Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Computed tomography (CT) and magnetic resonance imaging (MRI) play complementary roles in the pre- and postoperative evaluation of the various neoplasms that occur at the skull base. Typically, both imaging modalities are obtained in the diagnostic workup and treatment planning of skull base lesions. , Although CT provides excellent osseous detail with respect to the effect of the mass on adjacent bony structures, MRI gives better assessment of the tissue composition of the mass itself and its impact on adjacent soft tissue structures. Both imaging modalities can also play important roles in the postoperative surveillance after tumor resection as well as in identification of unexpected complications.
With its high spatial resolution, multidetector CT scanning now allows for submillimeter slice thickness imaging with multiplanar reconstructions and affords exquisite anatomic detail of the bony structures. , Bony lysis, sclerosis, erosion, and destruction can help identify the extent and aggressiveness of skull base tumors, and certain bony changes can help narrow the differential diagnosis. Bony widening or erosion of skull base foramina are useful features to indicate perineural tumor spread (PNTS). Bony defects along the skull base can reflect sites of cerebrospinal fluid (CSF) leak in the appropriate clinical context and can be confirmed after injection of iodinated contrast into the subarachnoid space by intrathecal injection (CT cisternography). CT angiography (CTA) is a CT study obtained following injection of intravenous (IV) contrast timed to the arterial or venous phase and can be a useful adjunct in imaging workup to identify the relationship between the vasculature and tumor. ,
Although CT imaging in soft tissue windows can provide some insight in the effect of skull base masses on the brain parenchyma and adjacent soft tissues, the soft tissue resolution provided by MRI is superior. Imaging characteristics, including T1 and T2 signal, enhancement pattern, and apparent diffusion coefficient (ADC) values, can help narrow the differential diagnosis in most cases. The presence of flow voids within a tumor can indicate its hypervascular nature, necessitating embolization before surgical resection. High-resolution MRI provides submillimeter slice thickness evaluation of skull base tumors that can be reconstructed in multiple planes, allowing for a more accurate assessment of extent of skull base masses and its impact on adjacent skull base foramina, cranial nerves (CNs), and vessels. Fat-suppression techniques such as short tau inversion recovery (STIR) may improve the conspicuity of soft tissue lesions embedded in fatty tissue. Highly fluid-sensitive thin-section (i.e., T2-weighted) sequences (high-resolution three-dimensional [3D] T2-weighted imaging, fast imaging using steady-state acquisition with phase recycling [FIESTA], or constructive interference in steady state [CISS]), provide high contrast and spatial resolution and can be used to depict the intracanalicular and cisternal portions of CNs, as well as structures of the fluid-filled labyrinth. These same sequences may provide an alternative to gadolinium-enhanced T1-weighted imaging for vestibular schwannoma (VS) monitoring. ,
By virtue of their anatomic location, tumors arising from the skull base are typically extraaxial masses that arise most often from bone, meninges, nerves, or vessels or from adjacent anatomic compartments. This section discusses the most commonly encountered lesions that should be considered in the preoperative assessment of tumors arising from the anterior, central, and posterior skull base.
The majority of tumors at the anterior skull base arise from invasive malignancy from the sinonasal cavity, but the anterior skull base may also be violated by tumors of the scalp, face, and orbits. Squamous cell carcinoma (SCC) is the most common histologic subtype of sinonasal tumors, making up more than 50% of cases. , Other subtypes, such as adenocarcinoma, undifferentiated carcinoma, minor salivary gland carcinoma, neuroendocrine carcinoma, and nonepithelial malignancies (i.e., lymphoma, olfactory neuroblastoma, sarcoma, and melanoma), are considerably less common.
The histopathologic diagnosis of various sinonasal malignancies is not easily rendered by imaging; however, the presence of unilateral sinus disease with associated bone destruction on CT, typically performed in the clinical context of rhinosinusitis, should raise suspicion for sinonasal malignancy ( Fig. 27.1 A). On MRI, precontrast T1-weighted images without fat suppression can elegantly demonstrate the soft tissue extent of the tumor, with violation of normal fat planes indicating invasive nature ( Fig. 27.1 B). T2-weighted MRI sequences can help distinguish the true extent of the mass from associated trapped secretions, which are nonenhancing and T2 bright ( Fig. 27.1 C). , Diffusion restriction within the soft tissue mass can confirm its hypercellular nature.
