Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]()
![]() Access video and video lecture content for this chapter online at Elsevier eBooks+
Access video and video lecture content for this chapter online at Elsevier eBooks+
Wounds of the posterior trunk can arise as a result of trauma, tumor ablation, chronic pressure, or congenital defects. Management of these wounds can be quite challenging for the reconstructive surgeon given the relative lack of soft-tissue laxity, dearth of microsurgical recipient vessels, and dependent anatomical location. Of note, there are the many vital structures that traverse this anatomical region, including structures involved in neurological signaling, respiratory function, visceral protection, and postural support, and exposure or violation of these structures increases the risk of postoperative morbidity and mortality. Compounding these challenges may be other elements of the patient history, including the presence of irradiation, spinal hardware, fulminant infection, or cerebrospinal fluid leak. As a result of the above challenges, healing by secondary intention or skin grafting are frequently suboptimal, especially for intermediate and deep posterior trunk wounds.
Muscle and myocutaneous flaps based on the latissimus dorsi and trapezius muscles are robust, well-vascularized flaps that are considered the traditional first-line options for reconstruction in the posterior trunk. Early studies demonstrated superior outcomes of muscle flaps compared to random pattern fasciocutaneous flaps or primary closure, especially in the presence of infection. This may be due to improved delivery of antibiotics and immune cells, superior tissue oxygenation and wound coverage, and inhibition of bacterial growth when compared to muscle-sparing flaps. Newer studies, however, have challenged these beliefs, producing data to support the use of perforator flaps, even for large wounds.
Perforator flaps can offer reliable wound healing, cosmesis, and an improved comorbidity profile when compared to muscle-based flaps. However, the steep technical learning curve and decreased tissue bulk compared to muscle-containing flaps may limit the use of perforator flaps in the posterior trunk. Flaps based on multiple perforators may address concerns related to limited tissue bulk and can efficiently recruit large areas of soft tissue in a relatively simple operation. Once mastered, perforator flaps offer a robust, secondary reconstructive option for soft-tissue defects in the posterior trunk, especially when muscle preservation is desired.
An algorithmic approach to posterior trunk reconstruction starts with categorization of the wound depth as superficial, intermediate, or deep. Superficial wounds can typically be managed with local wound care. Intermediate and deep wounds often require recruitment of soft tissues for filling of empty space and resurfacing. These wounds are further classified by their midline or non-midline position and by their location within the four posterior trunk regions (posterior cervical, upper thoracic, middle thoracic, and lumbar). Flap selection is then narrowed to locoregional options based on these coordinates. Free tissue transfer is typically considered an option of last resort. Wound characteristics such as size, depth, and the presence of hardware are then considered when deciding which soft and bony tissue component to include within the flap. Midline wounds differ from non-midline wounds in that there is a higher risk of complication by spinal involvement and/or spinal leak. As a result, midline wounds typically require two full layers of vascularized muscle or fascia for definitive closure. For non-midline wounds, or midline wounds without spinal exposure, soft-tissue needs within the wound will dictate the variable inclusion of vascularized muscle, fascia, subcutaneous tissues, and skin.
