Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Airway practitioners should be acquainted with common imaging modalities. A review of available imaging studies should always be included as part of the preoperative assessment of the airway.
The most useful imaging studies to review include neck, spine, and chest radiographs and cross-sectional imaging studies of the cervical spine or the soft tissue of the neck. A review of the scout image or topogram can provide a more complete assessment of the airway from the nares to the bronchi.
Computerized tomography (CT) is preferred in acute trauma because of the speed of acquisition, high-resolution imaging, bone detail, and detection of acute blood. The major disadvantage is the delivery of ionizing radiation to patients undergoing CT examination.
Magnetic resonance imaging (MRI) is the modality of choice for detection of acute stroke. No ionizing radiation is used, but only nonferromagnetic equipment can be used and patients must be carefully screened for a history of implants.
Assessment of the airway starts at the nose. The presence of septal deviation or bony spurs can affect the ease of nasotracheal or nasogastric tube insertion.
The presence of macroglossia, retrognathia, and large lingual tonsils at the tongue base may predict difficult intubation.
The degree of airway narrowing or deviation can be assessed on cross-sectional imaging of the neck.
The level of the larynx in an adult patient is usually between C4 to C6. Evidence of a low-lying larynx or anterior displacement of the larynx is a potential predictor of difficult intubation.
A number of imaging studies may be acquired in the work-up of a patient who presents with a new medical condition. A patient’s imaging library can include a chest radiograph, computed tomography (CT) of the chest, and/or CT or magnetic resonance imaging (MRI) of the brain and/or neck that provide some view of the airway, if only a cursory glance. This information about the airway can be aptly used for formulating an anesthetic plan. The main goal of this chapter is to introduce airway practitioners to normal airway anatomy, as visualized on radiography (plain film or digital radiograph) and cross-sectional imaging (e.g., CT and MRI), and to illustrate the anatomic variants and pathologic processes that can compromise the airway. The technology behind the different imaging modalities, as well as their technical differences, is briefly reviewed, with the main emphasis placed on evaluation of the airway using available radiologic studies, which most patients already have as part of their often extensive medical work-up. Familiarity with normal anatomy and its variants is useful to better understand how pathologic processes may affect and compromise the airway in ways that are most relevant to anesthesiologists.
The airway can be regarded as a tubular conduit for air inhaled from the nares to the tracheobronchial tree. The soft tissue structures bordering the airway may affect the integrity of the airway with respect to extrinsic compression, luminal encroachment, or airway displacement. Segmentation of the airway into the head, neck, and chest compartments is artificial but usually done, addressing the pathologies affecting these anatomic regions and for ease of discussion. Different imaging modalities, such as x-ray, CT, and MRI, can evaluate airway structures and the surrounding tissues with different levels of accuracy and spatial resolution. When analyzing these imaging modalities, one must be familiarized with the advantages and disadvantages of each imaging technique. This is especially important when selecting a study that will best depict the anatomic structures and pathologic processes affecting the airway that are of clinical interest.
A brief description of the different imaging modalities is presented here, starting with plain x-ray films and, more currently, digital radiographs. This will enable the reader to develop a good foundation for understanding how different imaging modalities are used in modern diagnostic imaging.
Wilhelm Conrad Roentgen, a German physicist, discovered x-rays on November 8, 1895, while studying the behavior of cathode rays (electrons) in high-energy cathode ray tubes. By serendipity, he noted that a mysterious ray that escaped the cathode ray tube struck a small piece of paper coated with fluorescent barium platinocyanide on a workbench 3 feet away, causing a faint fluorescent glow. Different objects placed between the cathode ray tube and the fluorescent screen changed the brightness of the fluorescence, indicating that the mysterious ray penetrated objects differently. When Roentgen held his hand between the tube and the screen and saw the outline of the bony skeleton of his hand, he quickly realized the significance of his discovery. For his work, he was awarded the first Nobel Prize for Physics in 1901.
