Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
According to the American Society of Anesthesiologists (ASA) practice guidelines, acute pain in the perioperative setting is defined as the presence of pain in a surgical patient after a procedure. The United States Institute of Medicine reported that despite advancements in perioperative pain control, 80% of patients who have surgery report postoperative pain, and 88% of these patients grade their severity as moderate, severe, or extreme. Uncontrolled postoperative pain may be associated with slower recovery times, prolonged hospital length of stay, increased readmission rates, increased time before ambulation, increased hospital costs, and decreased patient satisfaction. , Suboptimal analgesia may also have organ-specific complications, including poor respiratory effort from splinting and atelectasis, increased incidence of myocardial ischemia, impaired wound healing, delayed gastrointestinal motility with prolonged ileus, psychological distress with anxiety, and lack of sleep.
The concept of multimodal analgesia was introduced in 1993 to improve analgesia while concomitantly limiting opioid consumption and reducing the risk of opioid-related adverse effects. This involves the combined use of regional anesthesia or local anesthetic infiltration, opioid medications, as well as various classes of non-opioid analgesics, including nonsteroidal anti-inflammatory drugs (NSAIDs), selective-cyclooxygenase-2 (COX2) inhibitors, N-methyl-d-aspartate (NMDA)-receptor antagonists, and anti-epileptic drugs. Multimodal regimens may also be associated with the attenuation of the transition from an acute to a chronic postoperative pain state. ,
Regional anesthesia techniques include both the central neuraxial blockade and peripheral nerve blockade, both of which are frequently used either intraoperatively as the primary anesthetic or perioperatively as adjunctive therapy to manage acute postoperative pain, reduce opioid consumption, improve satisfaction and quality of life, decrease hospital expenditures, and improve other postsurgical outcomes. More recently, the use of ultrasonography in the fascial plane and neurolocalization has expanded the utilization of peripheral nerve blockade. Ultrasound guided regional anesthesia (UGRA) has been associated with improvements in performance time, reduction in doses of local anesthesia, and reductions in some complications (e.g. pneumothorax).
This chapter discusses the use of regional anesthesia techniques to control postoperative pain in various surgical types. The authors support a multimodal approach to the management of postoperative pain control, and more details on pharmacologic treatment in a multimodal approach can be found in Chapter 25 and adjuvant medications are addressed in Chapter 54 .
Surgical trauma activates peripheral nociceptors, including both high threshold neurons sensitive to mechanical, thermal, and chemical stimuli, which then relay afferent signals to the cerebral cortex via the spinal-thalamic tract. However, these afferent signals may be amplified at various points along the pathway. The presence of inflammation at the site of surgical trauma leads to local release of neurochemical factors, which increase sensitivity to endogenous ligands and decrease the threshold of afferent nociceptive neurons (peripheral sensitization). Inflamed neurons may also alter the expression of sodium channels, which may show an increased proportion of tetrodotoxin-resistant channels, which have greater spontaneous ectopic activity. Surgical insults may also lead to increased neuronal excitability within the spinal cord because of a persistent signal from peripheral afferent nociceptive neurons (central sensitization). , The nociceptive afferent neurons synapse in laminae I and II of the dorsal horn, and signals at this location may be modified as wide dynamic range neurons decrease their threshold for activation and widen their receptive field, and NMDA receptor activation is increased. This mechanism leads to a decrease in pain threshold at the surgical site (primary hyperalgesia) as well as surrounding uninjured tissue (secondary hyperalgesia). Thus even a tactile stimulus that is not painful may elicit a painful response (allodynia) in postsurgical patients because of sensitization.