Regardless of flap selection, the following principles serve to optimize soft-tissue reconstruction in the posterior trunk: debridement of all devitalized tissues, recruitment of well-vascularized tissue outside of the zone of injury, obliteration of empty space, coverage of vital structures/hardware with adequate tissue bulk, and a tension free closure. Appropriate flap selection minimizes patient morbidity while maximizing functionality. A sound reconstructive plan should include a multidisciplinary approach with neurosurgery and/or orthopedic surgery when indicated. This chapter synthesizes the current literature on reconstruction of the posterior trunk and provides an algorithmic approach, which can serve as a starting point when addressing the myriad challenges present within this anatomical region.
The back comprises 18% of total body surface area with anatomical boundaries beginning at the superior margin of the posterior trunk, demarcated by a line connecting the spinous process of the “ vertebra prominens” , or C7, to the acromial angle of both shoulders. The inferior margin lies just above the bilateral iliac crests, and lateral margins extend to the mid-axillary line on either side of the trunk.
The muscles of the back are organized into three groups. The extrinsic or superficial muscle group aids in limb movement and contains the trapezius, latissimus dorsi, levator scapulae, and rhomboids ( Fig. 12.1 ). The intermediate muscle group aids in respiratory effort and is composed of the serratus posterior inferior and serratus posterior superior (see Fig. 12.1 ). The deep or intrinsic group is further subdivided into two main subgroups: the erector spinae and the transversospinalis ( Fig. 12.2 ). The erector spinae include the iliocostalis, longissimus, and spinalis muscles, commonly referred to as the “paraspinous” muscle, and are the main spinal extensors, in addition to aiding in lateral bending and in rotation of the spine (see Fig. 12.2 ). The transversospinalis subgroup includes the semispinalis, multifidus, and spinal rotators, which aid in posterior and lateral bending of the spine and rotation (see Fig. 12.2 ). The gluteal muscles lay caudal to the inferior boundary of the back, and as a result of their proximity to this region, are frequently involved in locoregional posterior trunk reconstruction, especially for wounds in the lumbar region ( Fig. 12.3 ).
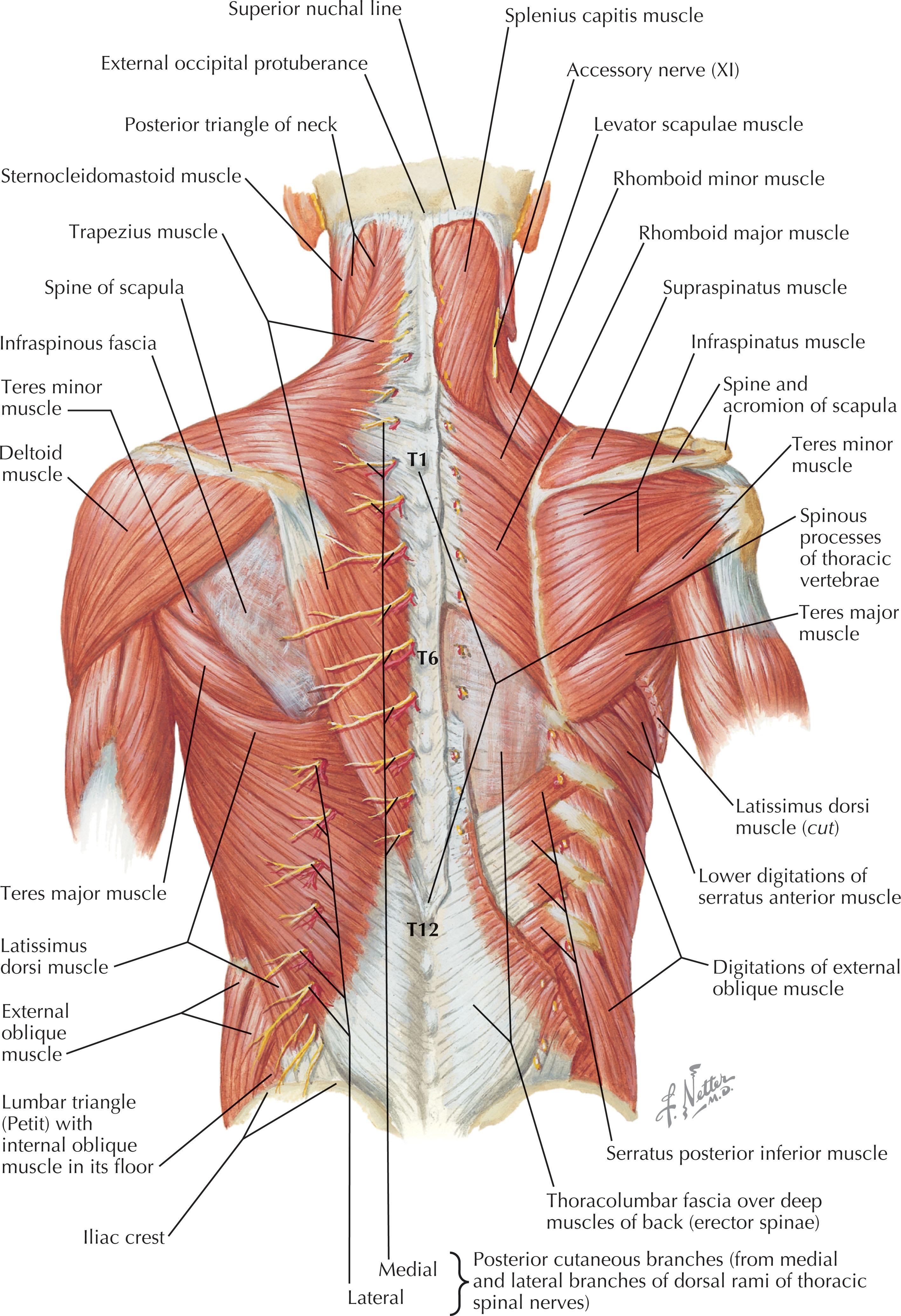
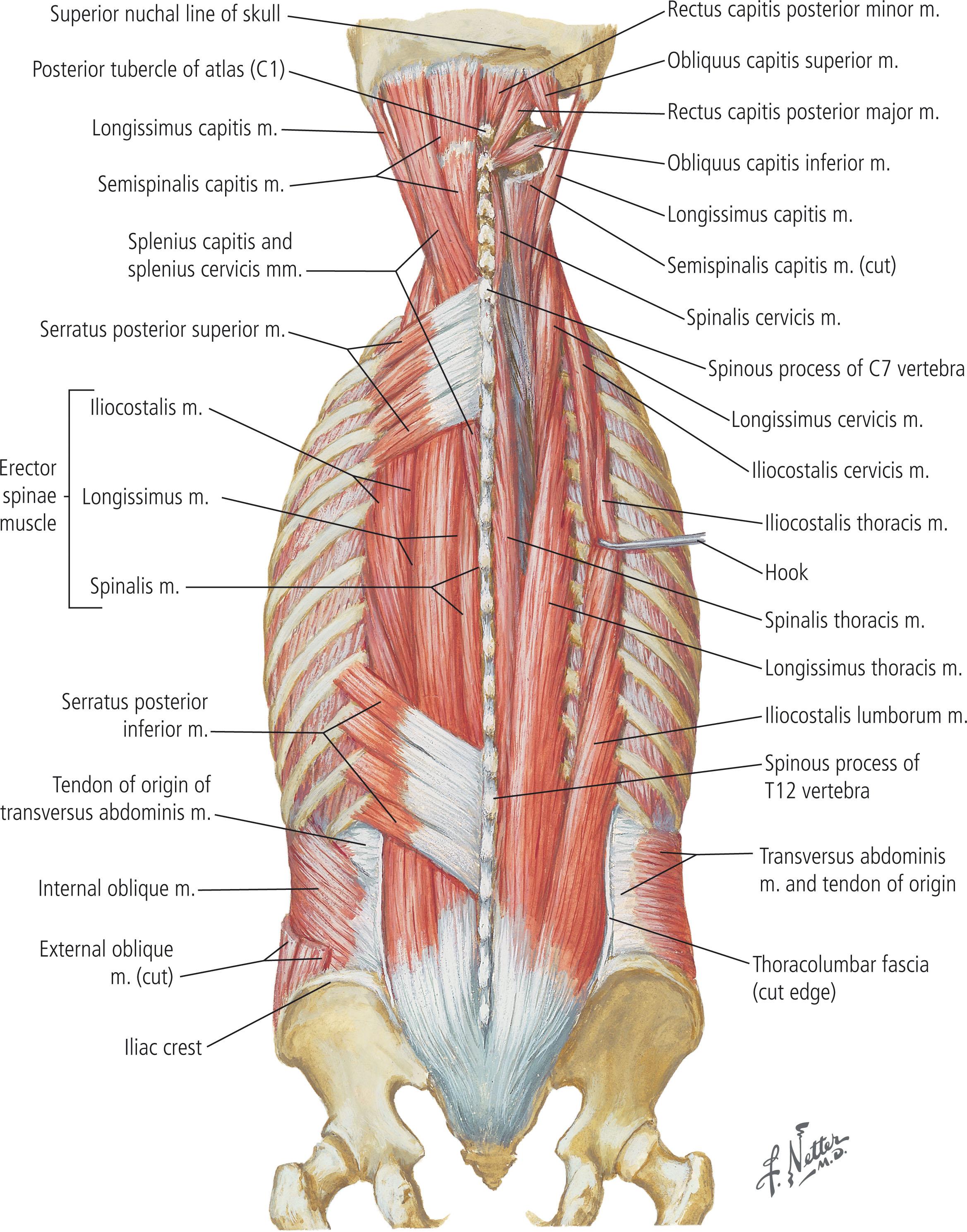
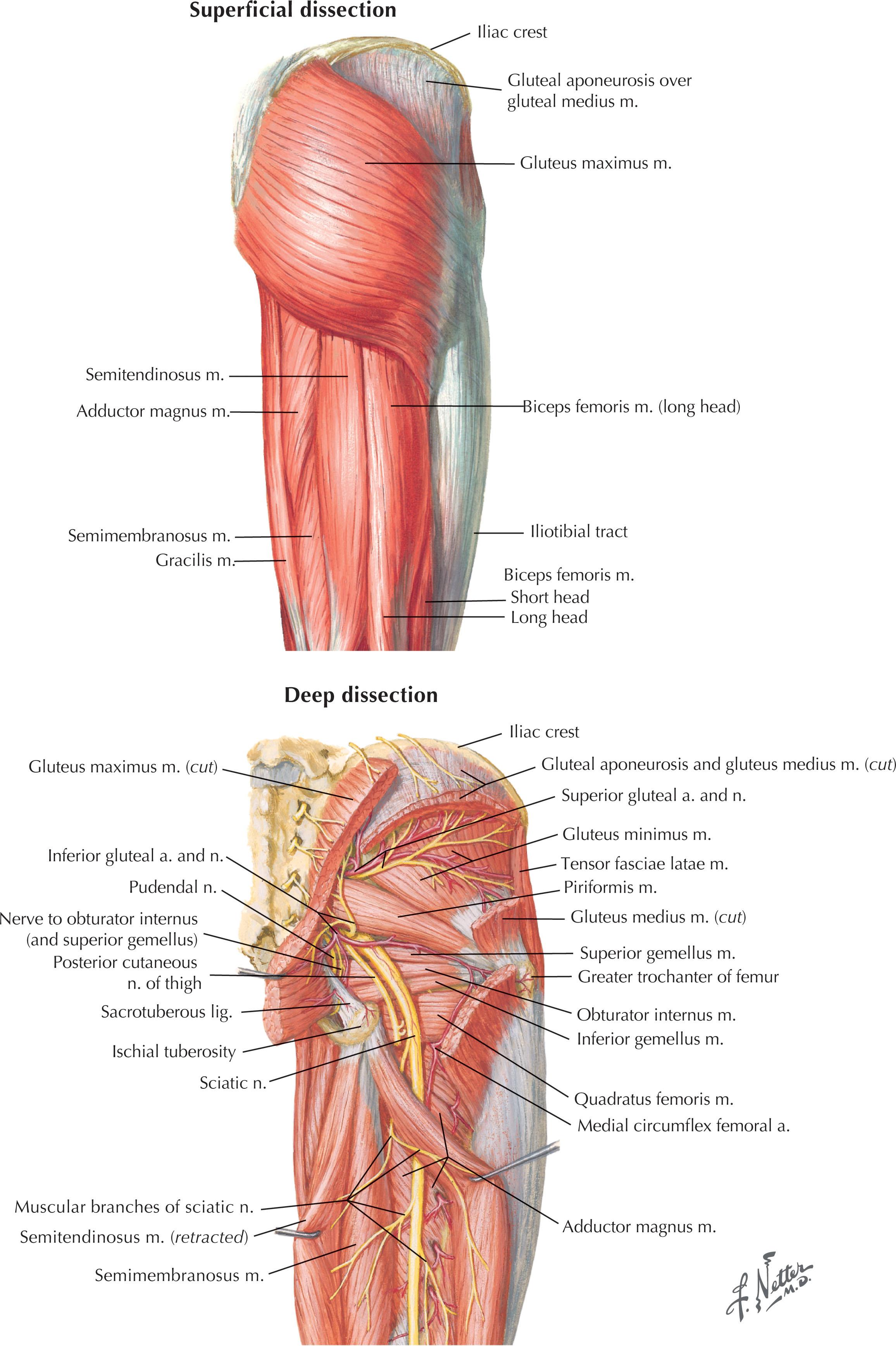
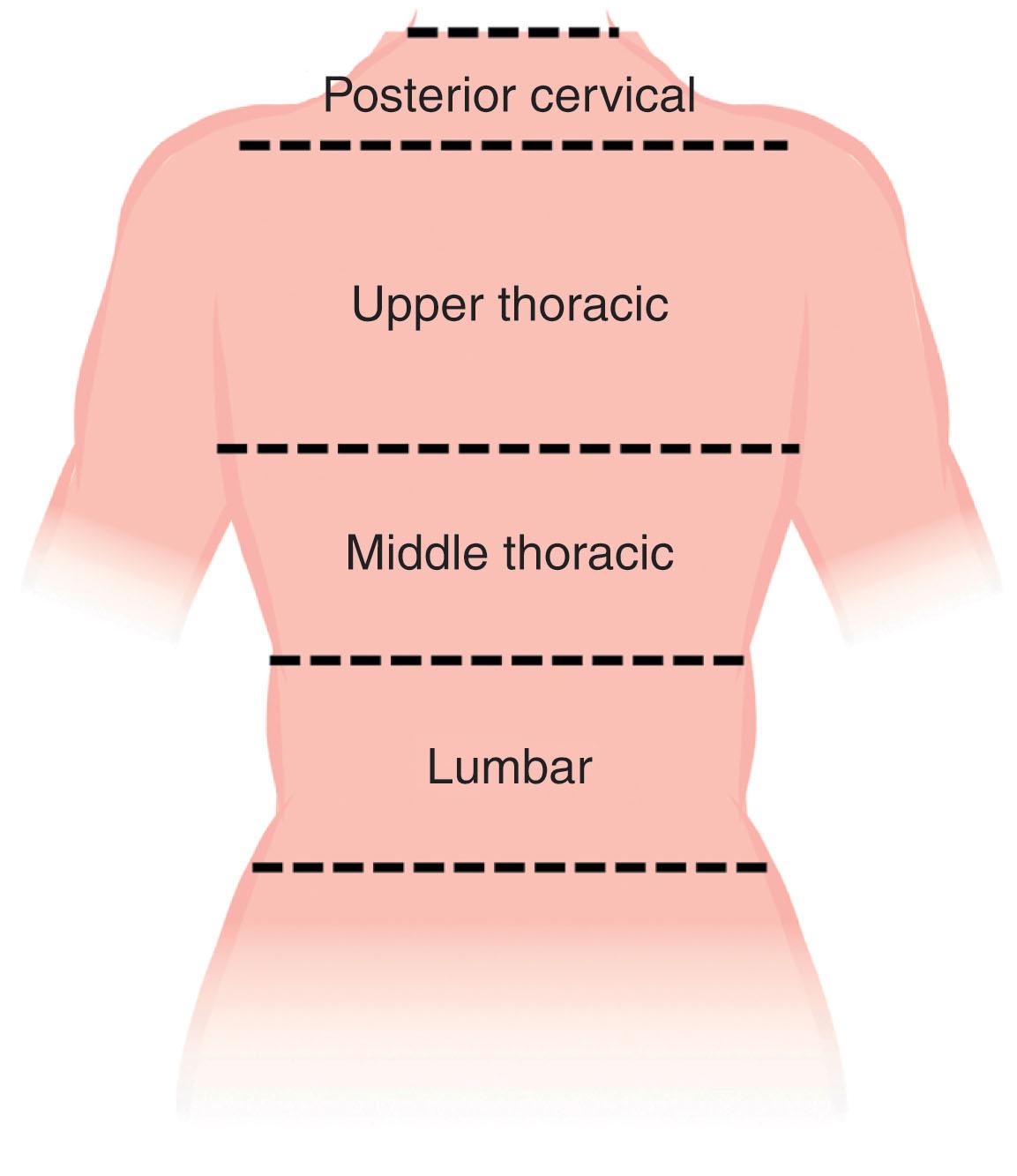
The muscles and soft tissues of the back are well vascularized and are fed both by axial vessels and dorsal branches of the intercostal and lumbar arteries. The skin and intrinsic muscles of the back are innervated primarily by the dorsal rami of the spinal nerves while extrinsic muscles are innervated by the ventral rami. Sensory innervation takes on a dermatomal pattern. The back can also be divided into four descriptive subregions from cephalad to caudad, for the purposes of grouping locoregional reconstructive options. This includes the posterior cervical, upper thoracic, middle thoracic, and lumbar subregions ( Fig. 12.4 ).
