2 and 17α-hydroxylase (CYP17). Expression of CYP11B2 is limited to the glomerulosa and this effectively prevents production of aldosterone in the other adrenocortical zones. On the other hand, CYP17 diverts pregnenolone and progesterone away from the pathway leading to aldosterone and into that leading to cortisol ( Fig. 24.1 ), explaining the reason for the lack of expression of CYP17 by the glomerulosa. Even in the presence of CYP17, the fasciculata produces large amounts of aldosterone precursors (in particular, progesterone and deoxycorticosterone) at levels that would lead to mineralocorticoid excess if they were even partially converted to aldosterone. Limiting expression of CYP11B2 to the outer zone in a situation where centripetal blood flow prevents these precursors from accessing CYP11B2 further controls the relative production rate of mineralocorticoids.
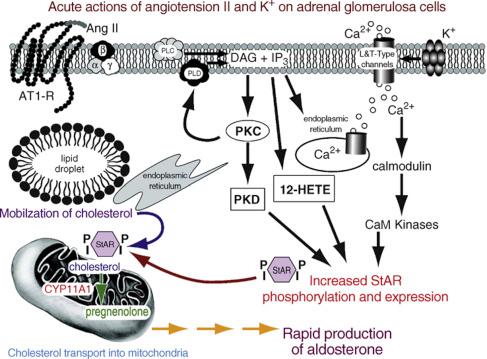
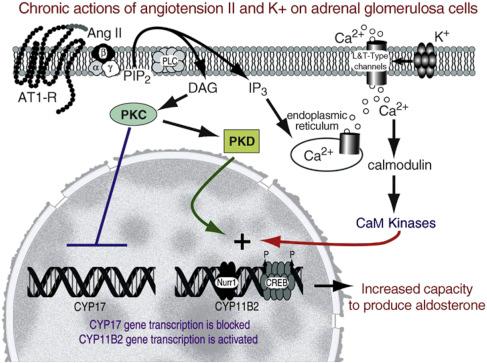
Historically, the regulation of aldosterone biosynthesis has been divided into two main phases. Acutely (minutes after a stimulus), aldosterone production is controlled by rapid signaling pathways that increase the movement of cholesterol into the mitochondria. This has been called the “early regulatory step” and as noted earlier is mediated by increased expression and phosphorylation of StAR protein ( Fig. 24.2 ). Chronically (hours to days), aldosterone production is regulated at the level of expression of the enzymes involved in the synthesis of aldosterone ( Fig. 24.3 ). This has been called the “late regulatory step” and is particularly dependent on increased transcription and expression of CYP11B2.
Renin is a protease produced and stored in the juxtaglomerular cells that surround the glomerular afferent arterioles. Renin release is controlled by three mechanisms. First, release is activated by a decrease in the perfusion pressure of blood traversing the renal afferent arterioles, which is sensed by the juxtaglomerular apparatus functioning as a baroreceptor. Second, renin secretion can be stimulated by secretions from the macula densa as a result of a drop in sodium concentration in the distal tubule. Third, a drop in blood pressure will cause sympathetic stimulation of juxtaglomerular cells to stimulate both renin release and afferent arteriole constriction.
Acting in the blood, renin mediates the rate-limiting step in the production of angiotensin II (AngII), cleaving the circulating precursor angiotensinogen to release the 10-amino acid peptide, angiotensin I ( Fig. 24.4 ). Thereafter, inactive angiotensin I is converted rapidly to the octapeptide hormone AngII by the action of angiotensin-converting enzyme (ACE). Circulating AngII is arguably the most important regulator of adrenal glomerulosa aldosterone production.