Biochemically, surgical insult leads to the release and upregulation of a pro-inflammatory cytokine profile, particularly an increase in interleukin-1b (IL-1b) and peripheral COX-2. Furthermore, IL-1b induces COX-2 in the central nervous system because of the presence of peripheral inflammation. There are also humoral signals released from the inflamed surgical tissue that cross the blood-brain barrier directly and induce central COX-2. Thus while peripheral-acting COX-2 inhibitors and local anesthetic blockade may work peripherally to block COX-2 expression and provide analgesia, only central-acting COX-2 inhibitors block the expression of central COX2. ,
A thorough patient evaluation, including history, medication review, and physical examination, is vital prior to perioperative anesthesia and analgesia. Preoperative pain levels, certain age groups, anxiety, and depression may be associated with heightened postsurgical pain. In contrast, in high-risk groups such as those with chronic pain (please refer to Chapter 36 ), regional anesthesia as an adjunct to analgesia may be beneficial. In some patients, regional anesthetic techniques may be inappropriate. For instance, in patients with a history of severe respiratory disease undergoing shoulder surgery, interscalene blockade may be disadvantageous because it will lead to phrenic nerve blockade and potential for subsequent respiratory decompensation. History should also be elicited on preexisting nerve injury, whether it is localized to the site of the surgical procedure, its chronicity, and its temporal change (e.g. recent improvement or worsening nerve function). Preexisting nerve damage or deficit is considered a relative contraindication to regional anesthesia. In some instances, if the patient’s preexisting neuropathy is stable, regional anesthesia may be pursued after a thorough discussion of the risks. The clinician should convey to a patient that a preexisting nerve injury may serve as a priming event (“first hit”) and that any sequential insults (“second hit”) can culminate in an increased risk of further nerve damage compared to the general population (please refer to Chapter 35 ).
As part of a multimodal approach to pain management, preventive analgesia should also be considered. Preventive analgesia is defined as analgesia administration prior to the occurrence of painful stimuli, which may prevent or reduce subsequent intraoperative and postoperative pain and analgesic requirements. Preventive analgesic techniques involve the use of pharmacologic agents that reduce the activation of nociceptors by blocking or decreasing receptor activation. Multiple studies have demonstrated that patients receiving preventive analgesia experience a decrease in total analgesic consumption (please refer to Chapter 24 ). ,
After ensuring that the patient is a good candidate for regional anesthesia, the clinician performing the regional anesthetic procedure should provide thorough informed consent. This is not only important from a medicolegal standpoint but also improves ethical practice that respects the patient’s autonomy and supports the patient-physician relationship. Studies in the regional anesthesia and perioperative setting demonstrate that patients desire to know the risks, benefits, and alternatives for the anesthetic modality that they will receive. ,
The utilization of regional anesthesia checklists is also an important part of the pre-procedural period. Checklists typically comprise of patient identification, review of allergies and anticoagulation, confirmation of informed consent and marked site, obtaining the necessary equipment and labeled drugs, application of standard ASA monitors, establishment of intravenous access, administration of sedation and oxygenation if indicated, preparation of site using sterile technique and use of gloves and mask, and performing a verbal “time out.” By implementing a regional anesthesia-specific pre-procedural checklist, studies have demonstrated a lower incidence of procedures being performed on the wrong side.
After administration of an analgesic, the clinician must follow up with the patient’s response, which is typically performed routinely after surgery using an 11 point (0–10) pain assessment scale or other pain measurement tools. Clinicians should assess patient satisfaction and tolerance of the current level of pain and whether more analgesia would be desirable. This patient-centered approach with reliable follow up of analgesic response and good communication is crucial for successful perioperative pain management.
Multimodal analgesia involves the use of multiple medications to treat pain, each with different mechanisms of action that may have synergistic or additive effects when combined. The combination of different types of analgesics captures analgesic efficacy at optimal doses while minimizing side effects from any one analgesic, particularly undesirable effects of opioids such as nausea, vomiting, and respiratory depression. It is important to note that the use of local anesthetic alone to block the neuronal pathways is not always sufficient and does not decrease the humoral biochemical responses that occur following surgery; systemic pharmacologic agents may be required to attenuate the humoral biochemical and inflammatory postsurgical response. In addition to opioids and regional anesthetic techniques, examples of medications commonly utilized during the perioperative period with different mechanisms of action include NSAIDs, acetaminophen, ketamine, α-2-agonists, glucocorticoids, gabapentinoids, and duloxetine ( Table 26.1 ). A sample multimodal analgesic plan is provided for perioperative management in Table 26.2 .
