Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Regenerative medicine is a continually developing field that combines the diverse disciplines of cellular and molecular biology, tissue engineering, and biomaterial science in order to design therapies to restore or maintain cells, tissue, and organs. While many other complex organisms retain the capacity to regrow limbs and repair organs throughout adult life, humans have traded in this regenerative potential for speed and strength of repair, which increases our vulnerability to scarring and its associated loss of functionality and aesthetic appeal. However, over the past decade, there has been tremendous progress toward the scientific underlying of stem cell biology and the clinical use of cell-based regenerative medicine to restore normal tissue and organ architecture and function. With continued success in clinical trials, tissue engineering has the potential to reduce the impact of organ shortages, donor site morbidity, and immune rejection, all of which are current limits to transplant surgeries. Developing technologies able to induce true tissue regeneration will enhance normal wound healing and minimize problematic repair through scarring. This chapter provides an overview of the current status of stem cell biology, tissue engineering, and clinical applications and describes the actions required to incorporate regenerative medicine into routine clinical practice.
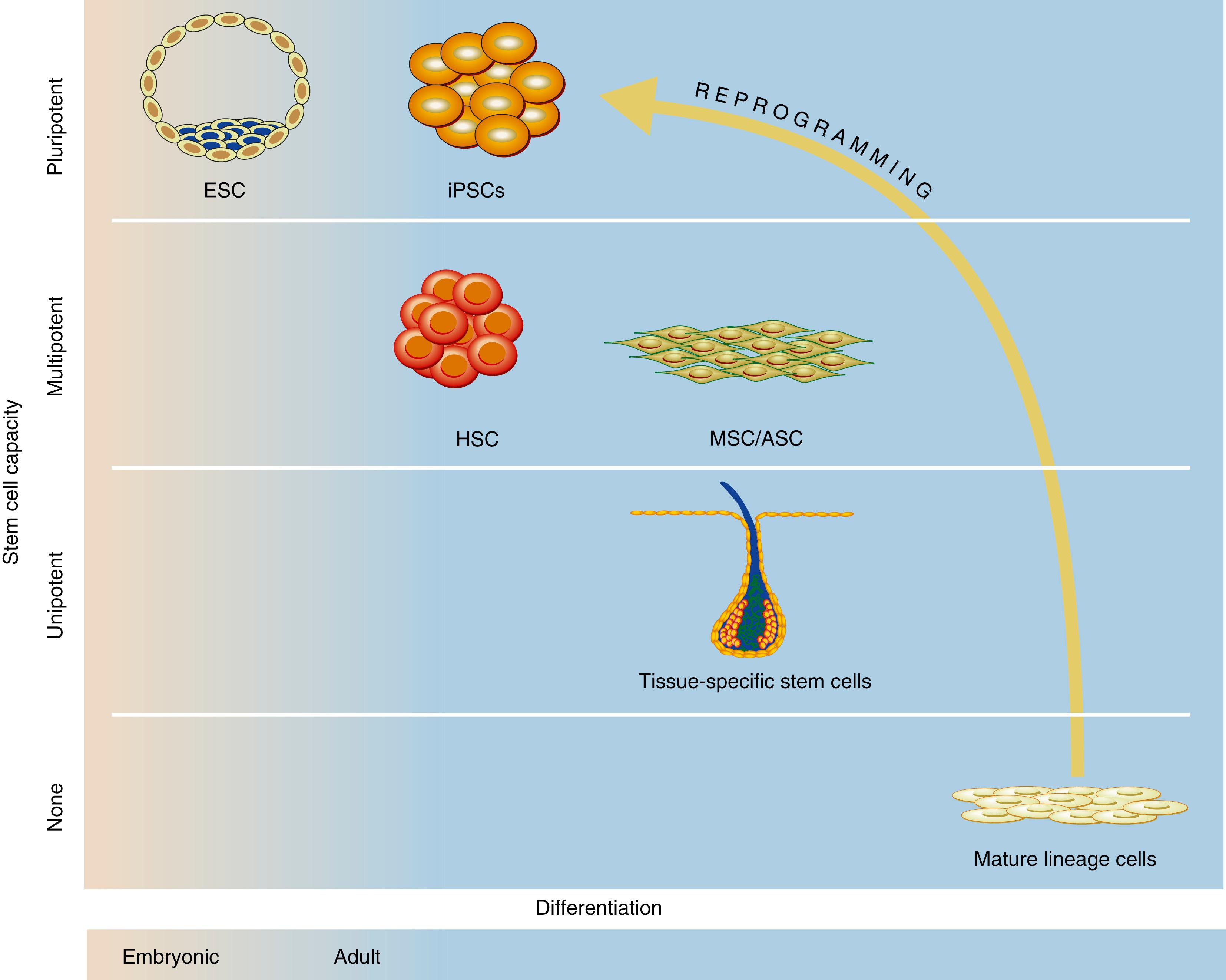
Stem cells are undifferentiated cells characterized by their unique capacity for long-term self-renewal and the ability to differentiate into multiple specialized cell types under the appropriate conditions ( Table 7.1 ). This potential to enter any desired differentiation program has made stem cells a strong focus of investigation within regenerative medicine. Traditionally, stem cells are classified as either pluripotent cells, which can differentiate into all three embryonic lineages (the ectoderm, mesoderm, and endoderm), or multipotent cells (“postnatal” or “tissue-specific”), which are more limited in their differentiation capacity and can only give rise to specialized cells of a specific tissue type ( Fig. 7.1 ). Multiple stem cells have been investigated within regenerative medicine.
| Term | Definition | Examples |
|---|---|---|
| Totipotent | Ability to form all differentiated cells in the embryo and extraembryonic tissue (e.g., placenta) | Zygote, morula |
| Pluripotent | Ability to form all lineages of the body but not extraembryonic tissue (e.g., placenta) | Embryonic stem cells (ESCs)—derived from the blastocyst inner cell mass |
| Multipotent | Adult stem cells that can form multiple cells in a particular cell lineage | Skeletal stem cells (SSCs) |