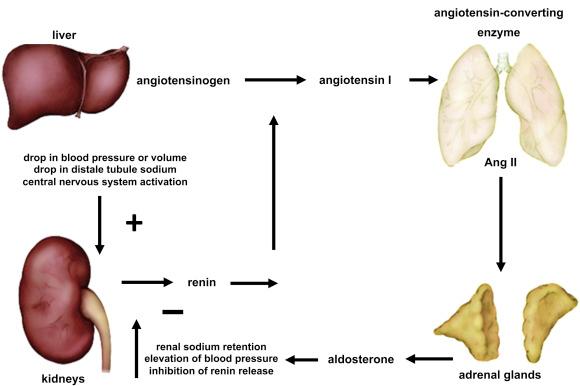
In humans, AngII has two G protein-coupled receptors, type 1 (AT1) and type 2 (AT2), through which this hormone can elicit intracellular responses. In the glomerulosa, AngII works primarily through AT1 receptors to regulate aldosterone production ( Fig. 24.2 ). AT1 receptors activate a variety of signaling pathways including phosphoinositide-specific phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP 2 ) to generate the two second messengers, inositol 1,4,5-trisphosphate (IP 3 ) and diacylglycerol (DAG). IP 3 is thought to initiate aldosterone secretion by eliciting a transient increase in the cytosolic calcium concentration and activating calcium/calmodulin-dependent protein kinases (CaM kinase), whereas DAG increases protein kinase C (PKC) activity. PKC activity has been suggested to underlie sustained aldosterone secretion from glomerulosa cells. This idea is supported by the ability of synthetic ligands for PKC to stimulate aldosterone production and inhibitors of PKC to inhibit aldosterone production. AngII also increases calcium influx in glomerulosa cells. Influx of extracellular calcium acts to increase PKC activity, enhance PKC-stimulated steroidogenesis and maintain aldosterone production. This increase in calcium influx is likely brought about by multiple mechanisms including AngII depolarization of glomerulosa cells via effects on potassium channels and voltage-gated calcium channels through G i . In fact, intracellular recording demonstrated that under basal conditions, adrenal zona glomerulosa cells are hyperpolarized with negative resting potentials determined by membrane permeability to K + . AngII decreases the activity of the leak K + channels and decreases the expression of the Kir3.4 channel, resulting in membrane depolarization.
Calcium influx is a critical component for sustained aldosterone production, but this signal alone does not appear to be sufficient to elicit maximal steroidogenesis. The other important signal for sustained aldosterone production is the PKC pathway. Indeed, activating PKC and calcium influx essentially reproduces AngII-stimulated aldosterone secretion, as well as the protein phosphorylation pattern. While generation of the DAG/PKC signal is initiated by activation of PLC, the phospholipase D (PLD) signaling system is also activated following AngII treatment of glomerulosa cells. PLD is activated in a sustained manner by AngII, suggesting the possibility that PLD may mediate prolonged generation of DAG and stimulation of the PKC pathway. PLD hydrolyzes primarily phosphatidylcholine to yield phosphatidic acid (phosphorylated DAG), which can then be converted to DAG by the action of lipid phosphate phosphatases, representing therefore a reservoir for sustained DAG production. In adrenal glomerulosa cells AngII stimulates the production of myristate-containing DAG ; since myristate is incorporated primarily into phosphatidylcholine, this result suggests the possibility that both PLC and PLD mediate AngII-induced DAG generation. PLD activity also appears to be necessary for AngII-induced aldosterone secretion, as demonstrated using the primary alcohol 1-butanol, which diverts production away from phosphatidic acid and DAG and instead forms phosphatidylbutanol (vs. the control tert-butanol, which does not affect PLD-generated lipid signals). 1-Butanol was found to inhibit the AngII-induced increase in DAG and phosphatidic acid levels, as well as AngII-elicited aldosterone secretion, in bovine adrenal glomerulosa cells and in H295R cells, whereas tert-butanol did not. In addition, the PLD inhibitor, fluoro-2-indolyl deschlorohalopemide (FIPI) inhibits aldosterone production stimulated by AngII in primary cultures of bovine adrenal glomerulosa cells and in the HAC15 human adrenocortical carcinoma cell line. More direct evidence arises from studies in which PLD was overexpressed using adenovirus-mediated transduction. Overexpression of wild-type PLD1 or PLD2, but not the lipase-inactive isoforms, increased PLD activity. However, only wild-type PLD2 enhanced AngII-stimulated aldosterone secretion, suggesting that PLD2 is the isoform mediating aldosterone secretion in response to AngII.
