Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews various x-ray–based techniques used in thoracic imaging, including radiography, fluoroscopy, digital tomosynthesis, and computed tomography (CT).
Merely 1 year after discovery of x-rays by Wilhelm Konrad Roentgen, calcium tungstate was chosen as the material for the first screen cassettes based on Thomas Edison's assessment of about 5000 chemicals for their light-producing capabilities upon exposure to x-rays. For the next 75 years, conventional radiography remained confined to the radiographic films sandwiched between two calcium tungstate–based fluoroscopic screens. It was not until the mid-1970s that the first computer-based processing of radiographic images began to emerge with concomitant introduction of rare earth chemicals into intensifying screens, which had better x-ray absorption and lower radiation dose requirements.
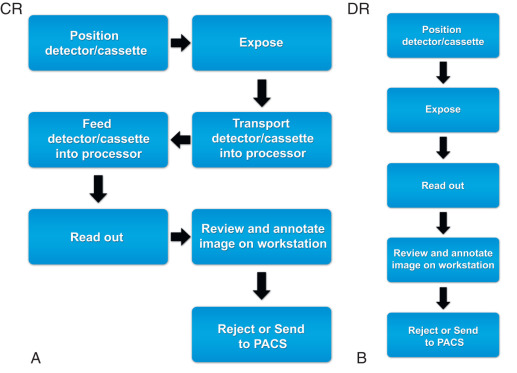
The earlier versions of computed radiography (CR) used the cassettes with new fluorescent materials, which retained the information of x-ray exposure as a latent image. This information was retrieved by stimulation with a thin focused laser beam, which resulted in re-emission of light. This light was captured with light-sensitive diodes and converted to electrical signals stored and processed with computers. Although initial versions of CR required much higher radiation exposure, subsequent refinements in the detectors resulted in considerable decreases in radiation doses. The cassette-based CR systems also required specific readout stations, which have also decreased in size and cost. These systems are in common use because of their flexibility and lower costs.
Developments in CR technology finally led to direct digital radiography (DR), with electronic detectors. In the direct conversion type of DR system, the incident x-ray photons interact with photodiodes and generate electrons, which form digital images. In the indirect conversion type, x-ray photons interact with a scintillator to generate light photons, which then subsequently interact with photodiodes to release electrons to form digital images ( Fig. 1.1 ).
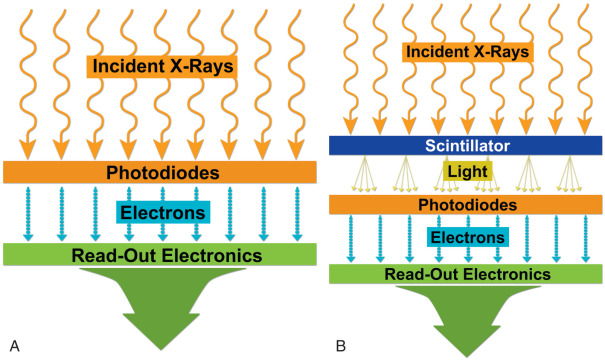
The indirect conversion type of DR system is most commonly used in chest radiography, and commonly used scintillation detectors are cesium iodide and gadolinium oxysulfide. The direct type of DR systems is used in mammography units. Compared with CR, DR systems are faster, allow more efficient throughput, have better image quality, and have higher radiation dose efficiency. For these reasons, mobile and wireless DR systems have immense applications in critical care settings, emergency departments, and intraoperative imaging.
Digitization of radiographic images with CR and DR has also enabled benefits in the form of electronic image storage and display on the picture archiving and communication system (PACS) as well as image manipulations such as adjustment of brightness and contrast, edge enhancement, inverted image display, zooming, and subtraction capabilities.
Modern radiography units are more radiation dose efficient and use automatic exposure control techniques to terminate the radiographic exposure when the desired quality is reached. It is also important that the operators use appropriate guidelines (e.g., centering, breath-hold instruction, and coning) to minimize unnecessary repetition of radiographs.
As opposed to readout workstations for CR, which implied some readout time, with DR systems, radiographs could be ready for interpretation in as little as 5 seconds after their acquisition ( Fig. 1.2 ).