X-rays are a type of electromagnetic radiation, which, as the name implies, transports energy through space as a combination of electric and magnetic fields. In diagnostic radiology, the predominant energy source used for imaging is ionizing radiation, such as alpha particles, beta particles, gamma rays, and x-rays. The science of electromagnetic waves and x-ray generation is very complex and exceeds the scope of this chapter. In principle, x-rays are produced by energy conversion as a fast stream of electrons is suddenly decelerated in an x-ray tube. The x-ray beam produced can be directed toward the part of the body being studied. The final image is dependent on the degree of attenuation of the beam by matter (e.g., soft tissues and bone). Attenuation, the reduction in the intensity of the beam as it traverses matter of different composition, is caused by the absorption or deflection of photons from the beam. The transmitted beam determines the final image, which is represented in shades of gray. The lightest or brightest area on the film or image represents the greatest attenuation of the beam by tissue and the least amount of beam transmitted to film. An example would be bone, a high-density material that attenuates much of the x-ray beam; images of bone on radiographs are very bright or white.
Conventional imaging (plain film or x-ray) is obtained using screen film cassette technology in which the film is processed using several chemical “washes” or chemical reactions to produce a two-dimensional image of the body part under examination on a large field of view film. With most radiology departments converting to an all-digital environment, heavily relying on various picture archiving communication systems (PACS) for workflow and archiving of radiologic studies, digital radiography has largely replaced screen film cassette plain film.
After the discovery of x-rays, it became apparent that images of the internal structures of the human body could yield important diagnostic information. However, the usefulness of the x-rays is limited by the projection of a three-dimensional object onto a two-dimensional display. With x-rays and radiographs, the details of internal objects are masked by the shadows of overlying and underlying structures. The goal of diagnostic imaging is to bring forth the organ or area of interest in detail and eliminate the unwanted information. Various film-based traditional tomographic techniques were developed, culminating in the creation of CT or computerized axial tomography (CAT). , The first clinically viable CT scanner was developed by Hounsfield and commercially marketed by EMI (EMI Limited, Middlesex, England) for brain imaging in the early 1970s. Since then, several generations of CT scanners have been developed.
As with conventional plain film or digital radiography, CT technology requires x-rays as the energy source. Whereas conventional radiography employs a single beam of x-rays from a single direction and yields a static image, CT images are obtained by using multiple collimated x-ray beams from multiple angles and the transmitted radiation is counted by a row or rows of detectors. A fan-shaped x-ray source rotates around the patient, who is enclosed in a gantry. The radiation counted by the detectors is analyzed using mathematical equations to localize and characterize the tissues within the imaged section based on density and attenuation measurements. A single cross-sectional image is produced with one rotation of the gantry. The gantry must then “unwind” to prepare for the next slice while the table with the patient moves forward or backward a distance predetermined by slice thickness. An intrinsic limitation of this technique is the time necessary for movement of the mechanical parts. ,
Introduction of slip-ring technology in the 1990s, along with development of faster computers, high-energy x-ray tubes, and multidetectors, enabled continuous activation of the x-ray source without having to unwind the gantry. With continuous movement of the tabletop, this process is known as the helical CT. Because the information acquired using helical CT is volumetric, as compared with the single slice obtained with conventional CT, the entire thorax or abdomen can be scanned in a single breath-hold. Volumetric information also makes it possible to identify small lesions more accurately and allows better three-dimensional reconstruction. Because of the higher speed of data acquisition, misregistration and image degradation caused by patient motion are no longer significant concerns. This is especially important when scanning uncooperative patients and trauma victims. The absorbed radiation dose used in a multidetector helical CT as compared with a conventional single-detector row CT is dependent on the scanning protocol and varies with the desired high-speed or high-quality study.
CT examination is best for depiction of bone and for detection of calcium and blood. The advantage of CT technology is that data acquisition is very quick, and the acquired data can be used for multidimensional reconstructions, which allow the display of different organs in an anatomic format that can be easily recognized by clinicians.