Aggressive sinonasal tumors have the potential to invade the orbit, especially via the lamina papyracea ( Fig. 27.1 D). The presence of visual symptoms or ophthalmoplegia should prompt urgent imaging in the appropriate clinical context. On CT, bone erosion and loss of normal orbital fat planes can be subtle markers of involvement. Orbit-tailored MRI sequences (e.g., coronal STIR, axial and coronal pre- and postcontrast T1-weighted imaging with fat saturation) can also be helpful to better define orbital invasion.
With anterior skull base invasion, cortical destruction of the cribriform plate, lateral lamella, or fovea ethmoidalis is best appreciated on CT. Loss of the normal T1 and T2 low-signal cortical margin, loss of the normal T1 hyperintense marrow signal, and nodular dural enhancement along the floor of the anterior cranial fossa on postcontrast T1-weighted imaging are good indicators of bone infiltration and transcalvarial spread of disease ( Fig. 27.1 E).
Olfactory neuroblastomas (ONBs), or esthesioneuroblastomas, arise from the specialized sensory neuroepithelial olfactory cells that are normally present in the cribriform plate, the superior turbinate, and the upper third of the nasal septum. The tumor can occur at any age, with bimodal peaks in the second and sixth decades of life. , , Patients typically present with unilateral nasal obstruction and hyposmia.
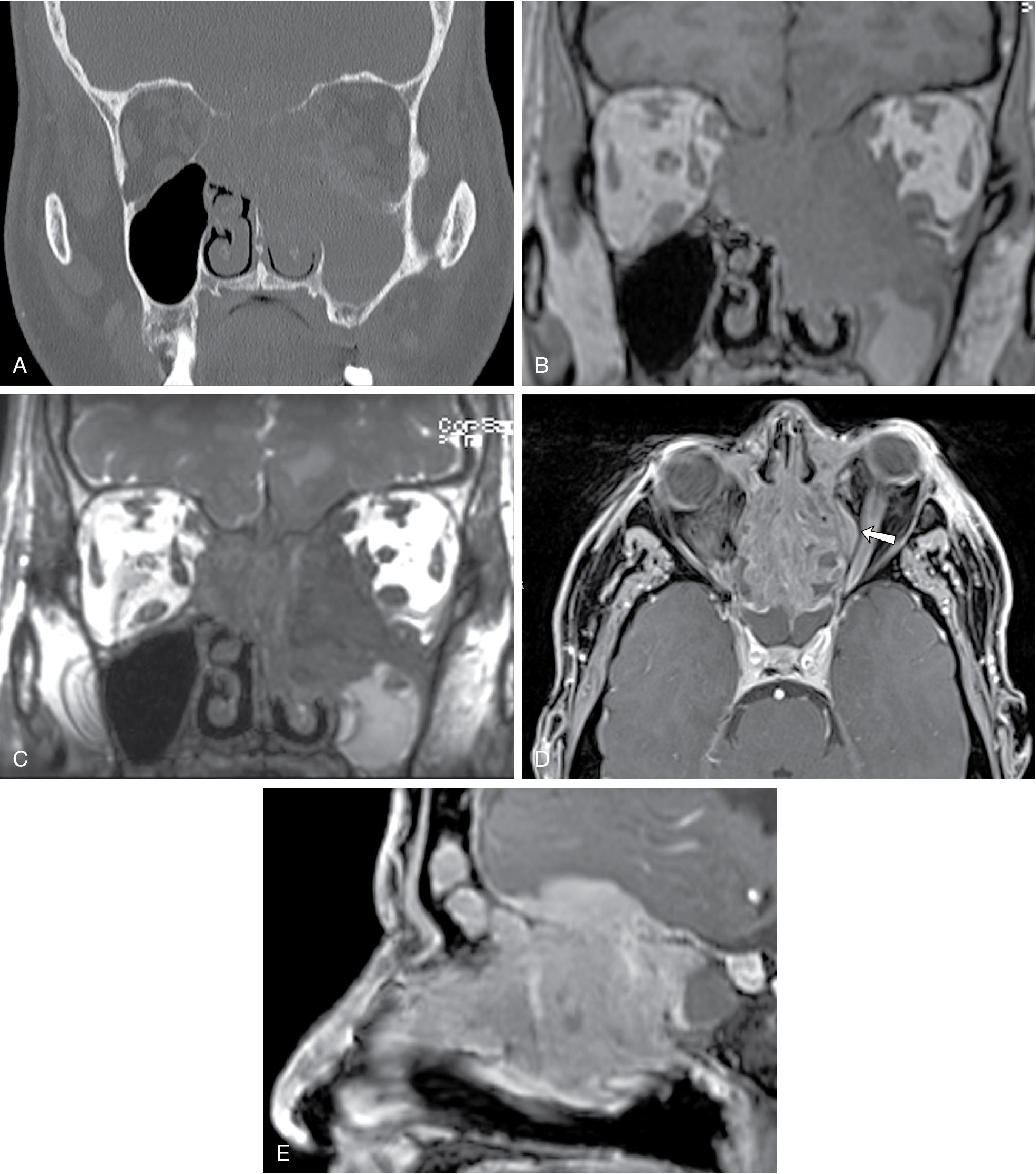
Olfactory neuroblastomas are typically located at the olfactory groove and are classically “dumbbell” shaped with the center portion of the mass formed at the cribriform plate. Direct tumor extension into the adjacent paranasal sinuses, cribriform plate, orbit, anterior skull base, and