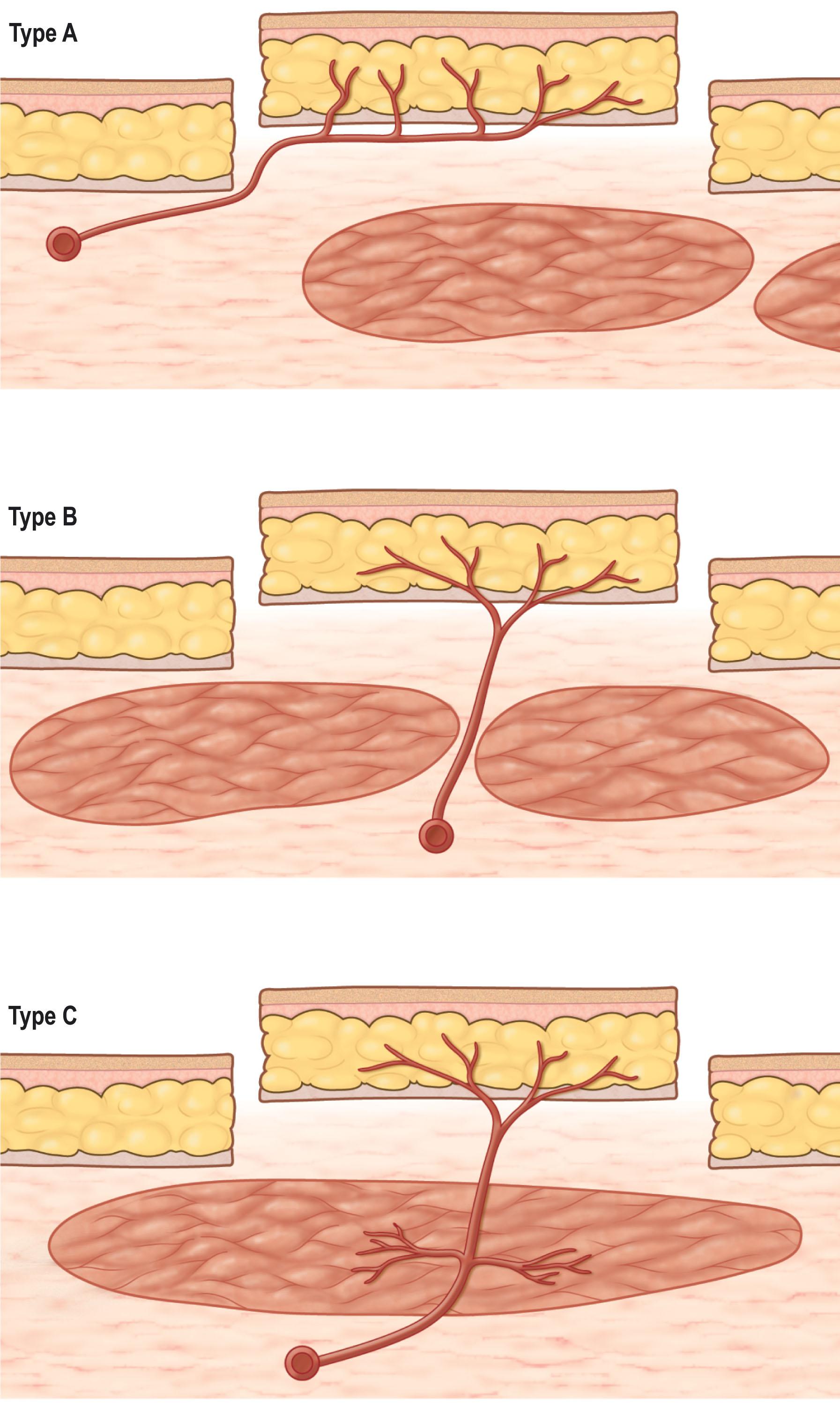
Perforating vessels in the posterior trunk branch off of the axial blood supply and are categorized by the course taken towards their termination point. There are three types of perforators ( Fig. 12.5 ):
Musculocutaneous (indirect muscle) perforators course through the muscle, perforate the outer layer of deep fascia, then supply the overlying skin
Septocutaneous (indirect septal) perforators course through the septum, perforate the outer layer of deep fascia, then supply overlying skin
Direct cutaneous perforators, which perforate the deep fascia only and then supply the overlying skin
Perforasomes are distinct regions of tissue within a vascular territory that is supplied by perforating blood vessels ( Fig. 12.6 ). A central dense vascular network progressively decreases in concentration towards the periphery of the perforasome. Multiple perforasomes are linked, directly or indirectly, by vessels that branch out radially from the periphery.
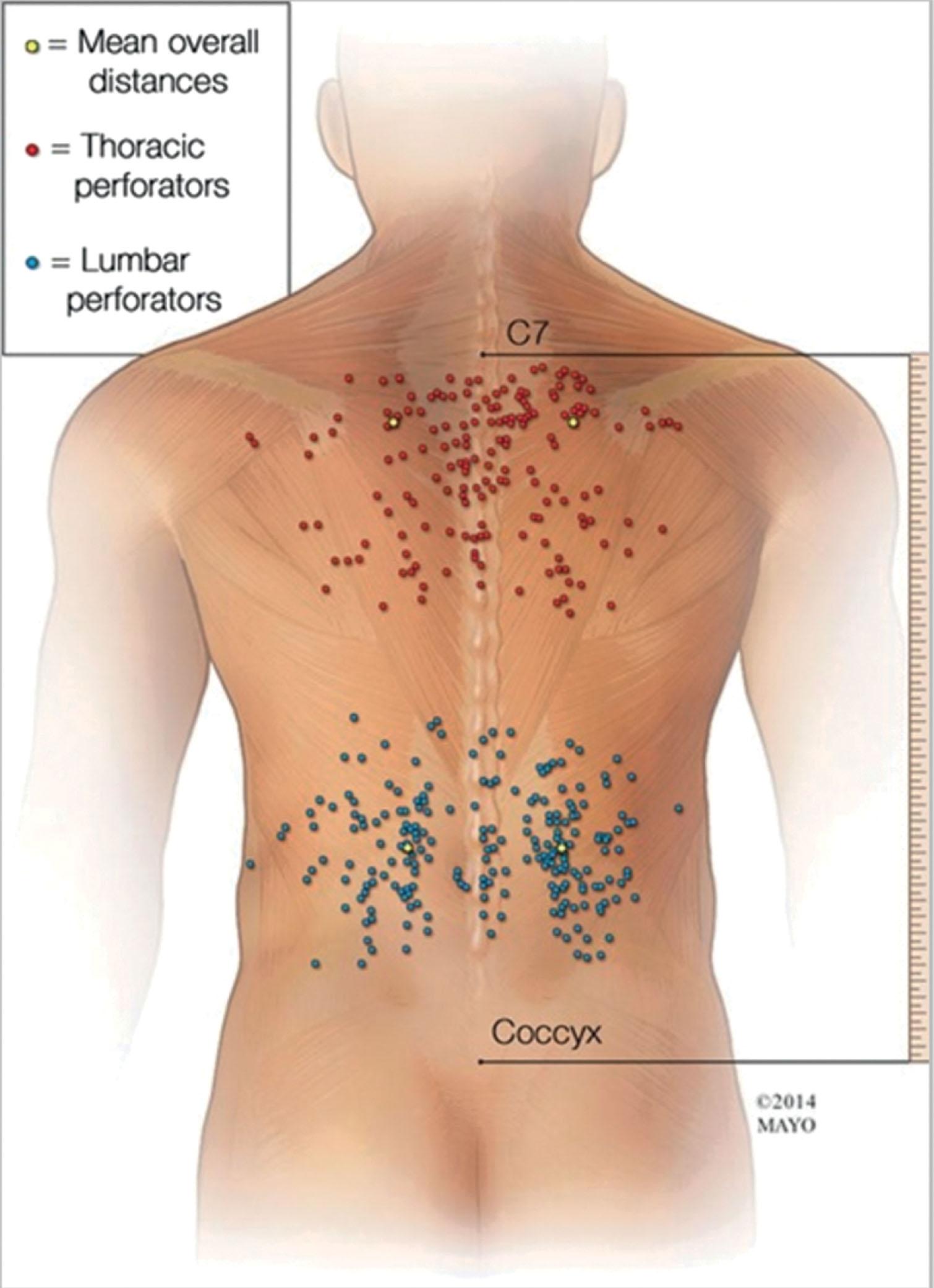
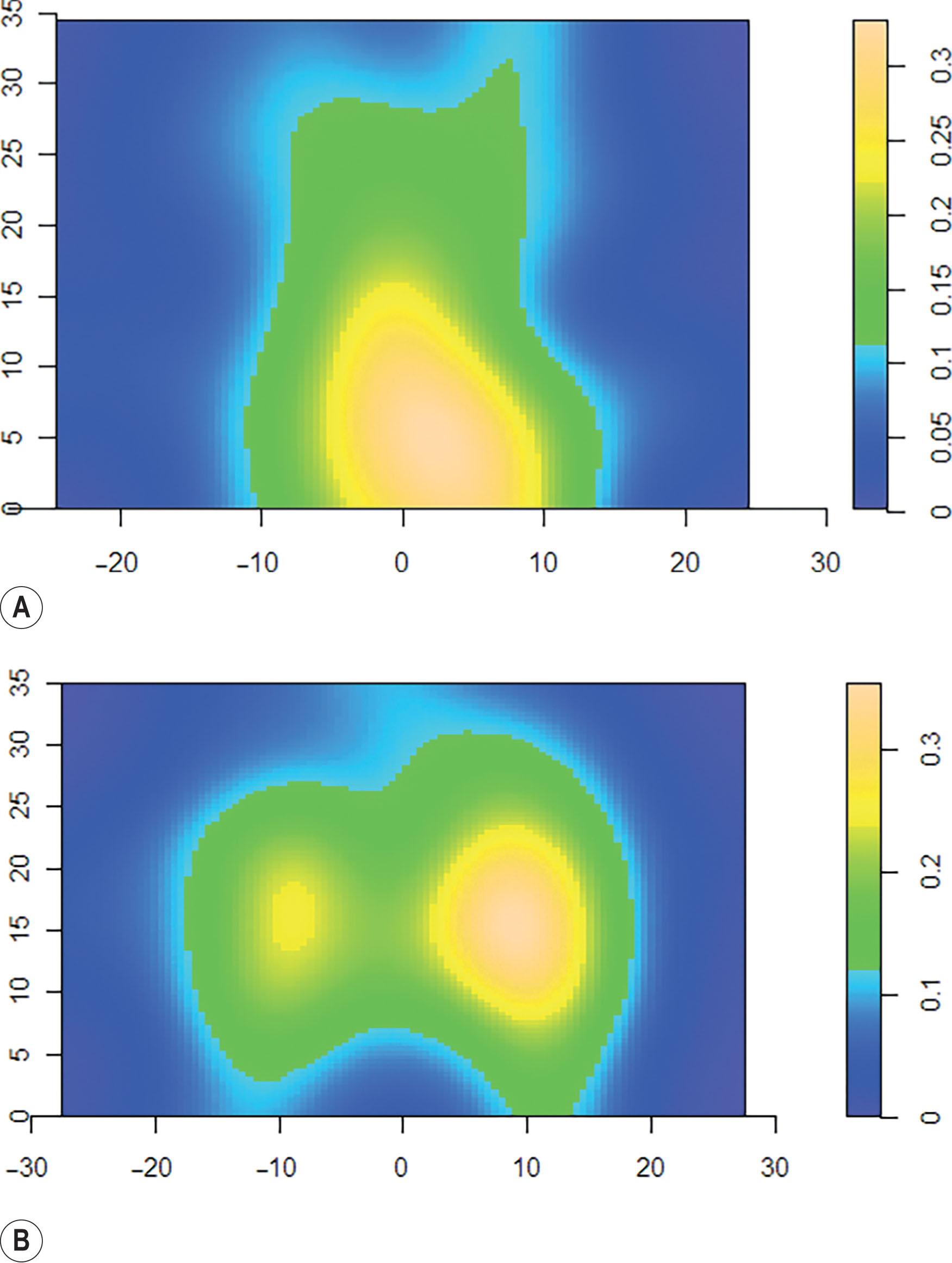
A line drawn transversely across the lower margin of the 12th rib divides the posterior trunk into the upper back (thoracic) and lower back (lumbar) regions, which correlate with the two major perforator densities within the region ( Figs. 12.7–12.9 ). In a cadaveric study of posterior trunk perforators, conducted by Aho et al ., 393 perforators were dissected to help elucidate their distribution within the two groupings in this region. The first perforator grouping is the thoracic cluster, which, based on cadaveric studies, has a mean of 17.8 perforators, located within 10–20 cm of the C7 reference point (see Figs. 12.7–12.9 ; Table 12.1 ). The center of the thoracic cluster has a perforator density of 0.3/cm 2 , reflecting a three-times higher average likelihood of containing a perforator than surrounding tissues (see Figs. 12.7 & 12.8 , Table 12.1 ).