In addition to activating PKC, the DAG produced by AngII-stimulated phospholipid hydrolysis also serves as a precursor for other signals regulating aldosterone secretion. Thus, arachidonic acid can be released by DAG lipase from arachidonic acid-containing DAG and is then metabolized by 12-lipoxygenase to generate 12-hydroxyeicosatetraenoic acid (12-HETE). 12-HETE appears to play an important role in mediating AngII-induced aldosterone secretion since blocking either arachidonic acid release with a DAG lipase inhibitor or its metabolism with a lipoxygenase inhibitor reduces AngII-stimulated steroidogenesis. In addition, this signal appears to be regulated and/or mediated by the PKC pathway, suggesting possible cross talk between these two signals. This arachidonate metabolite also seems to be involved in AngII-elicited increases in cytosolic calcium levels, and this effect is presumably at least part of the mechanism by which AngII-induced 12-HETE generation contributes to aldosterone production. Thus, DAG metabolism to 12-HETE and its activation of PKC appear to play important roles in aldosterone production. PKC can also activate protein kinase D (PKD), an enzyme that has some similarities to PKC in its ability to bind DAG and phorbol esters but has been classified as a separate kinase family. PKD is activated by AngII in the H295R human adrenal cell model, in primary cultures of bovine glomerulosa cells and in human aldosterone-producing adenomas (APAs). PKD has also been shown to phosphorylate and activate members of the cyclic AMP (cAMP) response element binding (CREB) protein family of transcription factors in glomerulosa cells and other systems. Activated CREB family members were found to bind to the StAR promoter and increase StAR transcription in glomerulosa cells, suggesting another way in which PKD might influence acute aldosterone biosynthesis. This result is consistent with data from Manna et al. demonstrating that PKD regulates StAR levels via CREB and AP-1 in a Leydig cell model. Alternatively, PKD may modulate longer term glomerulosa growth responses through regulation of mitogen-activated protein kinase activation.
Activation of the above signaling pathways initiates acute aldosterone biosynthesis, which requires two distinct processes to occur. As discussed above, cleavage of cholesterol’s side-chain is the rate-limiting reaction in steroidogenesis and this occurs inside the mitochondria. Therefore, cholesterol must first be mobilized to the mitochondria from storage sites in cytosolic lipid droplets. This first step is thought to be mediated by cytoskeletal rearrangements that allow juxtaposition of the mitochondria and lipid droplets. One protein that may be involved in this event may be the myrisotylated alanine-rich C kinase substrate (MARCKS) protein. MARCKS binds actin in a manner which is regulated by both protein kinase A (PKA) and PKC phosphorylation, and secretagogue-induced phosphorylation of this protein has been proposed to play a role in the cytoskeletal rearrangements associated with aldosterone production. Moreover, small GTPases like Rho can regulate the cytoskeleton and the role of these signaling molecules in glomerulosa cell function are currently under study.
The second and rate-limiting step of steroidogenesis involves transfer of the mobilized cholesterol from the outer mitochondrial membrane to the inner mitochondrial membrane, where CYP11A1 is localized and initiates steroid production. StAR possesses the characteristic properties of the labile acute regulator of steroidogenesis described in numerous studies from the laboratories of Orme-Johnson and Stocco. These criteria include:
The synthesis of StAR is increased upon hormone stimulation and is cycloheximide inhibited with a half-life of about 5 min.
StAR is localized to the mitochondria where it increases the transfer of cholesterol from the outer to the inner membrane.
StAR is specifically expressed in steroidogenic cells.
Increasing steroidogenic cell expression of StAR proteins using transgenes increases steroidogenesis.
Mutations in the StAR gene are found in the human disease lipoid congenital adrenal hyperplasia, which is characterized by a complete inability to synthesize adrenal and gonadal steroid hormones.
A mutant mouse generated to be deficient for the StAR gene demonstrated a phenotype similar to lipoid congenital adrenal hyperplasia. The StAR gene encodes a 37 kDa protein with a mitochondrial targeting sequence, which does not appear to be required for its role in the transfer of cholesterol into the mitochondria. This 37 kDa protein is processed to several mitochondrial proteins that range in size (30–32 kDa) and isoelectric point (6.1 and 6.7). The more acidic products of StAR proteolysis represent modifications by phosphorylation. Indeed, it is thought that phosphorylation is important to StAR’s function, presumably yielding the active form(s) of the protein. Thus, steroidogenesis is decreased when cells are incubated with amino acid analogs that cannot be phosphorylated or when cells are transfected with mutant StAR protein with alanine substituted for serine 194 (hamster) or 195 (human) that prevents phosphorylation. The amino acid residue mutated in this case represents a PKA rather than a PKC consensus phosphorylation site.
Because AngII, potassium, and adrenocorticotropic hormone (ACTH) differentially alter StAR phosphorylation, it is likely that both PKC- and PKA-activating agonists are capable of regulating StAR activity via phosphorylation. Nevertheless, a recent study in a Leydig cell model suggests that PKC alone increases StAR protein expression without triggering its steroidogenic activity. In this case the inclusion of low doses of cAMP analogs, which by themselves were without effect on StAR or steroidogenesis, resulted in an enhancement of StAR levels and steroid production. Another study examining the effect of sodium restriction in vivo, a manipulation that increases endogenous AngII, concluded that subtle changes in StAR processing (i.e., proteolysis and phosphorylation), rather than large changes in StAR protein expression, mediate acute steroidogenesis.