| Unipotent | Cells from one cell type | Osteocytes, chondrocytes |
Embryonic stem cells (ESCs) are cells derived from the inner cell mass of the blastocyst prior to implantation. They are pluripotent and have an unlimited capacity for self-renewal and the ability to differentiate into any somatic cell type. In the past, ESCs were cultured with animal material (e.g., mouse fibroblast “feeder” layers), which supplied the necessary growth factors to maintain ESCs in an undifferentiated state. Recently, human ESCs, developed for clinical use, are cultured and maintained in serum- and feeder-free conditions and undergo extensive microbiologic testing as per recommendations from the International Stem Cell Banking Initiative. The U.S. Food and Drug Administration also requires documentation of the source, potential genetically modified components, and any pathogenic agents used for all ESC-derived cells intended for therapeutic use.
A number of human ESC cell lines have been derived from embryos carrying monogenic inherited diseases or chromosomal abnormalities, including Huntington disease and cystic fibrosis. These cell lines are used to model diseases, thus providing a better understanding of their etiology and pathophysiology. Healthy human ESC lines have also been established in order to build cell banks that can differentiate into specific cells and tissues for reconstruction following congenital, traumatic, infective, or malignant lesions. Human ESCs are currently being investigated for the treatment of macular degeneration, cardiac diseases, and cancer.
Research into the use of human ESCs for regenerative medicine has been met with significant technical, political, and ethical hurdles. Despite a large number of human ESC lines established, only few peer-reviewed publications and a limited number of clinical trials have been conducted to date. Blastomere harvesting has raised concerns that this process damages potentially viable embryos. Although there are now techniques to isolate single cells from cleaved-stage embryos, at which point there are sufficient remaining cells for embryogenesis to proceed unperturbed, these processes have failed to satisfy those in opposition. Furthermore, the extensive pluripotentiality and capacity for unlimited self-renewal of ESCs put them at risk for dysregulated growth and tumorigenesis. In addition, the in vitro expansion of blastomeres required to transition to ESCs can profoundly alter their cellular and genetic biology and further predispose to tumor formation. While undifferentiated ESCs do not provoke an immune response upon transplantation, this is not true for more differentiated cells derived from ESCs, which begin to express major histocompatibility complex (MHC) types 1 and 2. MHC matching is indicated when ESCs are used clinically.