In contrast to radiography and CT, MRI uses no ionizing radiation. Instead, imaging is based on the resonance of the atomic nuclei of certain elements such as sodium, phosphorus, and hydrogen in response to radio waves of the same frequency produced in a static magnetic field environment. Current clinical MRI units use protons from the nuclei of hydrogen atoms to generate images because hydrogen is the most abundant element in the body. Every water molecule contains two hydrogen atoms, and larger molecules, such as lipids and proteins, contain many hydrogen atoms. MRI data are acquired by using powerful electromagnets to create a magnetic field, which influences the alignment of protons in hydrogen atoms in the body. When radio waves are applied, protons are knocked out of natural alignment, and when the radio wave is stopped, the protons return to their original state of equilibrium, realigning to the steady magnetic field and emitting energy, which is translated into weak radio signals. The time it takes for the protons to realign is referred to as a relaxation time and is dependent on the tissue composition and cellular environment. , The different relaxation times and signal strength of the protons are processed by a computer, which generates diagnostic images. With MRI, the chemical and physical properties of matter are examined at the molecular level. The relaxation times, T1 and T2, for each tissue type are expressed as constants at a given magnetic field strength. Imaging that optimizes T1 or T2 characteristics is referred to as T1-weighted or T2-weighted imaging. Tissue response to pathologic processes usually includes an increase in bound water, or edema, which lengthens the T2 relaxation time and appears as a bright focus on T2-weighted images. ,
MRI is more sensitive, but not necessarily more specific, in detecting pathology than CT, which depicts anatomy with unparalleled clarity. Imaging with MRI provides metabolic information at the cellular level, allowing one to link organ function and physiology to anatomic information. MRI and CT technologies also have other differences: (1) MRI shows poor bony detail, whereas CT provides excellent images of bony structures; (2) hemorrhage, especially acute, is clearly visible on CT scans as bright or white areas, but it may be difficult to diagnose with MRI because the appearance of blood varies temporally depending on the stage of breakdown of hemoglobin; (3) MRI is very susceptible to all types of motion artifacts, ranging from patient movement, breathing, swallowing, and phonation to vascular and cerebrospinal fluid pulsation and flow; and (4) MRI is time consuming—a typical MRI takes 30 to 45 minutes, whereas a CT scan can be performed in less than 1 minute—thus, patients with altered mental status or other difficulties sitting still can be imaged much more easily with CT.
MRI scanners operate in a strong magnetic field environment, and strict precautions must be observed. Because any item containing ferromagnetic substances introduced into the magnetic field environment can become a projectile and result in deleterious consequences for patients, personnel, and the MRI scanner itself, no metal objects should be brought into the MRI suite if one is not absolutely certain about their composition. Only specially designed nonferromagnetic equipment is used in the MRI suite, including anesthesia machines, monitoring equipment, oxygen tanks, intravenous poles, infusion pumps, and stretchers. One must also remove pagers, telephones, computers, credit cards, and analog watches because the strong magnetic field can cause malfunction or permanent damage. Patients must be carefully screened for implantable pacemakers, intracranial aneurysm clips, implants (e.g., cochlear implants, penile implants, or spinal cord stimulators), and other foreign metallic objects before entering the MRI environment. In addition to the risk of ferromagnetic objects acting as projectiles externally, producing unwanted movement internally, or causing equipment malfunction, there is also the risk of heating, which can cause severe thermal injuries to the patient.
The aim of this chapter is to review imaging of the airway. There is, however, useful information from imaging studies of other parts of the body. For example, imaging of the brain can give information regarding intracranial pathology such as masses and mass effect, including brain herniation, hemorrhage, and hydrocephalus. Readily apparent from imaging of the chest are aeration status of each lung; the presence or absence of pneumothorax, mediastinal shifts, or pleural effusion; diaphragm position; heart size; positioning of the endotracheal tube (ETT); and intravascular access catheters. Abdominal imaging provides information regarding the presence or absence of ileus, pneumoperitoneum, and mass effect.
To illustrate the usefulness of radiography in evaluating the airway, we focus our discussion on the interpretation of plain films or digital radiographs of the cervical spine, chest, and neck. These are probably the radiographic studies most frequently ordered in a hospital setting and also are the most relevant to anesthesiologists because a composite of these studies gives a picture of the entire airway. Although these radiologic studies are usually obtained for reasons other than airway evaluation, it is in this group of patients who are “normal” or “cleared for surgery” that one may glean important observations about the airway. In cross-sectional imaging (e.g., CT or MRI), the digital scout view of a neck, cervical spine, or chest study provides a similar image to a digital radiograph of the neck or chest and renders useful airway information. With a dedicated study of the neck or cervical spine, multidimensional reconstructions from those studies allow an excellent view of the airway, usually from the nares to tracheal bifurcation. The anatomy and pathology displayed by these imaging techniques may alert the anesthesiologist to potential difficulties in securing a patient’s airway and help him or her to develop an alternative anesthetic plan. The following sections address the basics of imaging interpretation with respect to airway anatomy and pathology.