| Cadaver | Location of thoracic perforators | |||||
|---|---|---|---|---|---|---|
| Left | Right | |||||
| No. of perforators | Mean (SD) distance (cm) | No. of perforators | Mean (SD) distance (cm) | |||
| From C7 reference point | From midline | From C7 reference point | From midline | |||
| 1 | 16 | 4.99 (3.10) | 8.96 (6.57) | 17 | 5.84 (3.92) | 9.92 (8.74) |
| 2 | 15 | 3.87 (3.53) | 20.27 (6.41) | 19 | 6.12 (3.62) | 15.89 (8.24) |
| 3 | 13 | 7.95 (7.06) | 9.28 (6.96) | 16 | 5.59 (4.54) | 5.49 (3.19) |
| 4 | 22 | 8.14 (5.05) | 19.60 (9.82) | 15 | 9.63 (4.85) | 17.27 (10.15) |
| 5 | 15 | 7.15 (8.25) | 15.96 (8.30) | 16 | 7.17 (5.94) | 16.16 (10.37) |
| Total | 81 | 6.51 (5.74) | 15.29 (9.14) | 83 | 6.80 (4.71) | 12.96 (9.44) |
The second perforator grouping is the lumbar cluster, which has a mean of 21.5 perforators located within 10–20 cm of the coccygeal reference point (see Figs. 12.7 & 12.8 ; Table 12.2 ). The center of the lumbar cluster has a perforator density of 0.3/cm 2 , reflecting a two-times higher average likelihood of containing a perforator than surrounding tissues (see Fig. 12.7 & 12.8 , Table 12.2 ). Some of the lumbar perforators cross the midline and join contralateral perforators via direct and indirect flow. It is important to appreciate the “watershed” areas of low perforator density between the thoracic and lumbar perforasomes during flap design (see Figs. 12.7 ).
| Cadaver | Location of lumbar perforators | |||||
|---|---|---|---|---|---|---|
| Left | Right | |||||
| No. of perforators | Mean (SD) distance (cm) | No. of perforators | Mean (SD) distance (cm) | |||
| From coccyx | From midline | From coccyx | From midline | |||
| 1 | 22 | 13.29 (4.89) | 6.98 (4.89) | 21 | 13.63 (3.83) | 6.40 (2.93) |
| 2 | 10 | 18.35 (4.36) | 8.07 (2.79) | 22 | 13.61 (6.75) | 9.27 (2.95) |
| 3 | 17 | 15.62 (8.32) | 7.69 (4.96) | 13 | 16.91 (10.27) | 8.69 (4.66) |
| 4 | 25 | 16.53 (9.58) | 14.69 (5.71) | 38 | 19.43 (7.07) | 11.65 (4.49) |
| 5 | 21 | 18.68 (8.35) | 11.33 (3.85) | 27 | 17.49 (7.95) | 7.70 (5.66) |
| Total | 95 | 16.28 (7.82) | 10.21 (5.61) | 121 | 16.66 (7.48) | 9.11 (4.70) |
There are four main principles that define perforasome theory.
Each perforasome is connected to adjacent perforasomes via two main mechanisms: direct flow via linking vessels or indirect flow via the subdermal plexus ( Fig. 12.10 ). Hypoperfusion, therefore, captures adjacent perforasomes.
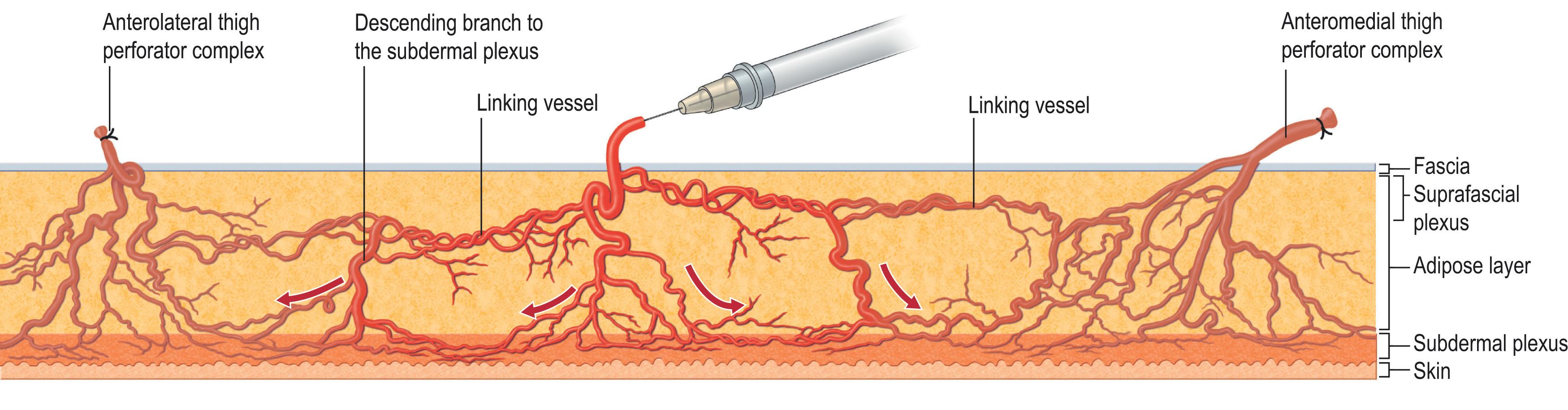
The vascular density of a given perforator is determined by the most proximal distance of the flap edge and flap width relative to the perforator location. This relationship subsequently defines the angle of perfusion. A decreasing number of linking vessels correlates with a decreasing angle of perfusion. The orientation of linking vessels corresponds to the orientation of maximal flap vascularity. Thus, the orientation of flap design should be parallel to the linking vessels to allow for optimal vascularity.
Preferential filling of perforasomes occurs within perforators of the same source artery followed by perforators of an adjacent source. When designing the optimal flap, it is important to center flaps over known perforators and to incorporate as many dominant linking vessels and multiple perforators from the same source artery. Further, larger flaps should incorporate tissues that originate away from the zone of injury, a practice which allows for more robust vascular support, but may not always prevent hypoperfusion in the distal tip.
Mass vascularity of a perforator is found adjacent to articulations and directed away from the same articulation. Midpoint perforators, also known as linking vessels, are found between two adjacent perforators and exhibit a bidirectional flow pattern.