| Medication Class | Example of Drugs | Mode of Delivery | Analgesic Ceiling | Side Effects | Comments |
|---|---|---|---|---|---|
| Acetaminophen | PO, IV | Yes | Hepatotoxicity, pruritus, rash | ||
| Non-selective NSAIDs | Ibuprofen, naproxen, meloxicam | PO, IV, IM | Yes | Platelet inhibition, GI bleeding risk, renal and hepatotoxicity, inhibition of bone healing and osteogenesis, cardiovascular risk (MI, stroke) | Provides opioid-sparing analgesia. Consider concurrent PPI use |
| COX-2 inhibitor | Celecoxib | PO | Yes | Renal and hepatotoxicity, cardiovascular risk (MI, stroke) | Provides opioid-sparing analgesia. Caution is advised in those with cardiovascular risk factors |
| Opioid | Fentanyl, morphine, hydromorphone | PO, IV, IM, SQ, NA, PNB, TD, TM, IN | No | Nausea/vomiting, respiratory depression, sedation, pruritus, constipation, urinary retention, dizziness | Choice of the agent should depend on potency and the expected duration of action. Use shorter-acting agents (e.g. fentanyl) for shorter hospital LOS or same-day discharge, and reserve longer-acting opioids (e.g. hydromorphone) for inpatients or opioid tolerant patients |
| Tramadol and tapentadol | PO, IV | Yes | Nausea/vomiting, dizziness, sedation, headache | Low affinity for opioid receptors; inhibits serotonin reuptake and may precipitate serotonin syndrome; contraindicated in patients taking MAOi; may lower the seizure threshold and caution is advised in those with a history of seizures; adjuvant in neuropathic pain | |
| Anti-convulsant/Gabapentinoid | Gabapentin, pregabalin | PO | Unknown | Dizziness, sedation, fatigue, tremor, ataxia, impairment in memory, edema, weight gain, vision abnormalities | Provides opioid-sparing analgesia, useful in both acute postoperative pain and chronic antihyperalgesia; adjuvant in neuropathic pain |
| NMDA Antagonist | Ketamine, methadone (opioid with NMDA receptor antagonism) | IV, IM, PO | Yes | Confusion, disorientation, dissociation, nausea/vomiting, sedation, ataxia, double-vision | Provides opioid-sparing analgesia; may attenuate chronic pain after surgery; implicated in preventing opioid-induced hyperalgesia; more effective in use in opioid tolerant patients but benefits seen in those opioid naïve |
| α-2-agonist | Dexmedetomidine, clonidine | PO, IV, IM, IN | Yes | Changes in blood pressure and heart rate (usually hypotension, bradycardia), nausea/vomiting | Suppression of opioid-withdrawal symptoms, preserves minute ventilation |
| Corticosteroid | Dexamethasone | IV, PO | Unknown | Edema, hypertension, elevated blood glucose, confusion, and delirium | Place in pain therapy is unclear, but evidence suggests analgesic effect as an adjuvant for opioid tolerance |
| Preoperative |
|---|
| Acetaminophen (paracetamol) 1000 mg PO |
| Caffeine 200 mg PO if the patient reports regular caffeine intake a |
| Celecoxib 400 mg PO (administered if aged 18–64 years and glomerular filtration rate >50 mL/min) b |
| Consider oxycodone immediate release 5 or 10 mg PO (administered if aged 18–64 years) in patients with preoperative opioid dependence |
| Intraoperative |
| Utilization of regional anesthetic techniques (may consider surgeon-administered wound infiltration and various PNB techniques including interscalene, anterior suprascapular, or supraclavicular block with supplementation of cervical plexus) |
| Consider dexamethasone up to 0.2 mg/kg IV |
| Consider dexmedetomidine 0.5 mcg/kg/min IV |
| Postoperative |
| Re-dose acetaminophen (paracetamol) 1000 IV every 6 hours until the patient starts taking oral medications |
| Ketorolac 7.5–15 mg IV every 6 hours up (up to five doses maximum; adjusted for age, creatinine clearance, and weight); avoid if age > 70 or creatinine clearance < 30 mL/min) b |
| Fentanyl 25 mcg IV prn or hydromorphone 0.2 mg IV prn for pain score> four |
| Oxycodone 5–10 mg PO every 4 hours prn and/or hydromorphone 1–2 mg PO every 4 hours prn when taking oral medications |
| Ketamine 10 mg IV every 5 minutes for severe pain (7–10) and consideration of initiating ketamine infusion 0.1–0.3 mg/kg/h for persistent severe postoperative pain |
a There is currently weak evidence for preoperative caffeine. Source: Hampl et al. Perioperative administration of caffeine tablets for the prevention of postoperative headaches. Can J Anaesth. 1995;42(9):789–792.