Somatic cell nuclear transfer (SCNT) involves the transfer of a nucleus from a differentiated somatic cell containing a desired genetic profile into an enucleated ovum. Mitotic divisions of the resultant cell in culture lead to the generation of a blastocyst capable of yielding a complete organism. Dolly the sheep was the first mammal to be cloned from a somatic cell in 1997. Since then, SCNT has been used to successfully clone more than 20 mammalian species, including monkeys. After significant optimization of SCNT protocols, human embryos have also been generated. SCNT enables generation of genetically matched stem cell lines, which provides a huge potential for human therapeutics in the screening of potentially useful treatments and as a source of replacement cells for damaged organs.
Numerous technical hurdles, however, have also limited the use of SCNT for regenerative therapies. First, the resultant hybrid cells are imperfect copies of the donor cell nucleus, which may explain the abnormalities often noted in the extraembryonic tissues and, if the embryo is viable, in cloned animals after birth. Second, cloning efficiency remains extremely low in all species. Third, the enucleated ovum contains mitochondrial deoxyribonucleic acid (DNA) that is genetically distinct from the nucleus donor’s mitochondrial DNA, and this mismatch may risk immune rejection following transplantation. Finally, there is also a scarcity of high-quality donor mature human metaphase II oocytes that are available for research. Before SCNT can be applied widely in clinical practice, these technological limitations need to be addressed.
In 2006, Takahashi and Yamanaka made a major scientific breakthrough and identified a set of four transcription factors (Oct4, Sox2, KLF4, and cMyc—the “Yamanaka factors”) able to reprogram mouse somatic cells (e.g., fibroblasts) back into ESC-like induced pluripotent stem cells (iPSCs). Shortly after this discovery, in 2007, the first human iPSCs were generated from human fibroblasts. , iPSCs sparked a huge interest in the field of regenerative medicine given the ease and reproducibility with which they can be generated and the relative lack of ethical or political concerns. Autologous iPSCs can theoretically reduce the need for immunosuppression posttransplantation. iPSCs are also reported to be more tolerance-inducing than other transplanted cells (such as ESCs). This may permit extensive cell banking and the use of allogenic cells without the need for long-term immune suppression. Since 2007, human iPSC technology has rapidly evolved, and human iPSCs are being used to model a variety of human diseases, develop cell therapies, and discover candidate drugs. CRISPR-Cas9 technology has helped advance iPSC-based disease modeling by introducing disease-causing mutations to wild-type iPSCs and eliminating the same mutations in patient iPSCs.
Although the first clinical trial using human iPSC products was launched in 2014, the translation of human iPSCs to clinical trials has not been straightforward. The risk of tumorigenicity is a major concern, and genetic mutations in iPSCs in the first human iPSC-mandated trial suspension. iPSCs, like ESCs, are maintained in culture for prolonged periods of time, which increases the risk of accumulating karyotypic abnormalities and of losing heterozygosity. Methods to rigorously test and purify iPSC-derived products and to monitor the tumor formation following transplantation are topics of current investigation. Preserving the self-renewing and pluripotent nature of iPSCs while eliminating tumorigenesis and directing the fate of these cells in vivo is a continuous challenge.
Fetal stem cells are primitive cells that can been derived from fetal blood, liver, bone marrow, amniotic fluid, and placental tissue. They are similar in immunogenicity to ESCs but more restricted in their differentiation fates. Under appropriate growth conditions, fetal stem cells can differentiate toward adipogenic, osteogenic, and chondrogenic fates. Significant ethical debate surrounds the collection and use of fetal tissue because the intrauterine collection of fetal blood can risk damaging or terminating a pregnancy. Currently, fetal stem cells are obtained from terminated fetuses, tissues that otherwise would be discarded. Fetal stem cells are unlikely to become a routine source of cells in regenerative medicine but may provide a means whereby future autogenous in utero cellular and genetic therapies can be devised.