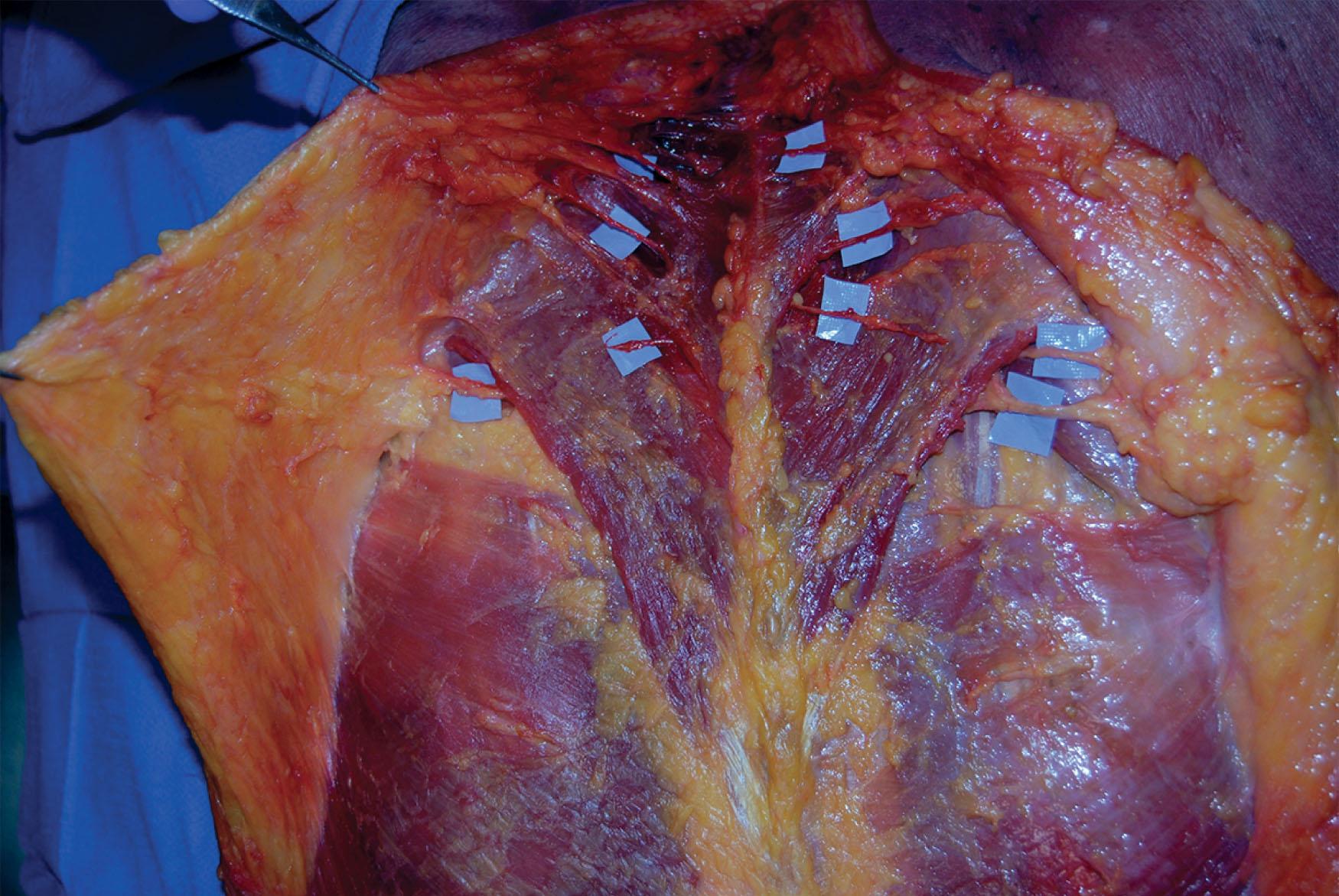
The trapezius, a Mathes and Nahai type II muscle, receives its blood supply from the ascending and descending branches of transverse cervical artery (TCA), with minor contributions from branches of the occipital artery and intercostal perforators ( Fig. 12.11 ; see Fig. 12.1 ). The spinal accessory nerve provides motor innervation (cranial nerve XI) and branches of C3 and C4 provide motor and sensory innervation. The external anatomical boundaries of the trapezius form a triangle, the base of which is marked by the midline of the back, extending from the external occipital protuberance to T12. This muscle then tapers laterally toward the base of the scapula, covering an area of 34 × 18 cm. The trapezius muscle originates along the superior nuchal line, the external occipital protuberance (nuchal ligament), the thoracic vertebrae, and the supraspinous ligaments. Insertion points include the scapular spine, acromion, and the lateral third of the clavicle. Elevation of the trapezius proceeds inferiorly to superiorly, and flap reach is increased by removing attachments to the scapular spine. The flap is typically utilized as an advancement, rotation, or turnover flap with muscle and myocutaneous variations (see Fig. 12.11 ). Care should be taken when harvesting the trapezial flap, as aggressive muscle harvest and/or nerve injury can result in winging of the scapula.
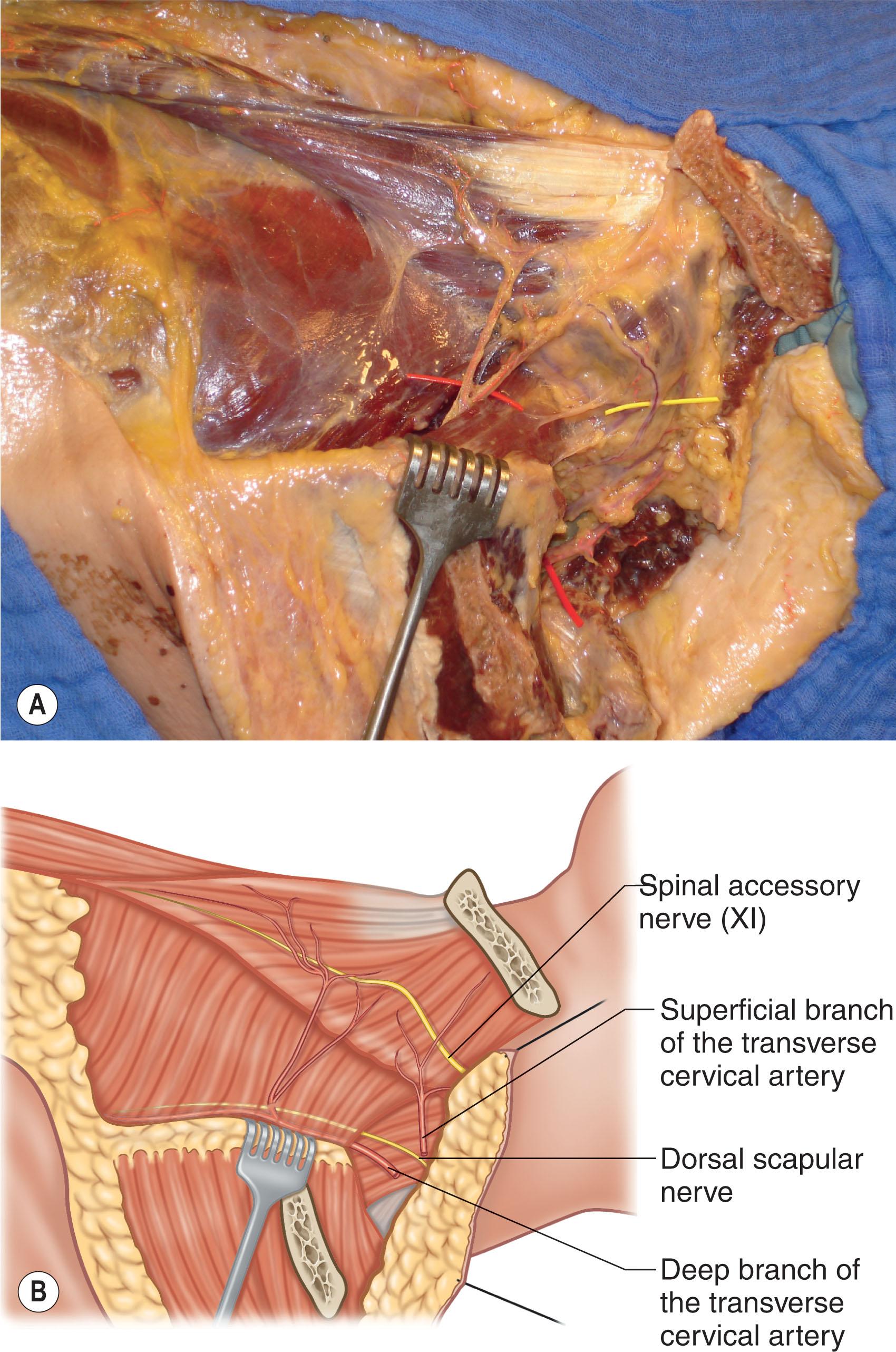
The dorsal scapular artery perforator (DSAP) and the superficial cervical artery perforator (SCAP) form the basis of the trapezial perforator flap, a muscle-sparing variation first described by Sadigh and colleagues in 2014 ( Fig. 12.12 ; see Fig. 12.9 ). There is also some contribution from the posterior intercostal arteries (see Fig. 12.9 ). The dorsal scapular artery (DSA) and the superficial cervical artery (SCA) are the deep and superficial branches of the TCA, respectively. As a result, flap harvest spares the subscapular system (see Fig. 12.13 ), which can be unavailable after tumor ablation or multiple prior attempts at reconstruction. The trapezial perforator flap not only preserves muscle function but has the added benefit of a limited donor site that can be well hidden under clothes. It provides a thin, flexible flap with skin paddles up to 25 × 15 cm that are ideal for coverage of superficial defects in the posterior cervical and upper thoracic regions, or even as a free flap. The DSA courses beneath the levator scapulae and rhomboid minor muscles, then branches between the rhomboid minor and major, perforating the fascia as the superficial dorsal scapular artery (SDSA). The SDSA then runs along the medial border of the scapula, deep to the trapezius, and sends out 1–2 perforators to the muscle and skin, located ~1–2 cm medial to the lower trapezial border. Alternatively, the DSA can originate directly from the third part of the subclavian artery. Due to its large arc of rotation, the trapezial perforator flap can reach ipsilateral and contralateral occipital, cervical, and upper thoracic wounds as a pedicled flap. Skin grafts can be utilized to aid closure if larger skin paddles are harvested.
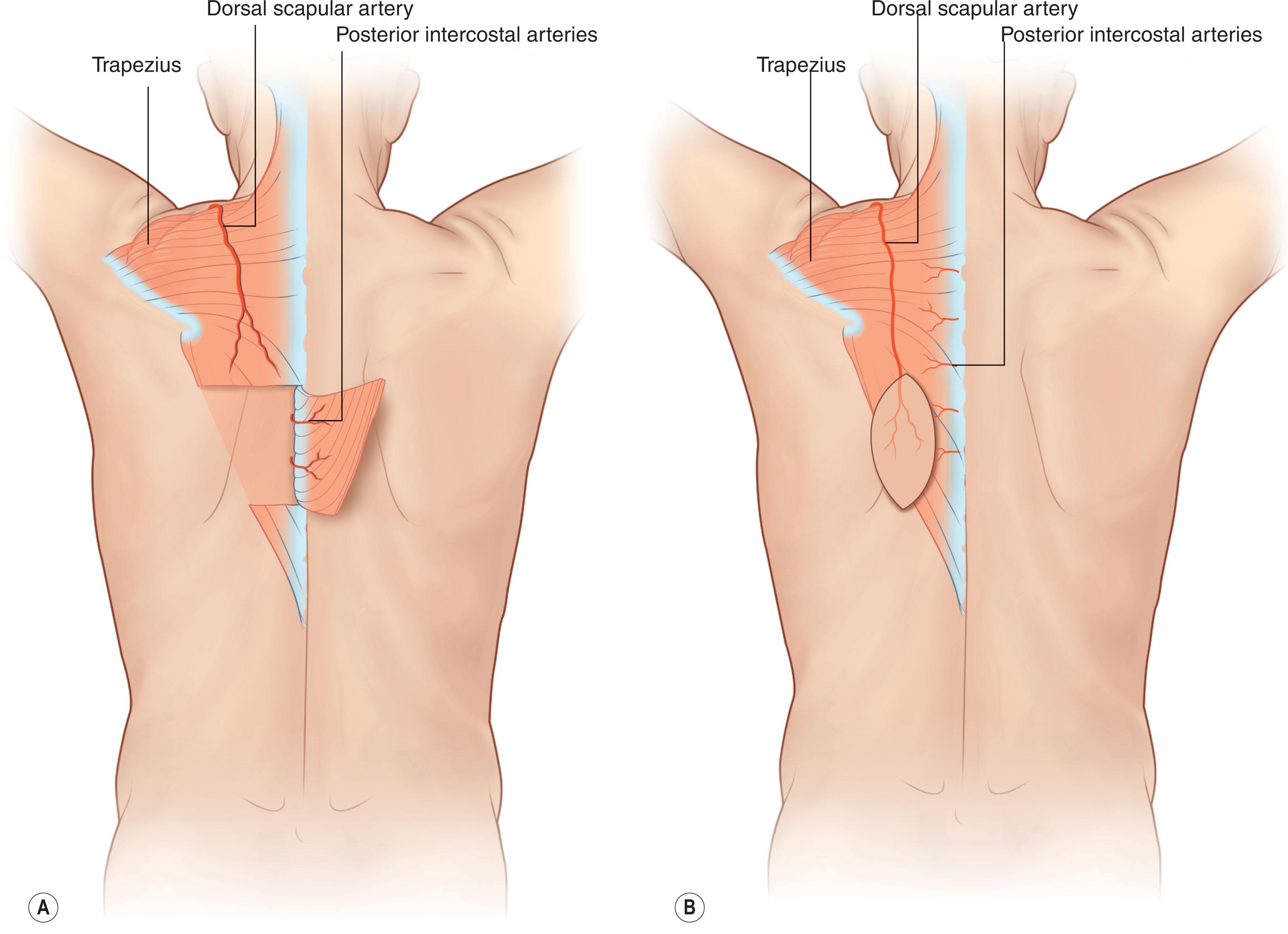
In a cadaveric study performed by Nyemb et al ., trapezial perforator location was assessed. In a majority of cases (78%), an average of 16 (range 9–27) perforating arteries can be found within 5–6 cm on either side of the midline, with an average diameter of 0.6 mm (range 0.1–2.6 mm). The line of perforators lies within fixed external landmarks, such as the scapular spine laterally, the scapular tip caudally, and the cranial margin of the trapezius, which can be used when designing trapezial perforator flaps.