b Careful patient selection for NSAID therapy is warranted; in certain patient populations undergoing colorectal surgery (e.g. Crohn’s disease and ulcerative colitis), NSAIDs may be contraindicated. PO, Mouth; IV, intravenous; PCA, patient-controlled analgesia; prn, as needed; GFR, glomerular filtration rate; PNB, peripheral nerve blockade. A sample multimodal pathway adapted from Panchamia et al. A 3-arm randomized clinical trial comparing interscalene blockade techniques with local infiltration analgesia for total shoulder arthroplasty. J Shoulder Elbow Surg. 2019;28(10):e325-e338.
Enhanced recovery after surgery (ERAS) perioperative care pathways, previously known as “fast-track” programs, are designed to achieve early recovery after surgery by maintaining preoperative organ function and decreasing the stress response associated with surgery. Studies demonstrate that implementation of ERAS pathways lead to a reduction in complications, decreased hospital stay, improved cardiopulmonary function, improved bowel function, and earlier resumption of physical and regular activities. The main elements of ERAS comprise providing preoperative counseling, optimizing nutrition, avoiding perioperative fasting, implementing multimodal analgesic pathways (regional and non-opioid analgesia), and early mobilization. A sample ERAS protocol pathway is provided in Table 26.3 .
| Preoperative |
|---|
| Fluid and carbohydrate loading, a and avoidance of prolonged fasting |
| Caffeine 200 mg PO if the patient reports regular caffeine intake |
| Antibiotic prophylaxis |
| Thromboprophylaxis |
| Acetaminophen (paracetamol) 1000 mg PO |
| Celecoxib 200–400 mg PO (adjusted for age, creatinine clearance, and weight) |
| Consider granisetron 1 mg IV or scopolamine patch if the patient reports a history of severe PONV |
| Intraoperative |
| Administration of short-acting anesthetic agents (e.g. fentanyl IV or remifentanil IV) |
| Utilization of regional anesthetic techniques (may consider neuraxial analgesia, liposomal bupivacaine wound infiltration, and various truncal blocks such as TAP blocks and rectus abdominis sheath block) |
| Avoidance of excessive fluid administration |
| Maintenance of normothermia |
| Re-dose Acetaminophen (paracetamol) 1000 IV if 6 hours since preoperative dose |
| Ketorolac 7.5–15 mg IV (adjusted for age, creatinine clearance, and weight) at skin closure; avoid if age > 70 or creatinine clearance < 30 mL/min) |
| Avoidance of surgical drain placement |
| PONV prophylaxis: dexamethasone 4–8 mg IV at induction, ondansetron 4 mg IV at the end of the case (if the patient has two or more PONV risk factors, b consider the addition of droperidol 0.625 mg IV or promethazine 12.5 mg IV or haloperidol 1–2 mg IV at the end of the case) |
| Postoperative |
| Maintenance of continuous regional anesthetic technique (e.g. epidural catheter) |
| Early removal of urinary catheter |
| Early oral nutrition |
| Bowel regimen and agents to promote stimulation of gut mobility |
| Early mobilization |
| Use of non-opioid analgesics:Re-dose acetaminophen (paracetamol) 1000 IV every 6 hours until the patient starts taking oral medicationsRe-dose ketorolac 7.5–15 mg IV every 6 hours up (up to five doses maximum; adjusted for age, creatinine clearance, and weight) at skin closure; avoid if age > 70 or creatinine clearance < 30 mL/min) |
| Oxycodone 5–10 mg PO every 4 hours prn when taking oral medications |
a Source describing evidence on preoperative carbohydrate loading. Bilku et al. Role of preoperative carbohydrate loading: a systematic review. Ann R Coll Surg Engl. 2014;96(1):15–22.
b PONV risk factors include female sex, young to middle age, history of PONV or motion sickness, and non-smoking status. PO, Mouth; IV, intravenous; PCA, patient-controlled analgesia; prn, as needed; PONV, postoperative nausea, and vomiting; TAP, transversus abdominis plane.