intracranial compartments can be seen. Erosive change and calcifications may be seen on CT ( Fig. 27.2 A). On MRI, ONBs usually are hypointense on T1-weighted imaging, enhance on postcontrast T1-weighted images ( Fig. 27.2 B), and appear heterogeneously hyperintense relative to the gray matter on T2-weighted imaging. , , The intracranial extension is often better delineated on MRI than on CT. With intracranial extension, marginal tumor cysts can be identified, and when present, are highly suggestive of ONB , ( Fig. 27.2 C).
Pituitary adenomas are common central skull base tumors that account for approximately 10% to 15% of all intracranial neoplasms. , The most common pituitary adenomas are prolactinomas, making up approximately 25% to 41% of all pituitary tumors, followed by adrenocorticotropic and growth hormone-secreting tumors in 5% and 2.8% of cases, respectively. Undetectable by CT, microadenomas are small hypoenhancing nodules embedded within pituitary tissue (<1 cm in size by definition) that can show increased conspicuity on MRI with dynamic contrast-enhanced imaging, which takes advantage of the differential rate of enhancement between the tumor and surrounding parenchyma early in the initial 60 seconds after contrast administration; subsequently, the tumor may show iso-enhancement with the pituitary gland, rendering it inconspicuous on more delayed postcontrast images.
Nonfunctioning adenomas compose 25% of pituitary adenomas. These tumors often grow to a significant size and cause optic chiasmal compression before they are detected. , Additional mass effect on inferior frontal lobes, ventricular system, and CNs coursing the suprasellar and prepontine cisterns can also be seen.