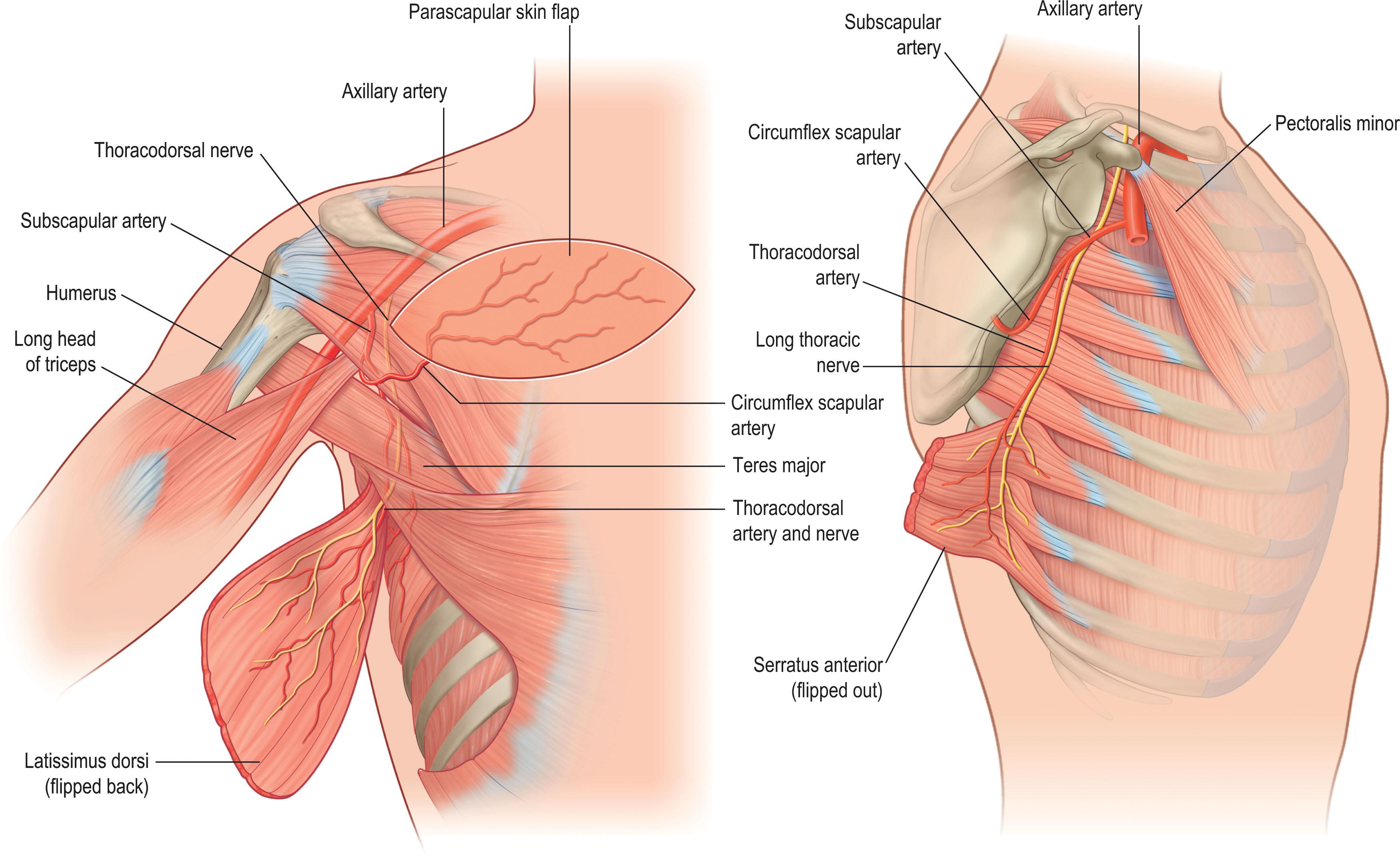
The scapular and parascapular flaps are part of the subscapular system ( Fig. 12.13 ). The scapular flap is an axial flap with blood supply provided by the transverse branch of the circumflex scapular artery (CSA). The CSA arises from the subscapular artery and occasionally directly from the axillary artery, before penetrating the triangular space formed by the teres muscles and the long head of the triceps. The flap is oriented parallel to the spine of the scapula ( Figs. 12.13 & 12.14 ). The midline of the flap can be visualized as a horizontal line extending from 2 cm above the posterior axillary crease to the vertebral column. The distal extent of the scapular flap extends midway between the midline of the back and the medial border of the scapula. The scapular flap is raised from medial to lateral and can include both fasciocutaneous and bony components, with dimensions typically up to 10 × 25 cm. A larger, 50 × 10 cm bi-scapular flap has been described by anastomosing the flap to the contralateral circumflex scapular artery. The vascular pedicle is typically 7–10 cm, which may be lengthened up to 14 cm by including the subscapular vessel. The arterial and venous caliber of the source vessels ranges from 2 to 4 mm. Donor sites less than 10 cm may be closed primarily, while larger donor sites may require skin grafting for coverage. Other regional flaps may be harvested simultaneously with the scapular flap, including the parascapular, latissimus dorsi, and serratus flaps. A thin, circumflex scapular artery perforator flap can also be raised based off perforating vessels arising within 1.5 cm of the CSA bifurcation, either from the main or descending branch. The CSA bifurcation is commonly found within the omotricipital space, a triangular region bordered by the teres minor and major muscles superiorly and the long head of triceps laterally.
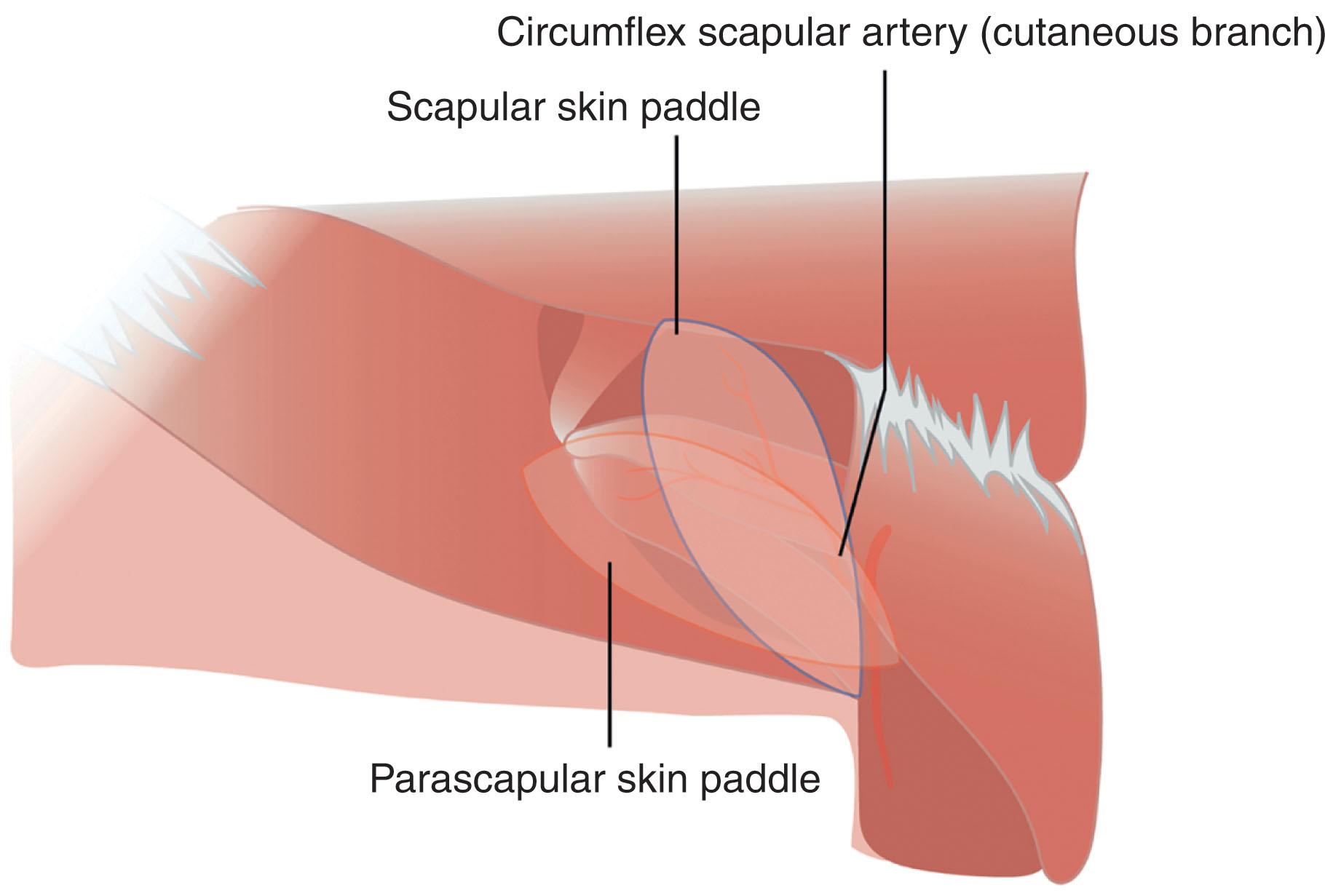
The parascapular flap is similar in characteristics to the scapular flap and provides another option for fasciocutaneous flap reconstruction from the subscapular system (see Figs. 12.13 & 12.14 ). It is supplied by the descending branches of the CSA, with multiple radial arteries off the descending branch providing blood flow for an inframammary extended circumflex scapular flap. The parascapular flap is oriented vertically, parallel to the lateral scapular border (see Figs. 12.13 & 2.14 ). The midline of the parascapular flap can be visualized as a vertical line extending from the triangular space towards the posterior iliac spine. Additional length can be obtained by following the contour of the inframammary crease. Based off radial branches of the descending branch of the circumflex scapular artery, the inframammary extended circumflex scapular flap can reach dimensions upwards of 10 × 30 cm and has the benefit of improved donor site scar aesthetics. Because their vascular pedicles arise from the same source artery, the parascapular flap can be raised simultaneously with the scapular flap and/or other flaps emanating from the subscapular system, with the convenience of a single anastomosis at the subscapular vessels (see Fig. 12.13 ). The resultant chimeric flaps can incorporate the latissimus dorsi and/or serratus anterior muscles, which can allow for coverage of larger defects. The arc of rotations for flaps in the subscapular system allows them to reach the shoulder, axilla, and lateral thoracic wall. The pedicle can be lengthened by anastomosing the distal end of the thoracodorsal artery and subscapular vein, forming a reverse flow flap orientation.