A life-threatening event from local anesthetic medication is local anesthetic systemic toxicity (LAST), which currently has an estimated incidence of 0.03% or 0.27 events per 1,000 peripheral blocks. While accidental injection of a local anesthetic directly into a vessel is a well-known cause of LAST, other predisposing factors may include increasing use of continuous catheter techniques, high volume fascial plane blocks, multiple peripheral nerve blocks in the same patient, and utilization of tumescent anesthesia. It is recommended that the proceduralist adhere to guidelines on the maximum recommended local anesthetic dose.
There has also been a growing use of non-local anesthetic adjuvants in regional nerve blocks, including epinephrine, α-2-agonists, neostigmine, and steroids (e.g. dexamethasone). Data on the additional analgesic benefit are mostly lacking, and caution is advised when using certain adjuvants. The addition of α-2-agonists (e.g. clonidine) may be associated with bradycardia and hypotension. Gastrointestinal side effects may be frequent with neostigmine.
The aforementioned ERAS pathways and the regional anesthetic techniques described below should be tailored to the individual patient while keeping in mind the surgical type, side effects of the individual medications, and the patient’s comorbidities. The regional anesthetic blocks described below include neuraxial regional techniques as well as peripheral/perineural nerve blocks commonly performed in the perioperative setting and may serve as rescue blocks for postoperative pain management in the wards and beyond. The goal of this section is not to provide a comprehensive list but instead provide examples of common regional anesthetic blocks and to appraise the evidence in the literature for their analgesic efficacy in the perioperative setting.
Generally, neuraxial techniques provide better analgesia than systemic opioids and have the advantage of decreasing adverse perioperative pathophysiology (e.g. stress response) while improving patient outcomes. For instance, continuous epidural infusion has been shown to be associated with decreased pulmonary, cardiovascular, and gastrointestinal complications in high-risk patients after high-risk procedures. Below, we describe the spinal (intrathecal), epidural, and caudal anesthesia techniques.
While intrathecal local anesthetic administration is often used as the primary anesthetic for many surgeries (e.g. cesarean deliveries, certain abdominal procedures) instead of general anesthesia, the focus of this section will be on intrathecal medications for postoperative analgesia only. Intrathecal opioids are potent, centrally acting analgesic drugs and are commonly administered to provide intraoperative and postoperative analgesia.
When administering neuraxial opioids, it is important to consider the lipophilic properties of opioids. Studies have demonstrated that the liposolubility of intrathecal opioids is inversely proportional to spinal selectivity and spinal-mediated analgesia, which is higher for hydrophilic opioids than for other more lipophilic opioids. While all opioids administered intrathecally produce some degree of spinal-mediated analgesia, hydrophilic opioids provide primarily spinal-mediated analgesia, while lipophilic opioids tend to provide analgesia via either a spinal or systemic mechanism. Hydrophilic opioids, including morphine and hydromorphone, remain in the cerebrospinal fluid (CSF) for a long duration and can produce a delayed but longer duration of analgesia. Because of this property, hydrophilic opioids may have a higher incidence of delayed respiratory depression because of greater CSF spread. Intrathecal morphine may lead to severe respiratory depression lasting up to 24 hours. The respiratory depression after intrathecal morphine administration usually follows a biphasic pattern with an initial onset in the first 1–3 hours and a late-onset at 6–12 hours because of their hydrophilic nature and cephalad spread. On the other hand, lipophilic opioids, such as fentanyl and sufentanil, have a faster onset of action but a shorter duration of analgesia because of more rapid diffusion from CSF. Common side effects of all intrathecal opioids include pruritus in up to 60% of patients, , nausea and vomiting in over 50% of patients, and urinary retention in up to 80% of patients.