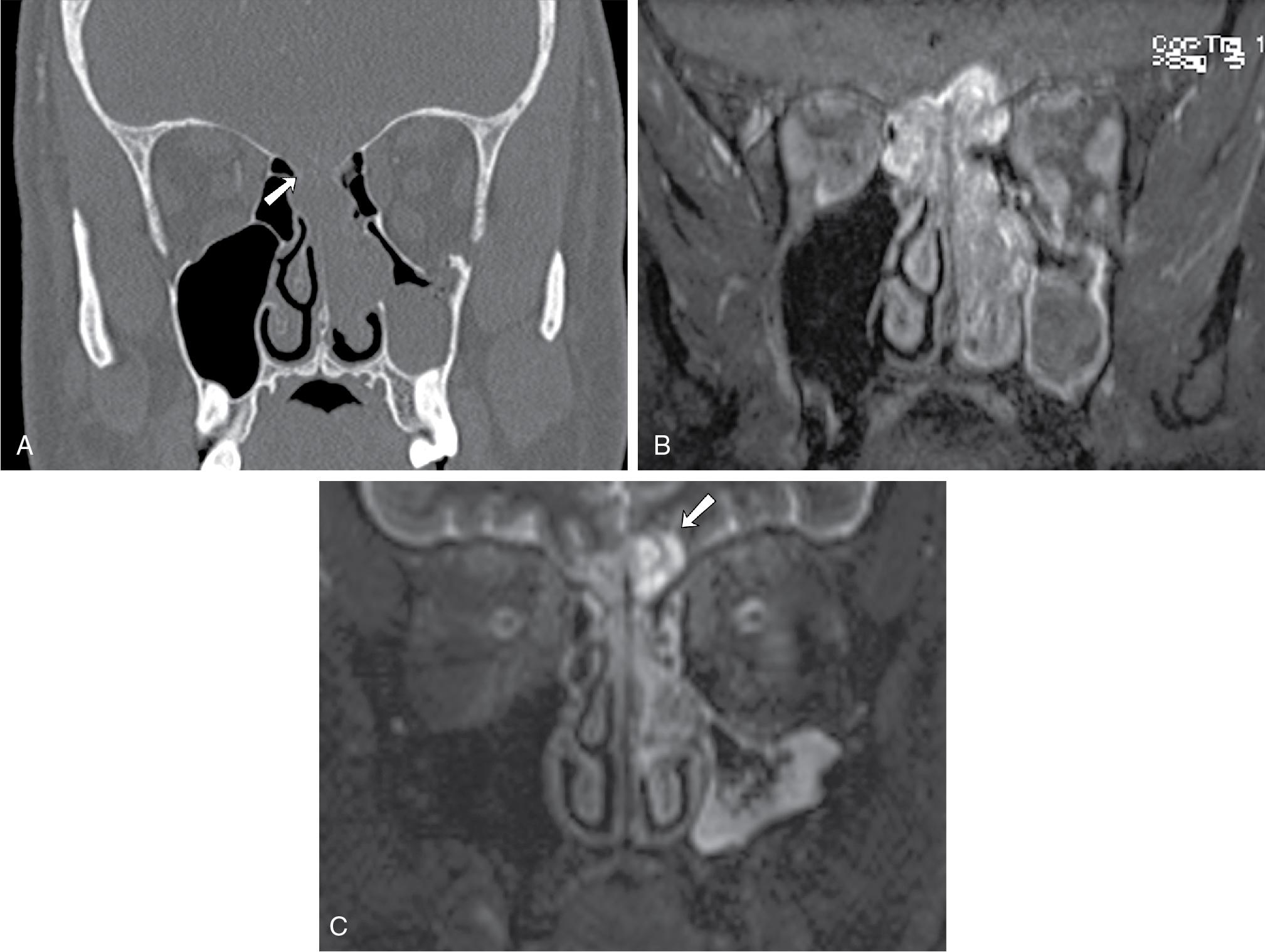
On CT, an expansile mass remodeling the floor of the sella should raise suspicion for adenoma ( Fig. 27.3 A, p. 183). On MRI, a macroadenoma is often demonstrated as a homogenously enhancing mass extending into the suprasellar compartment, classically giving rise to a “snowman” or a “figure of 8” morphology ( Fig. 27.3 B) and potentially compressing the optic chiasm or nerves. , Cystic nonenhancing components can be seen. The pituitary stalk is often deviated to the opposite side of the mass but with larger tumors can be undistinguishable from the adjacent mass.