The latissimus dorsi (LD), a Mathes and Nahai type V muscle, is another flap originating from the subscapular system ( Fig. 12.15 ; see Figs. 12.1 & 12.13 ). It receives blood primarily from the thoracodorsal artery via the subscapular artery, and secondarily from segmental perforating branches of intercostal and lumbar arteries. Innervation is from the thoracodorsal nerve, and it is the largest flap that can be harvested off a single vascular pedicle. External anatomical boundaries are formed by the midline of the back, the posterior superior iliac crest, and the inferior angle of the scapula. The LD originates from the outer region of the superior iliac crest and the thoracolumbar fascia and spinous processes (T7–L5) posteriorly; it then extends superolaterally as broad, fan-like muscle, with dimension up to 20 × 40 cm. The LD gradually tapers and inserts into the intertubercular groove of the humerus, forming the posterior axillary fold (see Fig. 12.13 ). The LD flap is typically raised inferiorly to superiorly and can include muscular and fasciocutaneous components. A vascular pedicle ranging from 5–15 cm can be harvested, depending on the degree of thoracodorsal artery dissection down to its origin at the subscapular artery. As a pedicled flap, the LD can reach as far caudally as the lumbosacral region. Within the substance of the LD muscle, the artery divides into a transverse branch and a descending branch. A split LD flap variation, based on one of these branches, or a muscle sparing LD, can be raised to minimize muscle morbidity. Skin paddles up to 22 × 15 cm can be included for coverage. Because the LD can survive on its secondary segmental blood supply, a medially based, “reverse flow” musculocutaneous LD flap is an alternative for reconstruction in the middle thoracic and lumbar regions (see Figs. 12.1 & 12.15 ). In this version, the thoracodorsal pedicle is sacrificed, and the flap is fed by the T9–T11 posterior intercostal artery perforators. The pedicle may also be lengthened via placement of a vein graft.
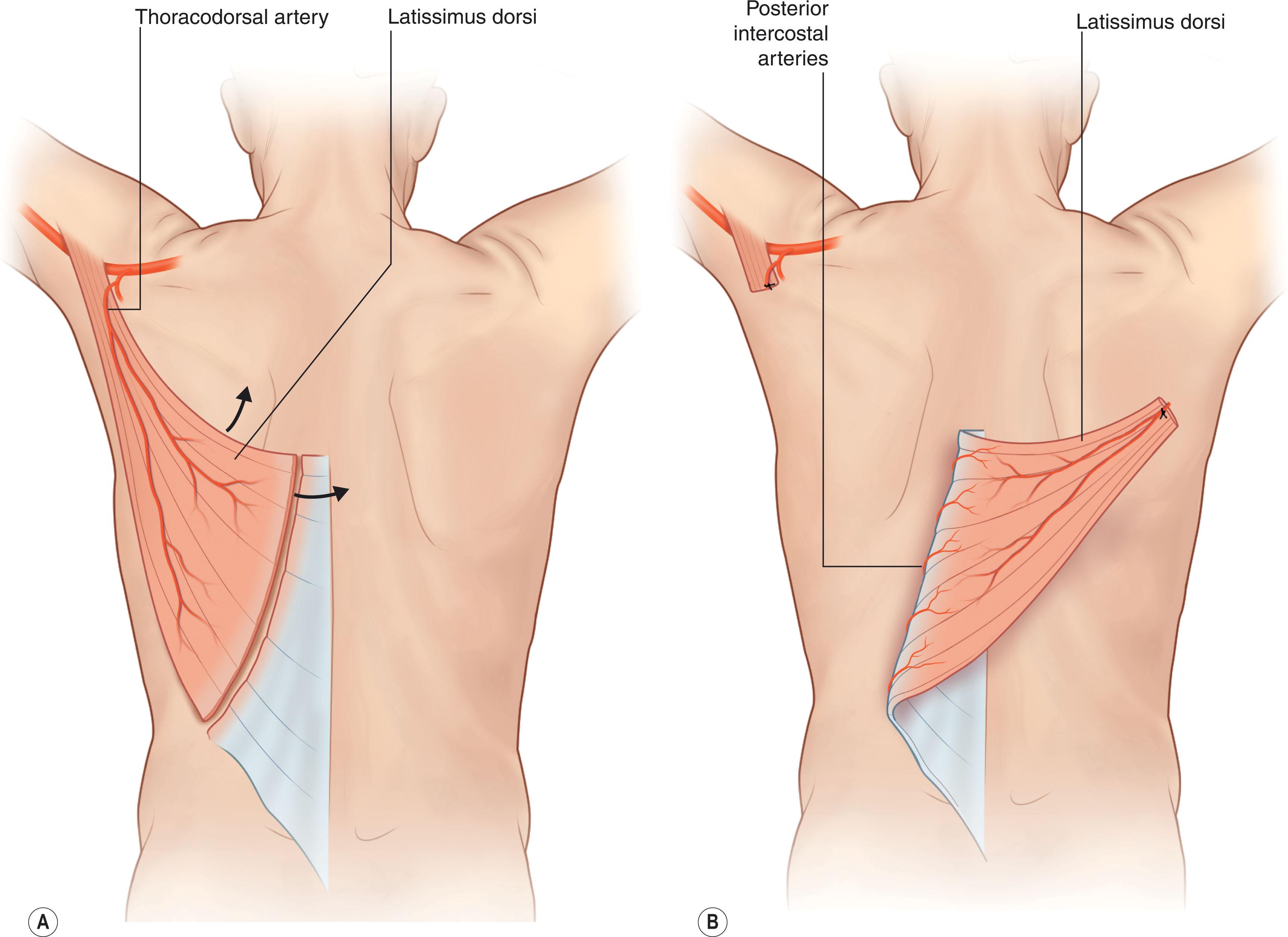
The LD is also the most common flap used in free tissue transfers within the posterior trunk, most commonly for reconstruction of lumbosacral defects. This is due to the large thoracodorsal artery diameter, with arterial and venous calibers ranging from 2 to 5 mm. Free tissue transfer with the LD is commonly successful even after the muscle has been irradiated. If greater length is required to bridge the distance between the flap and a non-local recipient vessel, vein grafts or arteriovenous loops can be utilized (see Free tissue transfer below). The LD can also be raised as a chimeric flap for coverage of larger defects by including other flaps within the subscapular system, including serratus, scapular, and/or parascapular flaps.
The thoracodorsal artery perforator (TDAP) flap can be raised as a fasciocutaneous or cutaneous flap, sparing the LD muscle ( Fig. 12.16 ). The TDAP flap offers consistent, long perforators, with single perforators reportedly able to support flaps up to 18 × 55 cm in size. Like the LD flap, the long pedicle of the TDAP flap makes it favorable for use in free tissue transfer. The thoracodorsal vessels course along the deep surface of the LD muscle, divide into the transverse branch and the lateral or vertical branch, typically situated at 45° angles from one another. A cadaveric dissection conducted by Heitmann et al . identified a total of 64 musculocutaneous perforators larger than 0.5 mm in 20 specimens. Of the 64 perforators, 36 were from the descending branch and 28 from the transverse branch. A direct cutaneous branch was also noted in 11 dissections. These branches follow an intramuscular course before continuing as skin perforators. The first perforator typically arises 6–8 cm below the posterior axillary fold and 2 cm medial to the posterior axillary line. Each of roughly three subsequent perforators arise at 1.5–4 cm intervals inferiorly. The perforators range from 0.3 to 0.6 mm in diameter. After locating the perforator, dissection proceeds until the desired pedicle length is obtained. Care must be taken to preserve the thoracodorsal nerve.