There is limited evidence on the use of other intrathecal medications for postoperative pain control, including α-2-agonists, steroids, and neostigmine. A randomized clinical study by Sarma and colleagues showed that a low dose of intrathecal dexmedetomidine or clonidine with bupivacaine produced a shorter onset and longer duration of motor and sensory blockade than bupivacaine alone in lower limb surgeries. Another prospective, double-blind study showed that intrathecal dexmedetomidine with bupivacaine provided earlier onset of sensory and motor block, longer duration of analgesia, and preserved hemodynamic stability compared to bupivacaine alone in infraumbilical surgeries. Of all adjuvants for neuraxial analgesia, only clonidine is approved by the FDA. Future well-designed randomized controlled trials (RCTs) assessing postoperative analgesia with intrathecal adjuvant medications are warranted.
In summary, intrathecal administration of lipophilic opioids produces short-term analgesia for approximately 1–4 hours and may be helpful for immediate postoperative pain. Although hydrophilic intrathecal opioids provide longer duration analgesia (for up to 24 hours) useful for more invasive and painful surgeries, we recommend that it be limited to patients expected to remain hospitalized in an appropriately monitored setting for at least one postoperative day.
Continuous epidural analgesia offers postoperative pain management for a longer duration than a single injection spinal injection and analgesia superior to systemic opioids. In terms of pharmacokinetics, medications placed in the epidural space must cross the dura prior to reaching the spinal cord. In addition, because of the high vascularity of the epidural space, there may be a significant redistribution of epidural medications into the systemic circulation. Furthermore, the presence of epidural fat may serve as a repository for lipophilic drugs.
Notably, compared to the intrathecal space, significantly larger amounts of medication need to be delivered via the epidural route to obtain equivalent analgesic efficacy because diffusion of drugs across the dura is concentration and time dependent. This pharmacokinetic concept is complex and involves inherent drug properties, primarily lipophilicity. For instance, lipophilic opioids (fentanyl and sufentanil) readily cross the dura to reach spinal cord receptors, whereas hydrophilic opioids (morphine) would cross the dura more slowly; thus when calculating the epidural dose for a medication that is equianalgesic to the intrathecal route, a higher epidural dose would be required for hydrophilic medications than for lipophilic medications. As a general rule of thumb, 10 mg of epidural morphine (hydrophilic opioid) would be equianalgesic to 0.1 mg of intrathecal morphine (10:1 ratio), whereas 33 mcg of epidural fentanyl would be equianalgesic to 6–10 mcg of intrathecal fentanyl (5:1 to 3:1 ratio). However, further well-designed dosing studies are warranted to validate these findings.
Typically, epidural local anesthesia with or without opioids offers better physiologic benefits after most surgeries than opioids alone and may decrease opioid-related side effects. The choice of local anesthetic may vary based on provider preference, but a local anesthetic with a longer duration of action with preferential sensory blockade and limited motor blockade is preferable for postoperative analgesia. However, in certain circumstances, such as persistent hypotension, epidural opioids alone may be more advantageous because of sympathectomy often associated with epidural local anesthetic.
When initiating epidural analgesia, small epidural bolus doses (5–10 mL) are usually administered first over a short period of 5–10 minutes to achieve an adequate depth of analgesia prior to initiating a continuous epidural infusion rate that can be titrated to effect. Furthermore, different delivery modes of epidural analgesia may be instituted, including fixed continuous infusion or patient-controlled epidural analgesia (PCEA). Similar to intravenous patient-controlled analgesia (PCA), PCEA allows analgesic requirements to be individualized to the needs of the patient and improves patient satisfaction. , Various combination settings may be employed, including continuous epidural infusion rate with PCEA together or a programmed intermittent epidural bolus technique with PCEA.
Similar to the intrathecal route, there is limited evidence of certain epidurally delivered adjuvant medications. Clonidine may provide analgesia via the epidural route by activating the descending noradrenergic pathway but may be limited by hypotension, bradycardia, and sedation. Epinephrine may increase the intensity of sensory blockade when administered with a local anesthetic.