The plain chest radiograph is the most commonly performed imaging procedure in most radiology practices, constituting between 30% and 50% of studies. The standard routine chest radiograph consists of an erect radiograph made in the posteroanterior (PA) projection and a left lateral radiograph, both obtained at full inspiration. In the standard PA projection, the patient faces the cassette, and the x-ray tube is located 6 feet behind the patient. The arms are directed away from the chest with the hands resting on the waist. This technique prevents magnification of anterior structures such as the heart. The full inspiration allows complete expansion of the lungs and separation of pulmonary blood vessels to improve detection of pathology. In an ideally performed standard radiograph, the trachea is in midline projection over the spine and is equidistant from the heads of both clavicles. The lung apices, the lateral costophrenic (CP) angles of the lungs, and the diaphragm are completely visualized, and the diaphragm lies at or below the anterior sixth and posterior ninth ribs. Chest radiographs should be exposed using a high kilovoltage (kV) technique, usually in the range of 100 to 140 kV. With this technique, a grid or air gap is required to reduce scatter radiation. The main advantage of this technique is that the bony structures appear less dense, permitting better visualization of the underlying parenchyma and the mediastinum. The only drawbacks are the decreased detectability of calcified lesions and loss of bony detail.
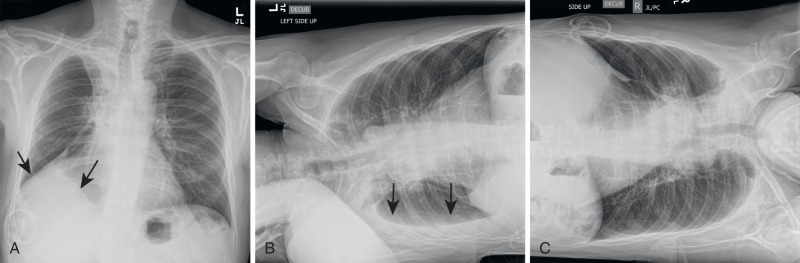
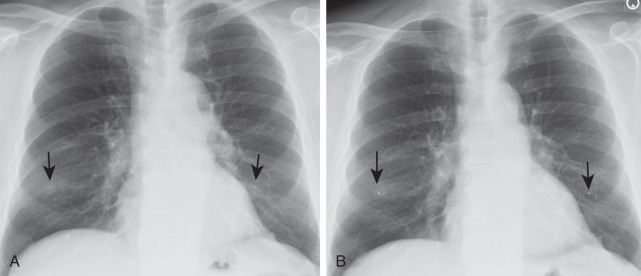
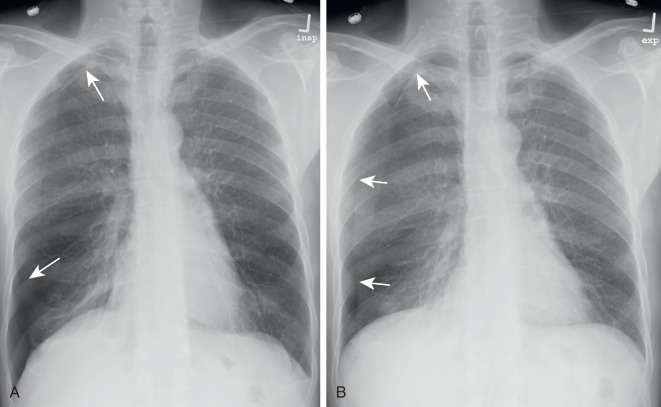
With the emergence of CT, the use of additional projections and views in chest radiography has precipitously decreased because additional information in doubtful circumstances is typically resolved with cross-sectional imaging. Furthermore, emerging literature also demonstrates the use of digital tomosynthesis in these situations. Additional views of the chest may be required in special instances. Shallow oblique radiographs (15 degrees) may be useful in confirming the presence of a suspected nodule. Forty-five–degree oblique radiographs can be used for the detection of asbestos-related pleural plaques. Apical lordotic views project the clavicles above the chest, improving visualization of the apices and the middle lobe, particularly in cases of middle lobe atelectasis. Lateral decubitus radiographs ( Fig. 1.3 ) can be used to determine the presence or mobility of pleural effusion and to detect small pneumothorax, particularly in patients who are confined to bed and unable to sit or stand erect. A PA radiograph done with nipple markers is useful in differentiating nipple shadows from true lung nodules ( Fig. 1.4 ). Paired inspiratory and expiratory radiographs ( Fig. 1.5 ) can improve the conspicuity of small pneumothorax in doubtful cases.