In 6% to 10% of cases, macroadenomas may invade the cavernous sinus (Fig, 27.3 C), often unilaterally, limiting surgical resection. Determination of cavernous sinus invasion on imaging, as described by Micko, is made based on the tumor margin relative to the margins of the cavernous internal carotid artery (ICA). If the tumor margin abuts the medial margin of the ICA, then no tumor invasion is present; if the tumor margin extends beyond the lateral margin of the ICA, then tumor invasion is probable. Between these two points, tumor invasion is best described as possible. Complete encasement of the intracavernous ICA is a definitive sign of cavernous sinus invasion, but it is a late sign. , Macroadenomas rarely narrow the cavernous carotid artery, which helps distinguish them from meningiomas, though this is not always the case.
Prior studies have explored the MR features of macroadenoma that may correlate with tumor consistency, usually focusing on T2-weighted signal intensity as a measure of water content. Most pituitary adenomas are soft (nonfibrous) and therefore can be adequately removed by aspiration and curettage via the transsphenoidal route. However, approximately 5% to 15% of pituitary adenomas are fibrous and firm and may not be amenable to this surgical approach. Relatively low T2-weighted signal intensity and homogeneous contrast enhancement may be predictive of dense consistency. However, this remains controversial. , A mosaic pattern with intratumoral hyperintense foci on post contrast high-resolution 3D T2-weighted imaging (FIESTA) has been shown to be a significant predictor of tumor consistency; when present, it is indicative of lower collagen content compared with solid-type adenomas.
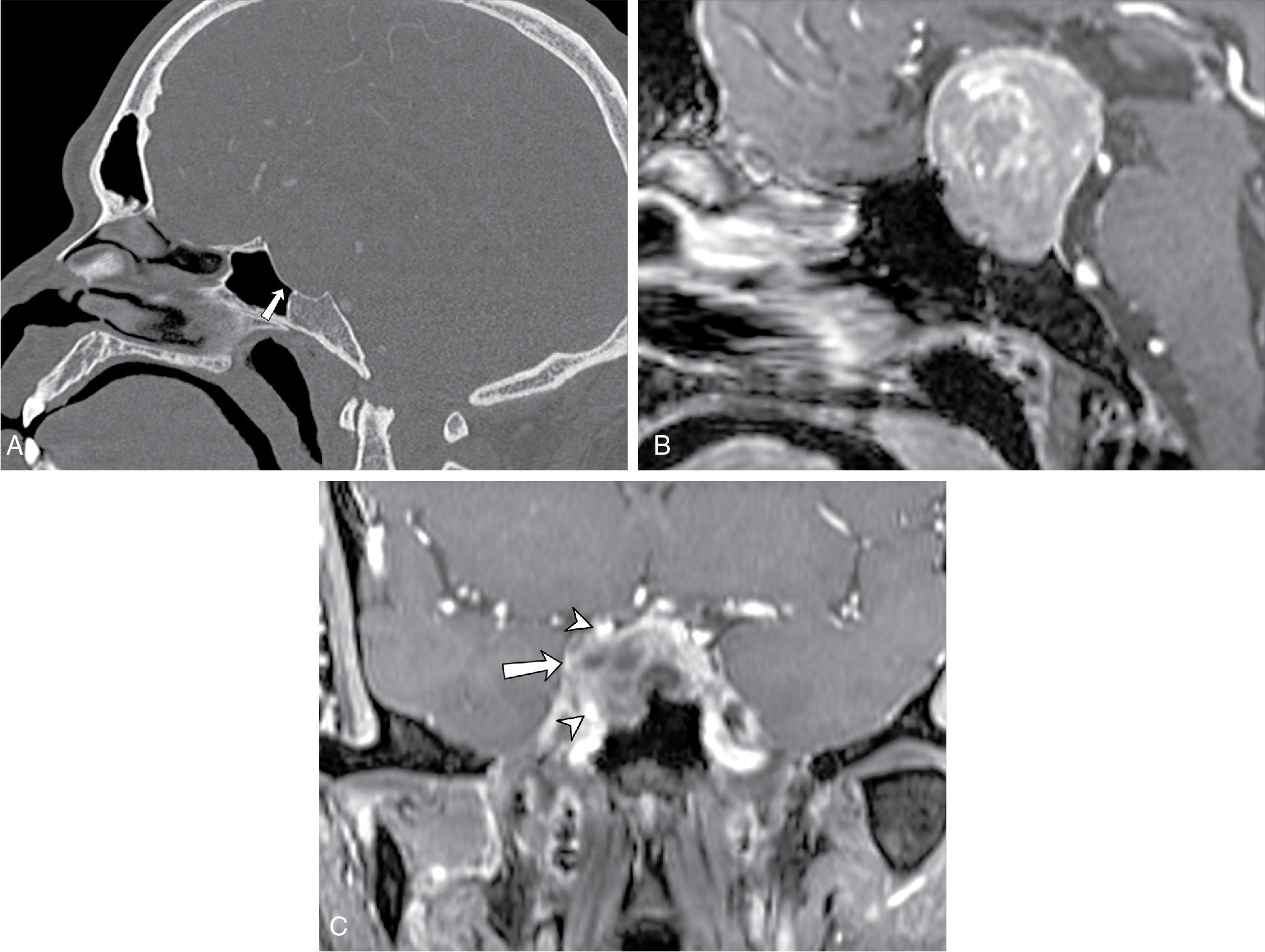