In summary, continuous epidural analgesia provides superior analgesia and better patient outcomes than intravenous opioids and can provide postoperative pain control with epidural local anesthetic alone or a combination of epidural local anesthetic and opioids. Continuous infusion and other delivery modes can provide prolonged postoperative analgesia and may be titrated to an analgesic effect.
Caudal analgesia involves injection through the sacrococcygeal ligament with entrance into the epidural space. There is limited utility of caudal analgesia for acute postoperative pain control in adult patients because it is more technically challenging to perform caudal injection compared to lumbar or thoracic epidural blocks. Caudal analgesia is useful in the pediatric population and provides excellent postoperative analgesia for inguinal hernia repair, urologic interventions (e.g. circumcision, hypospadias correction, orchidopexy), anal atresia repair, and lower extremity surgeries. , Additional details on pediatric analgesia may be found in Chapter 28 .
The most common risks from neuraxial analgesia include hypotension, nausea and vomiting, and back pain. Transient neurological syndrome (TNS) is severe lower back pain localized in the buttocks and lower extremities after recovery from spinal anesthesia with no evidence of localized nerve damage. Risk factors for TNS include intrathecal lidocaine administration, lithotomy position, and ambulatory surgery. Postdural puncture headache (PDPH) is because of lumbar puncture with subsequent CSF leak through the puncture site causing symptoms related to traction of pain-sensitive central nervous system structures. To decrease the risk of PDPH, it is recommended to use a non-cutting needle. When utilizing a pencil-point spinal needle, a meta-analysis by Zorrilla-Vaca et al. demonstrated no difference in PDPH rates between 22-gauge and 25-gauge needles. Thus this study concluded that providers might consider using larger-caliber pencil-point spinal needles in all but the youngest patients to maximize technical proficiency instead of risking a failed spinal anesthetic by using a smaller sized spinal needle. Epidural catheters may be misplaced or may migrate after placement, leading to ineffective or inadequate analgesic coverage. Epidural placement closer to one side may result in a one-sided blockade with inadequate coverage of the contralateral side. Inadvertent placement of an epidural catheter in the subdural space may lead to unpredictable block characteristics, including an inappropriately high block because of extensive spread, delayed onset, segmental distribution, and potential for motor blockade. The feared complication is total spinal anesthesia, which occurs if excess local anesthetic is administered intrathecally, leading to coma, paralysis, hypotension, bradycardia, and apnea. Other rare adverse events include infection, hematoma, and nerve injury.
Utilization of peripheral nerve blockade in the perioperative setting is associated with adequate pain control, reductions in hospital length of stay, decreased hospital costs, better rehabilitation, and other improved postsurgical outcomes. , Peripheral nerve blockade can be established with a single injection of local anesthetic or with a continuous infusion through a perineural catheter. A perineural catheter with continuous infusion may provide equivalent analgesia to an epidural for certain surgeries while carrying fewer side effects than epidural analgesia. Patients may be discharged home with continuous local analgesic medications delivered via a perineural catheter and may remove the perineural catheter themselves without requiring a hospital visit. ,
Techniques for peripheral nerve blockade localization include paresthesia, peripheral nerve stimulator, and UGRA, although there has been a recent preference for UGRA. UGRA may be associated with a greater block success rate, faster onset of sensory block, fewer needle passes, and less discomfort during the block. , Lidocaine and mepivacaine are commonly used to provide short to intermediate duration peripheral nerve blockade. In contrast, bupivacaine, levobupivacaine, and ropivacaine are preferred for longer duration peripheral nerve blockade. , Other adjuvant medications are not currently approved but are commonly added to local anesthetics, including epinephrine (detects inadvertent intravascular uptake, denser and prolonged blockade related to vascular constriction) and clonidine (prolongs the duration of analgesia with added risks for hypotension and cardiac arrhythmias). , Below, we discuss various peripheral nerve blocks of the upper extremity, lower extremity, and trunk.