Bedside portable chest radiographs may account for up to 50% of chest radiographs in a hospital setting. Many critically ill patients, including patients in intensive care units (ICUs), must be radiographed at the bedside, resulting in radiographs with limited diagnostic information. The diagnostic quality of these images is often limited because of the increased exposure time needed, which results in respiratory motion. Because portable chest radiographs are acquired with reduced target film distance (<6 feet) and in anteroposterior projection, magnification occurs, particularly of the heart and anterior structures. In ICUs, often patients have lines and tubes overlying the chest; these must be moved to the side if possible. At the very least, coiling or bunching up of wires and electrodes over the chest should be avoided. Better quality radiographs can be obtained in large patients, with attention to centering, coning, and use of antiscatter grids to minimize scattering in large patients.
With advances in DR and display systems, it is possible to apply subtraction techniques in chest radiography that include dual-energy subtraction and temporal subtraction. The former refers to removal of bone structures from the chest radiograph to improve the detectability of pulmonary findings, which can easily be missed ( Fig. 1.6 ). There is a wide variation in the frequency of missed findings ranging from 10% to 50% as reported in different studies. To reduce the frequency of missed findings because of overlapping bones, dual-energy subtraction radiography has been explored in the chest. Such radiographs can be acquired with either single exposure of the patient using layered detectors (each detecting different energy spectra) or sequential exposure of the patient with high and low kV with minimum interval. Subtraction of bones from the soft tissue with dual-energy radiography helps improve visibility of pulmonary findings. Conversely, subtraction of soft tissues from bones can help assess the presence of calcifications in pulmonary nodules and pleural plaques. The visibility of coronary and pericardial calcifications as well as chest implants is also improved with subtraction of soft tissues from the bones.
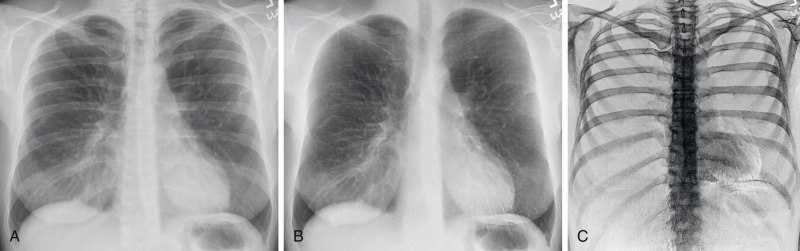
Temporal subtraction implies subtraction of chest radiograph at two different time points for the purpose of identifying changes in abnormalities over time. Studies have reported the effectiveness of this technique for the progression or regression of pulmonary findings as well as for the detection of new abnormalities. Although subtraction techniques have been around for several years, their clinical use in the United States remains low because of the cost of adopting these techniques, lack of reimbursement, and concerns over increases in interpretation time. Edge enhancement improves the conspicuity of pneumothorax and outlines of supporting devices and access lines ( Fig. 1.7 ).
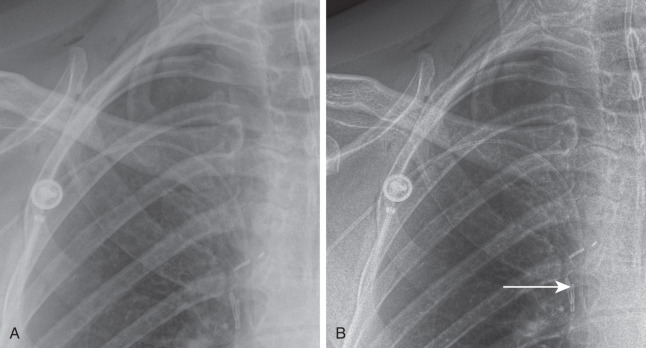
With the widespread application of CT, chest fluoroscopy is mainly restricted to the evaluation of diaphragmatic motion in patients with suspected diaphragmatic paralysis. Ideally, fluoroscopy for this purpose should be performed with the patient standing so that both hemidiaphragms can be visualized simultaneously. After the diaphragm is localized and centered, patients are instructed to perform a short quick inspiratory maneuver (e.g., a sniff). In patients with diaphragmatic paralysis, the affected hemidiaphragm moves up during a rapid inspiration instead of moving down.