The mechanism by which StAR induces cholesterol movement from the outer to the inner mitochondrial membrane is poorly understood; nevertheless, StAR is clearly required for the translocation of cholesterol from the outer to the inner mitochondrial membrane, and this rate-limiting step is controlled by signaling pathways (e.g., PKA, PKC, casein kinase 2) that are activated by aldosterone secretagogues.
Chronically, AngII increases adrenal aldosterone production through two major actions: first, increasing the expression of the enzymes needed to produce aldosterone, particularly CYP11B2 and second, causing hypertrophy and hyperplasia of the adrenal glomerulosa. In vivo studies have provided strong evidence that sodium restriction increases renin/AngII levels causing an induction of glomerulosa CYP11B2 expression. The ability of low sodium diets to increase aldosterone production and CYP11B2 expression can be largely (but not completely) inhibited by angiotensinogen inhibitors or antagonists of the AT1 receptor. Taken together, these studies suggest an important role for AngII in regulating glomerulosa expression of CYP11B2 and the long-term production of aldosterone.
In vitro studies have focused on defining the intracellular signals involved in AngII-directed CYP11B2 expression. As noted above, adrenal AT1 receptors couple to several signaling pathways. The most characterized of these is the activation of PLC which increases intracellular calcium and DAG. These second messengers activate calmodulin and PKC, respectively. PKC does not increase transcript levels of CYP11B2 but can act as an inhibitor for the expression of the fasciculata enzyme, CYP17 ( Fig. 24.3 ). This effect has been recently attributed to Fos-mediated repression of the CYP17 transcriptional activator, steroidogenic factor 1 (SF1). Therefore, PKC activation may play an important role in the zonation of the adrenal by blocking glomerulosa cell expression of CYP17. Several lines of evidence suggest that calcium signaling acts to increase glomerulosa cell expression of CYP11B2. For example, calcium signaling appears to be the major pathway used by extracellular potassium to increase CYP11B2 levels based on the ability of inhibitors of calcium influx, calmodulin, or CaM kinase to block completely the ion’s ability to induce CYP11B2 mRNA levels. However, AngII-mediated increases in CYP11B2 mRNA are only partially sensitive to the inhibition of calmodulin or CaM kinase. Thus, AngII may induce CYP11B2 expression through calcium and other signaling pathways ( Fig. 24.3 ).
The increase in CYP11B2 expression apparently results from increased transcription of the gene. Activation of transcription seems to rely on the activation of transcription factors that bind to a cAMP response element (CRE) found in the proximal region of the CYP11B2 promoter. In addition, both AngII and potassium rapidly induce the expression of the nuclear hormone receptor NR4A3, which also binds the promoter and activates CYP11B2 transcription. This factor’s expression is also increased in adrenal aldosterone-producing tumors and may play a role in the increase in tumor CYP11B2 levels.
AngII regulation of long-term aldosterone production relies on other signaling pathways. AngII treatment also activates adrenal cell PKD and this activation is associated with increased CYP11B2 expression, suggesting that this pathway may be involved in CYP11B2 regulation. Further, AngII-induced PKD activation is dependent upon PKC. However, PKD is known to phosphorylate and stimulate transcriptional activity of the CREB protein transcription factor in kidney HEK 293 cells, in MA-10 Leydig cells and in primary cultures of glomerulus cells. As noted above, the promoter region of CYP11B2 is highly dependent on CREB response elements, suggesting that this PKD-mediated CREB activation may be important in regulating chronic aldosterone synthetic capacity.
The role of potassium in the regulation of aldosterone production is often underestimated. Infusion of potassium will cause an acute increase in aldosterone production, and a high potassium diet will increase aldosterone levels as well as the capacity of the adrenal to produce aldosterone. The development of angiotensinogen-deficient mice is perhaps the most convincing evidence for the role of potassium in the regulation of the adrenal glomerulosa. These animals are devoid of AngII but maintain adrenal zona glomerulosa aldosterone production that appears to be regulated predominantly by circulating potassium. This potassium effect seems appropriate, as aldosterone increases renal excretion of potassium. If levels of plasma potassium are suddenly raised, as after a large meal of potassium-rich foods, aldosterone is secreted and acts on the kidney to induce the excretion of the excess potassium load. As described below, however, the effects of aldosterone to increase renal sodium retention and potassium excretion require an hour or more; thus, there must be more rapid ways to remove high levels of potassium entering the circulation, because a significant increase would be life threatening. The rapid transfer of potassium from extracellular fluid into cells is accomplished by a combination of insulin and epinephrine effects on potassium transport across cell membranes. Renal clearance through both acute and chronic mechanisms will also act to regulate potassium levels.