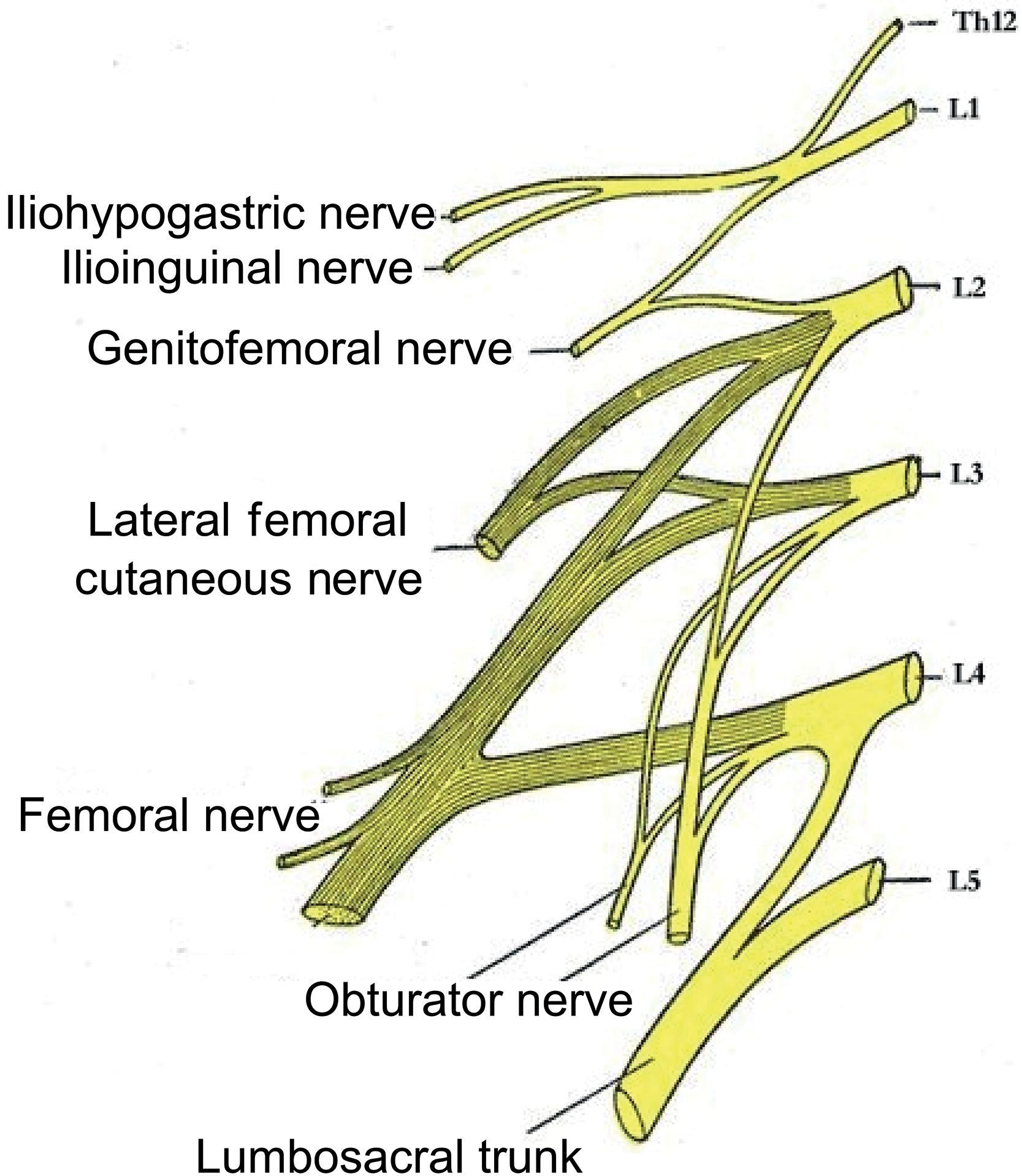
The ventral rami from the lumbar plexus (L1–4) and the sacral plexus (L4–5, S1–3) provide innervation to the lower extremity. The specific nerves derived from the lumbar plexus ( Fig. 26.1 ) include the lateral femoral cutaneous nerve (L2–3), obturator nerve (L2–4), femoral nerve (L2–4); the lumbar plexus also gives rise to the iliohypogastric, ilioinguinal, and genitofemoral nerves, which innervate the inguinal and genital areas. The sciatic nerve (L4–5, S1–3) is derived from the sacral plexus.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here