When performing a fluoroscopic examination, it is important to limit the exposure duration to minimize the radiation dose to the patient and operator. Automatic brightness control should be used to allow adjustment of exposure to body habitus. Fluoroscopic time should be kept to the minimum, and the exposure factors should be recorded.
In digital tomosynthesis, multiple projections (about 60) of very low-dose x-rays are obtained through the region of interest during a breath-hold of 10 seconds. The acquired data are then reconstructed into contiguous coronal images. At total estimated effective dose of about 0.12 mSv, the radiation dose from digital tomosynthesis is higher than typical PA chest radiographs (0.02 mSv) but significantly lower than most chest CT examinations (2–6 mSv). Compared with plain radiography, digital tomosynthesis provides superior lesion detectability from noise reduction, better depth assessment, and superior contrast resolution.
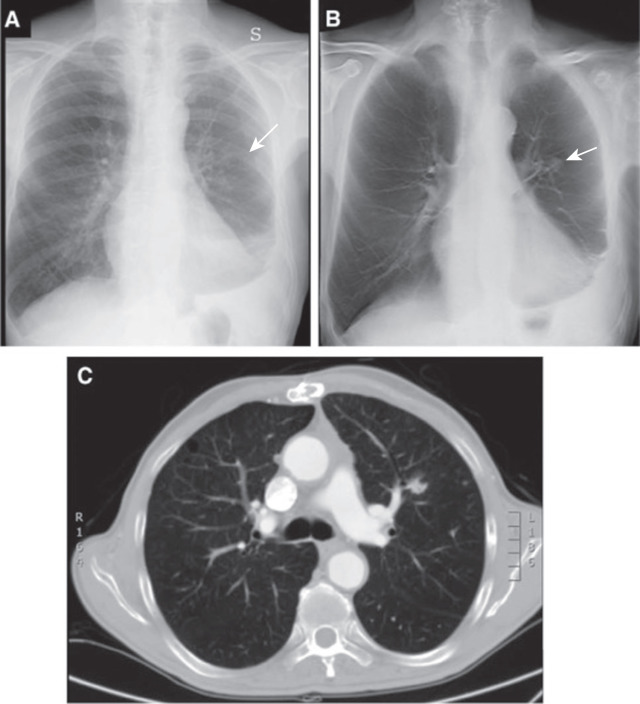
Although the driving application of chest digital tomosynthesis is improved detection of pulmonary nodules over conventional radiography, several other applications have been assessed as well—for example, suspected interstitial lung disease, cystic fibrosis, airway evaluation, and evaluation of thoracic skeleton fractures. Studies have shown that digital tomosynthesis can help detect more pulmonary nodules smaller than 9 mm in diameter compared with combined PA and lateral chest radiographs ( Fig. 1.8 ).
Similar to conventional radiography, CT also uses x-rays generated from an x-ray tube. Although x-rays in conventional radiography are projected from one direction only, the x-ray tube in CT revolves 360 degrees around the patient while continuously projecting x-rays so that cross-sectional images of the body can be generated. These x-rays traverse through the patient's body and reach the detector assembly, where they create a signal. This signal is represented by attenuation value or coefficient, which is a measure of attenuation of x-rays as it traverses through the material or body part being scanned. The Hounsfield units (HU) or CT numbers are arbitrary units that represent the average of all attenuation values in a voxel of image. The voxel represents a three-dimensional (3D) volume element in the object consisting of each individual number in the image matrix. The Hounsfield value of water is considered “0.” Fat, lung, and air have negative values (-50 to 100, -850 to -910, and -1000, respectively). Soft tissues, bone, and metals have positive values (45 to 65, 700 to 1500, and over 2000, respectively).