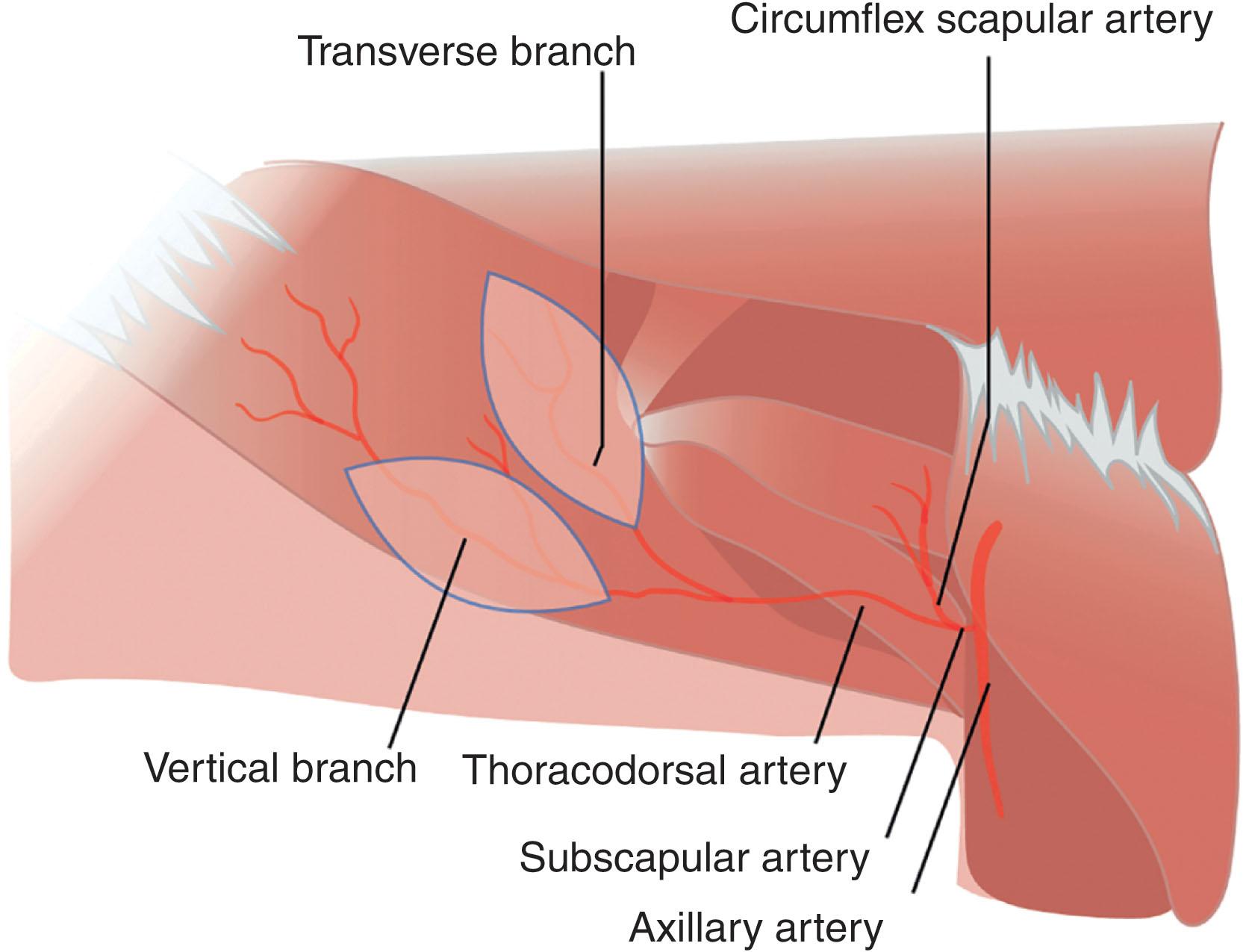
Three flap variations can be raised from the thoracodorsal artery perforators including the musculocutaneous perforator, the thoracodorsal artery perforator (TDAP) flap based on the septocutaneous perforator, and the lateral thoracic artery perforator (LTAP) flap based on the direct cutaneous perforator from the lateral thoracic artery (see Fig. 12.16 ). The TDAP, however, predominates due to a long vascular pedicle (18 cm) and large diameter vessels. This provides a greater ease of anastomosis to recipient vessels outside the zone of injury, with an option of end-side placement, thus preserving flow in receptor vessels. Modifications can be made to the TDAP flap to increase flap length and bulk, including pre-expansion. More than one perforator from the same thoracodorsal vessel branch can be incorporated, and the surrounding subcutaneous fat can be harvested by angling the subcutaneous dissection obliquely from the skin paddle. The TDAP flap orientation is configured along the axis of the linking vessels, allowing for inter-perforator flow. Further benefits to utilization of the TDAP flap for reconstruction of the posterior trunk include the availability of chimeric variations with other flaps in the subscapular system (see Figs. 12.13 & 12.16 ). Common chimeric flap combinations include the TDAP and LD and the TDAP and serratus anterior muscle. Scapular bone may also be added when osseous reconstruction is required. The TDAP is an ideal perforator flap in the posterior trunk due to its ease of harvest, soft-tissue bulk, and minimal morbidity.
The paraspinous (PS), or erector spinae, are a group of intrinsic muscles that includes the longissimus, iliocostalis, and spinalis ( Figs. 12.17 & 12.18 ; see Fig. 12.2 ). They are located within the paravertebral gutters on either side of the spinal column. The PS muscles extend from the lumbosacral to thoracic region, originating from the sacrum, iliac crest, lamina, and transverse processes of the vertebrae and inserting into the posteromedial aspect of the ribs, with insertions into the occiput at the most superior extent (see Fig. 12.2 ). The blood supply to the PS muscles is provided by the intercostal arteries, segmental branches from the aorta, which give deep and lateral perforators to the muscle. Thus, the PS is classified as a Mathes and Nahai type IV muscle.
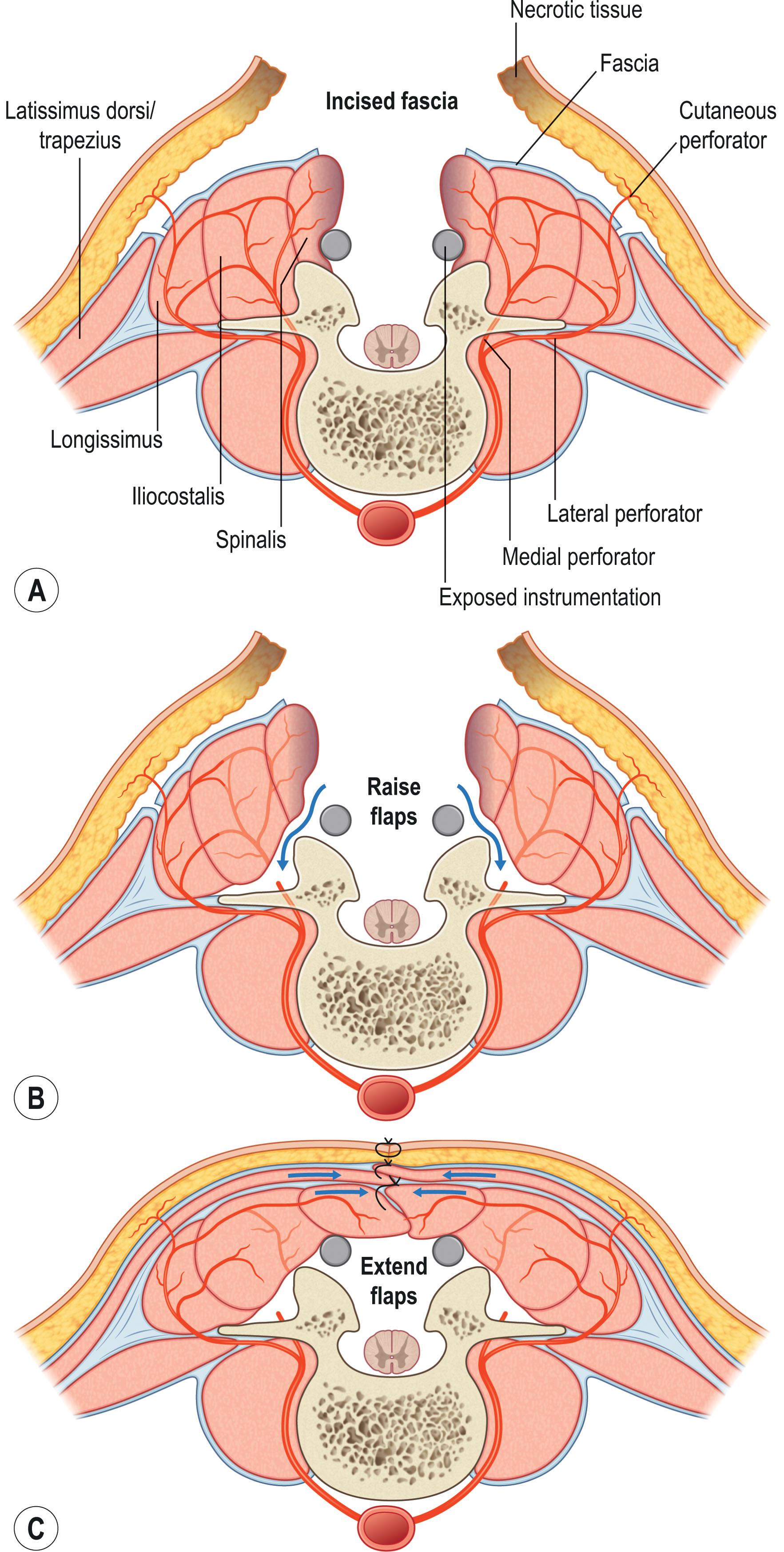
Exposure and harvest of the PS flap for closure of upper thoracic defects requires dissection of the overlying trapezius, rhomboid major, and serratus posterior superior muscles (see Figs. 12.1 & 12.2 ). Alternatively, dissection of the thoracolumbar fascia, latissimus dorsi, and serratus posterior inferior muscles is necessary for exposure and harvest of the paraspinous muscle in the middle thoracic region (see Figs. 12.1 & 12.2 ). The PS flap is commonly utilized as a bipedicled or turnover flap, although osseo-muscular flaps, as initially described by Mustardé, can also be harvested by fracturing of the transverse processes of the vertebrae. Flap design should incorporate well-perfused tissues with perforators based outside the zone of injury.
Bipedicled PS flap elevation incorporates the lateral column perforators and proceeds in a medial to lateral direction (see Fig. 12.18 ). Attachments to the medial spinal process and the transverse process are removed (see Figs. 12.17 & 12.18 ). The flap remains in continuity at the superior and inferior muscle segments, ensuring adequate segmental perfusion while allowing the medial portion of the flap to advance toward the midline defect (see Fig. 12.18 ). Flap advancement can be increased by cauterization of medial perforators. Because the bipedicled design can limit flap excursion, a superiorly based, unipedicled flap based on intramuscular longitudinal vessels is another alternative, which can be raised bilaterally up to T10. Removal of attachments from the posterosuperior iliac crest, quadratus lumborum, and thoracolumbar fascia can also increase flap advancement.
When the fascial incision is placed on the lateral aspect of the erector spinae fascia, a turnover flap can be performed. The flap is raised from lateral to medial, and the medial perforator row is preserved. The lateral flap edges are then transposed toward the midline, while ensuring good opposition under minimal tension. A history of bilateral laminectomies increases the likelihood of damaged medial intercostal perforators and segmental pedicles from the posterior intercostals. In such cases, a turnover PS flap may be unreliable.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here