Meningiomas constitute the most common primary central nervous system (CNS) neoplasms, (female-to-male ratio, 2–3 to 1) with most occurring beyond the fifth decade of life. , Although the majority of meningiomas are benign, they do grow slowly over time and can compress adjacent brain and critical structures, necessitating removal. These dural-based tumors occur anywhere along the skull base, with approximately half of skull base meningiomas arising at the sphenoid wings; these can grow in a multicompartment fashion, invading the parasellar and paracavernous region, clivus, and tentorium cerebelli, and thus frequent involvement of the optic canals, cavernous sinuses, and sella is observed. Sphenoid wing meningiomas may also have bulky components projecting into the extraconal orbit, temporalis fossa, and middle cranial fossa, compressing the adjacent temporal and frontal lobes. Arterial encasement leading to stenosis may be present ( Fig. 27.4 A and B). Venous sinus invasion can be seen as well.
On noncontrast CT, meningiomas appear to be iso- to hyperdense to the cerebral cortex and may show associated hyperostosis ( Fig. 27.4 C and D). Their isodensity to brain tissue can make small meningiomas undetectable on noncontrast CT. With contrast-enhanced CT, classic imaging findings of homogeneous enhancement with dural tail can be readily appreciated. On conventional angiography, the “mother-in-law sign” (comes early; stays late) can be observed, in which the tumor enhances early and continues to enhance late into the venous phase. On MRI, meningiomas are classically iso- to hypointense on T2-weighted imaging and show avid contrast enhancement, though this may vary according to the degree of calcification or cystic degeneration. An enhancing dural “tail” is usually present, but this is not a necessary or pathognomonic feature.