The mechanism by which potassium regulates aldosterone production relies on the extreme sensitivity of the glomerulosa cell membrane to small increases in potassium concentrations. Indeed, small increases in potassium stimulate calcium influx, via depolarization of the plasma membrane and activation of voltage-dependent calcium channels. As with AngII stimulation, this influx is also thought to activate CaM kinase, and this influx is required for elevated potassium-induced aldosterone secretion, since inhibition of calcium influx abolishes the elevated potassium-stimulated secretory response. DAG and PKC are generally not thought to play a role in potassium stimulation of aldosterone secretion. This idea was based on studies showing that—as opposed to AngII treatment—potassium does not induce glomerulosa cell phosphatidylinositol hydrolysis, increases in DAG content, or PKC translocation to the plasma membrane. However, a previous report presented indirect evidence using a nonspecific inhibitor of PKC for a possible effect of increased potassium on the activity of this enzyme. In addition, it has been shown that small elevations in potassium trigger the phosphorylation of MARCKS, an endogenous PKC substrate whose phosphorylation is thought to be a marker for PKC activation in intact cells. Furthermore, elevations in potassium induce PLD activation, suggesting a possible mechanism for the observed MARCKS phosphorylation. Thus, PKC activity may also contribute to the aldosterone production induced by calcium-signaling pathways activated by elevated potassium.
It is accepted that small changes in circulating potassium can act directly on the adrenal glomerulosa to stimulate aldosterone biosynthesis; however, the direct influence of circulating potassium on glomerulosa production of aldosterone has not been studied as extensively as the influence of sodium balance. A recent study in mice also reported a slight increase in the thickness of the zona glomerulosa with increased dietary potassium and suggested the role of several genes in this process. These genes, including Mtus 1, Smoc 1, and Grp 48, were observed to be upregulated within 28 days of the initiation of a high potassium diet, although the in vitro experiments did not completely parallel these microarray results. In addition, rats on high potassium diets exhibit increased circulating aldosterone levels and adrenal expression of CYP11B2. Furthermore, studies using mice with targeted deletion of genes in the renin–angiotensin system have demonstrated that potassium can substitute for angiotensin and increase CYP11B2 expression and aldosterone production in the adrenal. Potassium signaling in glomerulosa cells involves a depolarization of the membrane leading to influx of calcium through T- and L-type channels. Consistent with this, pharmacologic elevation of cytosolic calcium increases expression of CYP11B2 mRNA in adrenal cell models and calcium channel blockers (CCBs) such as nifedipine block potassium induction of CYP11B2. Thus, a role for calcium in potassium induction of chronic aldosterone production and CYP11B2 expression appears likely.
Intracellular calcium signaling often occurs through the action of the calcium-binding protein, calmodulin. Calmodulin is a widely expressed protein that, in its calcium-bound form, activates a variety of enzymes and kinases. The tissue-specific expression of these kinases appears to be an important mechanism controlling the responses of different tissues to this signaling pathway. Of the various CaM kinases described to date, the multifunctional family of CaM kinases (types I, II, and IV) are most likely to be involved in AngII and potassium induction of aldosterone production. These kinases phosphorylate a wide variety of substrates and thus are distinct from the dedicated calcium-activated kinases such as myosin light chain kinase or phosphorylase kinase. Antagonists of calmodulin and CaM kinases completely inhibit potassium induction of CYP11B2 mRNA. In addition, the level of CaM kinase type I is elevated in the outer zones of the adrenal cortex. Increases in transcription appear to rely on the activation of transcription factors that bind to a CRE found in the proximal region of the CYP11B2 promoter. In addition, both AngII and potassium rapidly induce the expression of the nuclear hormone receptor NR4A3, which also binds the promoter and activates CYP11B2 transcription. Within the adrenal cortex, the expression of NR4A3 is highest in the adrenal glomerulosa layer, and its expression is elevated in aldosterone-producing adrenal tumors. These findings suggests that potassium stimulation of chronic aldosterone secretion relies on a relatively straightforward pathway involving an increased cytosolic calcium signal that activates CaM kinases, via the upstream regulator CaMKK2, thereby increasing expression of CYP11B2 ( Fig. 24.3 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here