The earliest “step-and-shoot” CT scanners involved x-ray tube and detector assemblies with direct wire attachments, which meant that after each revolution, the wires had to “unwind” before rescanning. In the early 1990s, the x-ray tube and detector elements were mounted on a “slip ring” gantry system that was connected to the wires. This arrangement meant that both x-ray tube and detector elements could continuously revolve around the patient while the table moved in or out of the gantry aperture. Dubbed as spiral or helical CT, this enabled a much faster coverage compared with its predecessor step-and-shoot CT systems. Because there was only a single row of detectors along the patient length in the earlier helical scanners, only one image could be created per 360-degree gantry revolution.
Helical CT experienced a breakthrough in the 1990s, when multiple rows of finer detectors were introduced into the detector assembly along the patient length, enabling multiple images or slices per gantry revolution of the x-ray tube. These multidetector-row CT (MDCT) scanners began with a modest 2 to 16 rows, enabling two to four images per 360-degree revolution and led to slice wars among different CT vendors in the early 2000s. Modern MDCT scanners ( Box 1.1 ) can now generate 64 to 640 overlapping images per gantry revolution, encompassing a length of up to 16 cm per gantry revolution!
Heavy patients can be adequately scanned
Longer scan lengths without tube heating issues
Allows use of higher mA and lower kV to reduce contrast volume requirements
Wide area detector assemblies enable faster scanning
Fine detector rows enable thinner images
Efficient detector assembly reduces image noise at low doses
Reduces motion artifacts
Enables better temporal resolution for cardiac and vascular CT
Reduces motion artifacts in patients unable to hold breath during CT
Enables image noise reduction at low radiation doses
Improves image quality without need for higher radiation doses
Reduces certain artifacts
CT, Computed tomography.
In addition to wide area coverage, the finer detector rows in modern MDCT scanners can generate submillimeter images (up to 0.5 mm thickness) of the whole chest in under 1 second. Wide area coverage, fast gantry revolution times down to a quarter of a second, and high scanning speeds have enabled users to substantially reduce or eliminate motion artifacts from CT images. In fact, some MDCT scanners can now enable free-breathing scanning of the entire chest in less than half a second.
X-ray tubes in newer MDCT scanners have also overcome tube heating issues for nonstop extended coverage examinations (e.g., multiple body part CT angiography) and for large patients. Simultaneous improvements in detector assemblies in certain MDCT scanners with better integration of electronics have helped improve scanner efficiency and have decreased image noise at low-dose levels as well as in very large patients.
Because of simultaneous improvements in computational capabilities, thanks to the video gaming industries and more accurate and efficient methods of image reconstruction, iterative reconstruction techniques have been introduced on most modern MDCT scanners. These techniques result in less image noise and fewer artifacts compared with the conventional filtered back projection methods, which in turn enables users to reduce radiation dose while maintaining or even enhancing image quality.
Although chest CT is the imaging modality of choice for the evaluation of several clinical indications, chest radiography remains the most commonly performed test in the radiology department for assessment of the chest in both inpatient and outpatient settings. Compared with chest CT, chest radiography has a much lower cost and is associated with a substantially lower radiation dose.
Some important clinical indications for chest CT are persistent cough, dyspnea, chest pain, abnormal chest radiograph findings, and cancer staging workup. Recently, low-dose CT for lung cancer screening has been approved for screening eligible patients at risk of developing lung cancer. A vast number of CTs are also performed for follow-up of indeterminate lung nodules seen on initial chest CT; these can be performed at low radiation dose levels in the absence of recent malignancy.
Chest CT angiography of pulmonary arteries is the imaging modality of choice for evaluation of patients with suspected or known pulmonary embolism (PE) and pulmonary arteriovenous malformations. A diffuse lung disease protocol (also referred to as high-resolution CT of the chest) is used for a host of clinical indications such as interstitial lung diseases, bronchiectasis, and post–lung transplantation evaluation. Abnormalities of the central airway and chest wall are also well assessed with CT. Indication-specific CT protocols can be designed to confine scan length to the area of interest and control the number of scan phases (inspiration, expiration, and prone images).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here