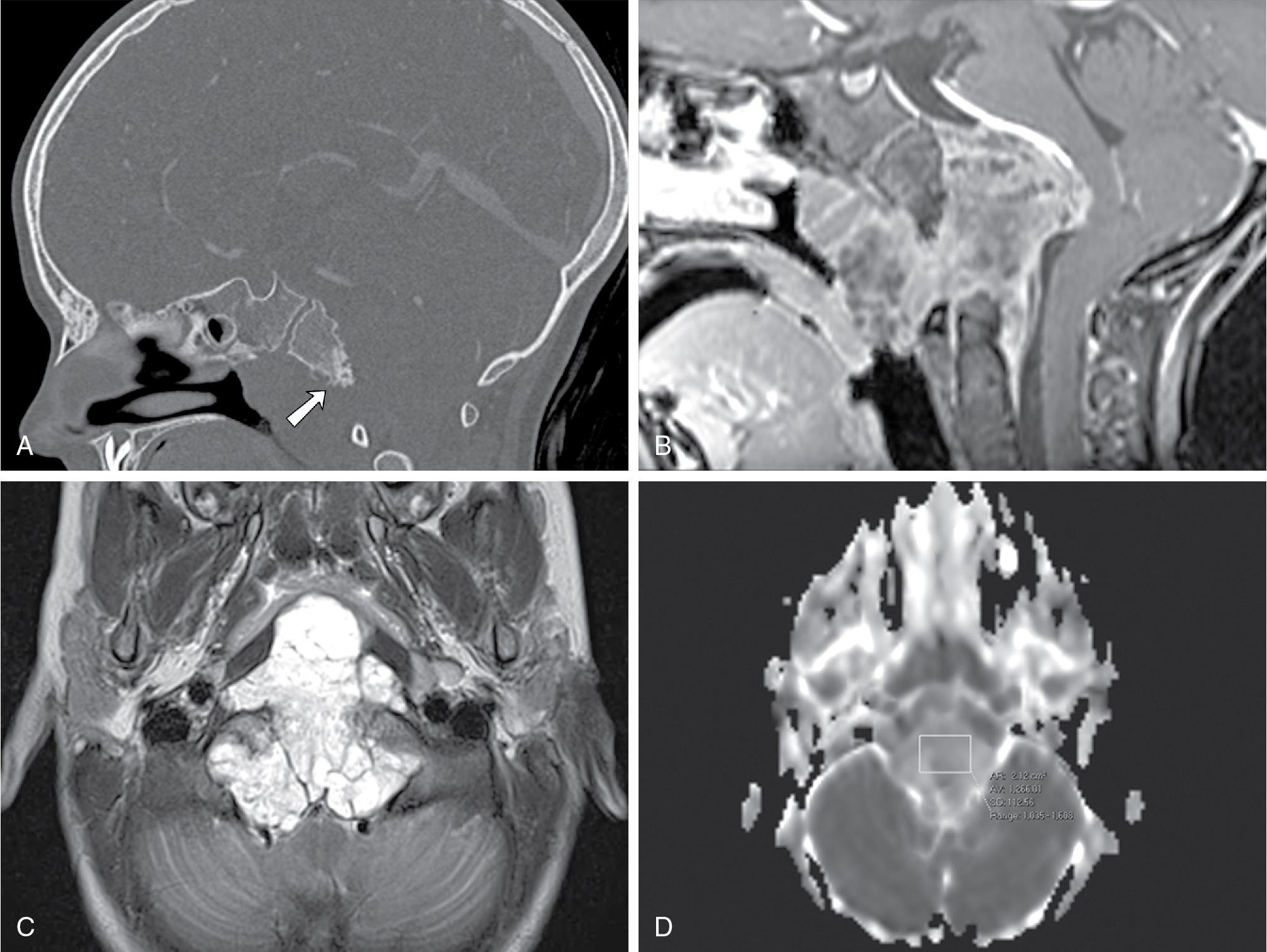
Although chordomas are not common tumors, one-third of all chordomas occur in the central skull base, and their characteristic imaging features are worth including here. Chordomas are histologically benign but locally invasive tumors, arising from midline embryonic remnants of the primitive notochord. The majority are located in the sacrococcygeal region (50% of cases), with a minority of cases involving other segments of the spine. ,
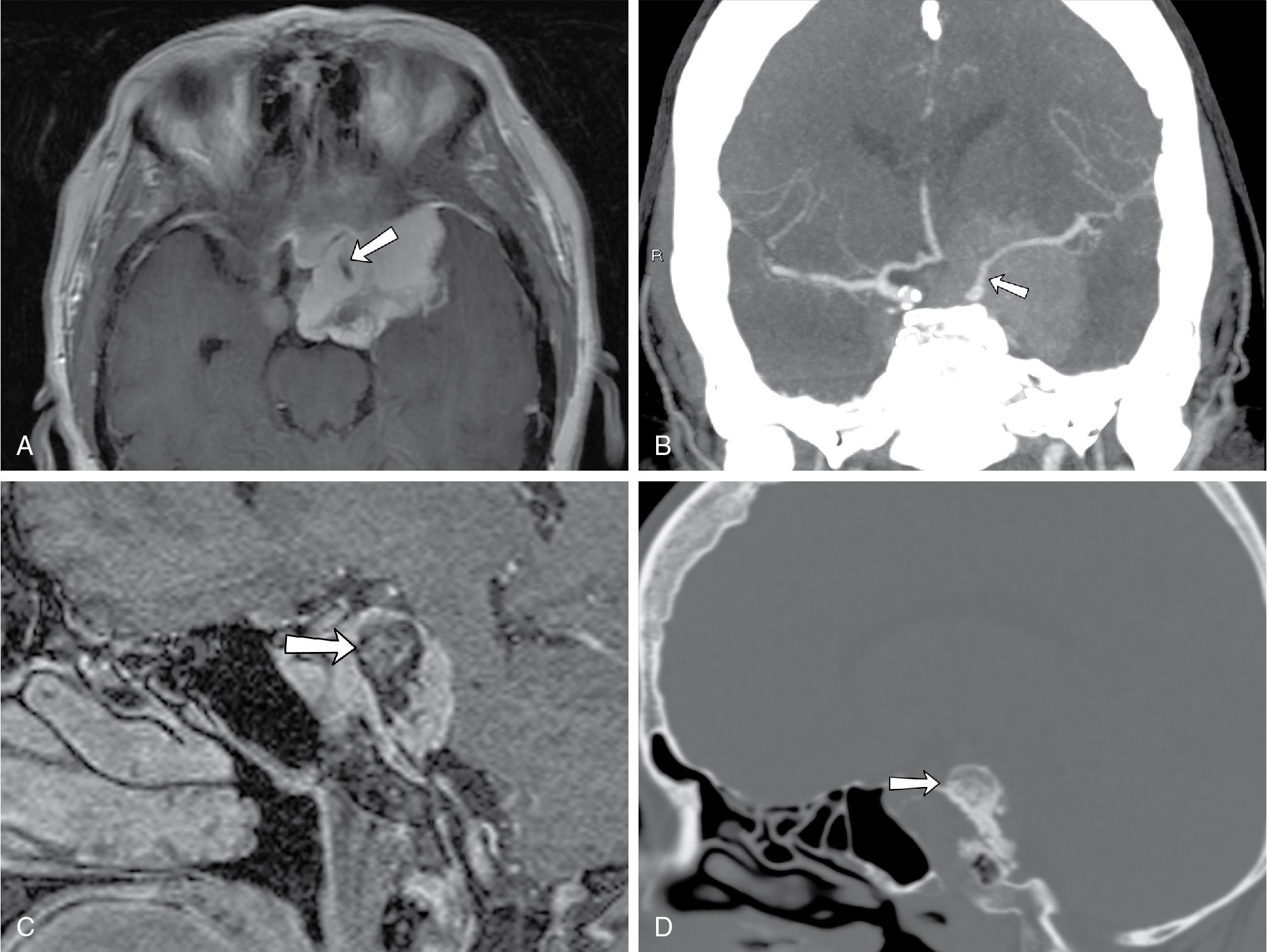
Computed tomography typically demonstrates a soft tissue mass centered in midline, with osseous lytic destruction of the clivus and central destroyed bone fragments (bony sequestra) ( Fig. 27.5 A). On MRI, chordomas are lobulated masses that characteristically demonstrate variable degrees of heterogeneous contrast enhancement ( Fig. 27.5 B) and a marked T2 hyperintense signal (probably reflecting a high fluid or mucin content or necrosis), as well as T2-weighted hypointense interlobular septa ( Fig. 27.5 C). These are soft tumors that can extend intracranially, most frequently into the posterior cranial fossa with compression on the brainstem, tending to displace or encase adjacent vessels, often without stenosis. , , Diffusion-weighted imaging (DWI) may be helpful in differentiating chordomas from chondrosarcomas; the latter usually associated with higher mean ADC values (2051 ± 261 × 10 −6 mm 2 /s vs 1474 ± 117 × 10 −6 mm 2 /s in classic chordomas; P < .001) ( Fig. 27.5 D).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here