In adult humans and other complex organisms, the regenerative capacity of tissues and organs is maintained by adult (or “tissue-specific”) stem cells. Adult stem cells are multipotent and can differentiate into some, but not all, tissue lineages. Typically, differentiation fates are limited to cells found in their tissue of origin and are influenced by the microenvironment or “stem cell niche” ( Fig. 7.2 ). Despite a more limited differentiation, potential adult stem cells can be isolated without ethical concerns, and this has helped establish their relevance for clinical applications. Hematopoietic stem cells (HSCs) were the first adult stem cells to be described. More recently, mesenchymal stem/stromal cells (MSCs), adipose-derived stem/stromal cells (ASCs), and skeletal stem cells (SSCs) also have become the focus of research in the field of regenerative medicine. It is likely that many additional tissue resident stem cells soon will be discovered.
![Fig. 7.2, Adult multipotent mesenchymal stem cells (MSCs) can be isolated from adipose tissue (adipose stem cells [ASCs]) or from bone marrow (MSCs). These cells have been shown to differentiate into multiple tissue types in vitro, including adipose (adipogenesis), bone (osteogenesis), cartilage (chondrogenesis), skeletal and cardiac muscle (skeletal and cardiac myogenesis), and nerve (neurogenesis) tissues. There has been varying success in experimentally differentiating these cells into these tissue types in vivo, a necessary step before adult multipotent stem cells can be used clinically for regenerative medicine applications. Fig. 7.2, Adult multipotent mesenchymal stem cells (MSCs) can be isolated from adipose tissue (adipose stem cells [ASCs]) or from bone marrow (MSCs). These cells have been shown to differentiate into multiple tissue types in vitro, including adipose (adipogenesis), bone (osteogenesis), cartilage (chondrogenesis), skeletal and cardiac muscle (skeletal and cardiac myogenesis), and nerve (neurogenesis) tissues. There has been varying success in experimentally differentiating these cells into these tissue types in vivo, a necessary step before adult multipotent stem cells can be used clinically for regenerative medicine applications.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RegenerativeMedicine/1_3s20B9780323640626000074.jpg)
HSCs have been the most studied and best characterized adult multipotent stem cell since their definitive isolation in mice several decades ago. HSCs have subsequently served as the experimental paradigm for basic studies into the biology of all adult stem cells. HSCs are blood-forming cell that reside in specialized niches within adult bone marrow and function to maintain homeostasis of all lineages of hematopoietic cells throughout adult life. Transplantation of HSCs for hematologic diseases and malignancies remains the most widely used stem cell therapy to date. HSCs transplanted into patients with cleared bone marrow niches engraft and function to repopulate all lineages of the hematopoietic system.
MSCs represent the collection of nonhematopoietic progenitor cells of mesodermal origin that have characteristic spindle shapes and can derive colonies from single cells (“colony-forming units-fibroblastic”). MSCs are considered immune-privileged and able to generate various types of connective tissue cells, including osteoblasts, adipocytes, chondroblasts, fibroblasts, and pericytes, which has made them attractive candidates for cell-based therapy. MSCs are thought to mediate their regenerative effects primarily through paracrine signaling, specifically the release of immunomodulators, antioxidant, antiapoptotic, angiogenic, and chemotactic agents.
However, there have been substantial inconsistencies in the definition of MSCs and the methods of isolation. Because there are no well-defined universal surface markers to prospectively isolate MSCs, they have been typically isolated by their inherent ability to adhere to polystyrene tissue culture plastic, a nonspecific technique. The development of standardized definitions and isolation techniques is essential in the continued use of MSCs for regenerative medicine. Because of this heterogeneity, there is a growing trend toward using the term “MSC” as an umbrella for many tissue-specific multipotent stem cells with unique differentiation abilities.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here