The cervical spine articulates with the occiput cranially and the thoracic vertebrae caudally. The bony elements, muscles, ligaments, and intervertebral discs support and provide protection to the spinal cord. On a lateral radiograph of the cervical spine, one can appreciate the bony morphology of the vertebrae and the disc spaces and assess the alignment of the vertebral column very quickly. This indirectly provides information regarding the integrity of the ligaments, which are crucial in maintaining alignment of the cervical spine. Individual ligaments and muscle groups, however, all have the same or similar attenuation and cannot be differentiated from one another on a radiograph. Soft tissue pathology, including ligamentous injury, is better assessed by MRI. Regardless of the type of imaging study, a systematic approach is recommended to evaluate the spine for alignment, bony integrity, cartilage, joint space, and soft tissue abnormalities.
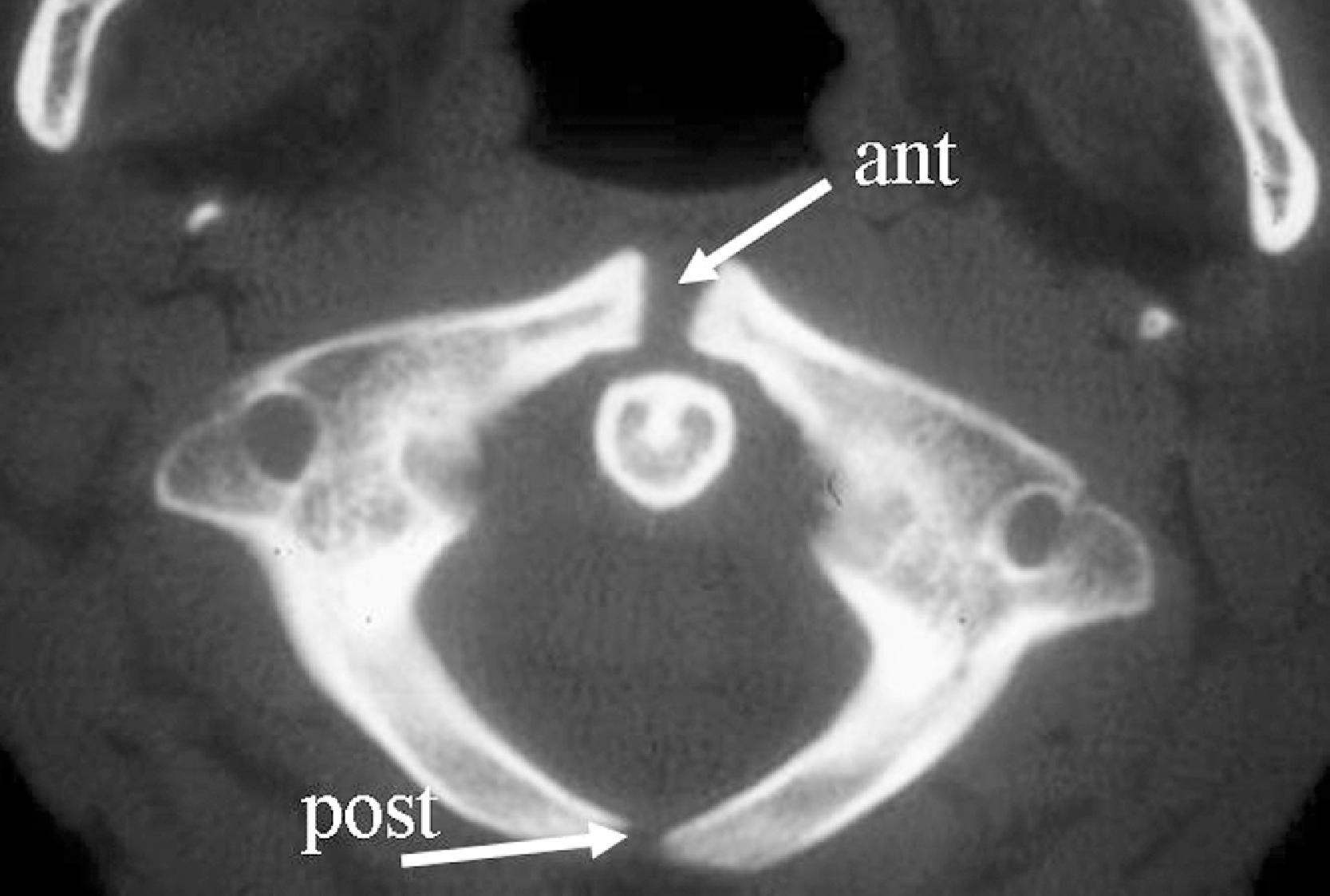
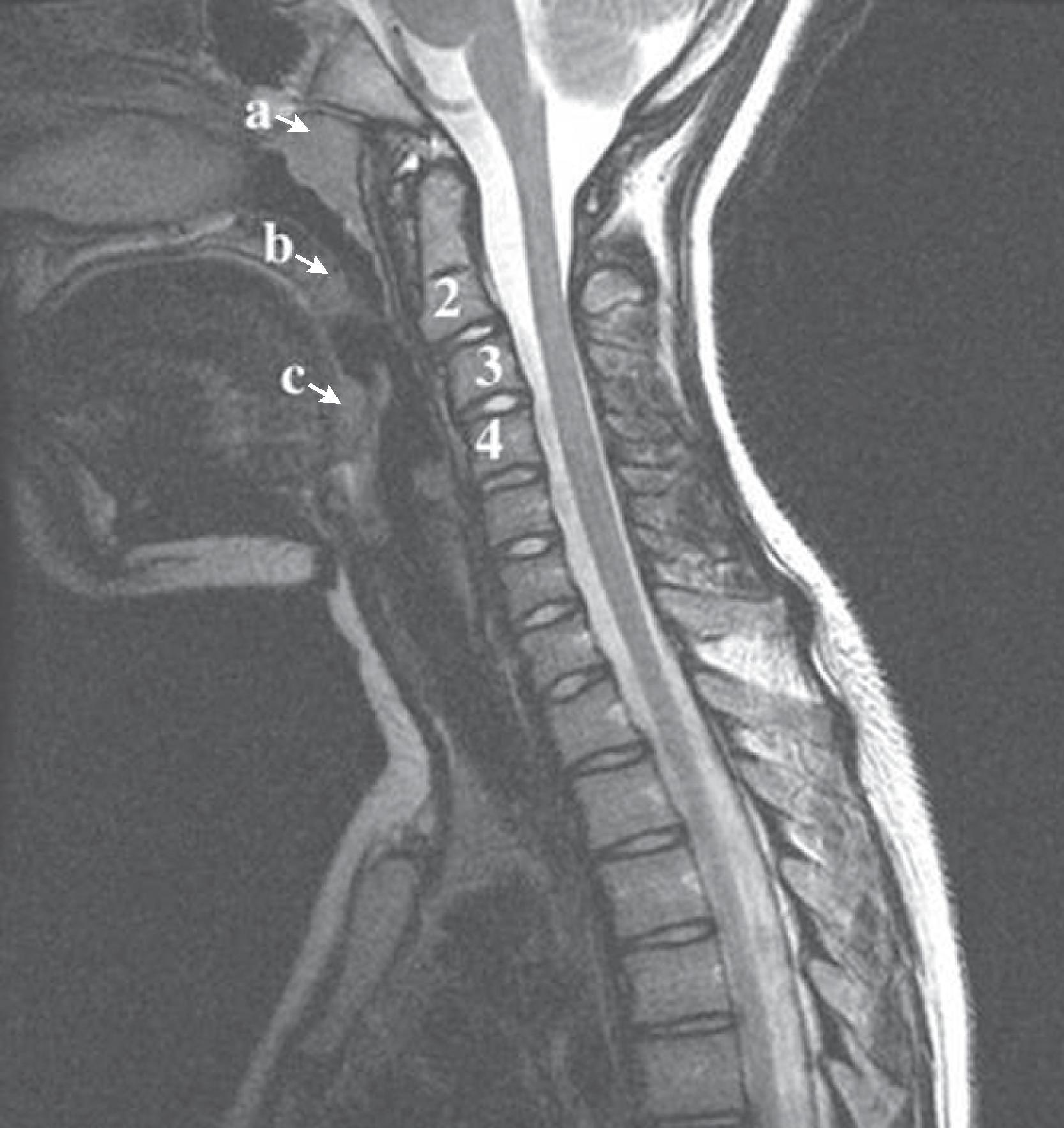
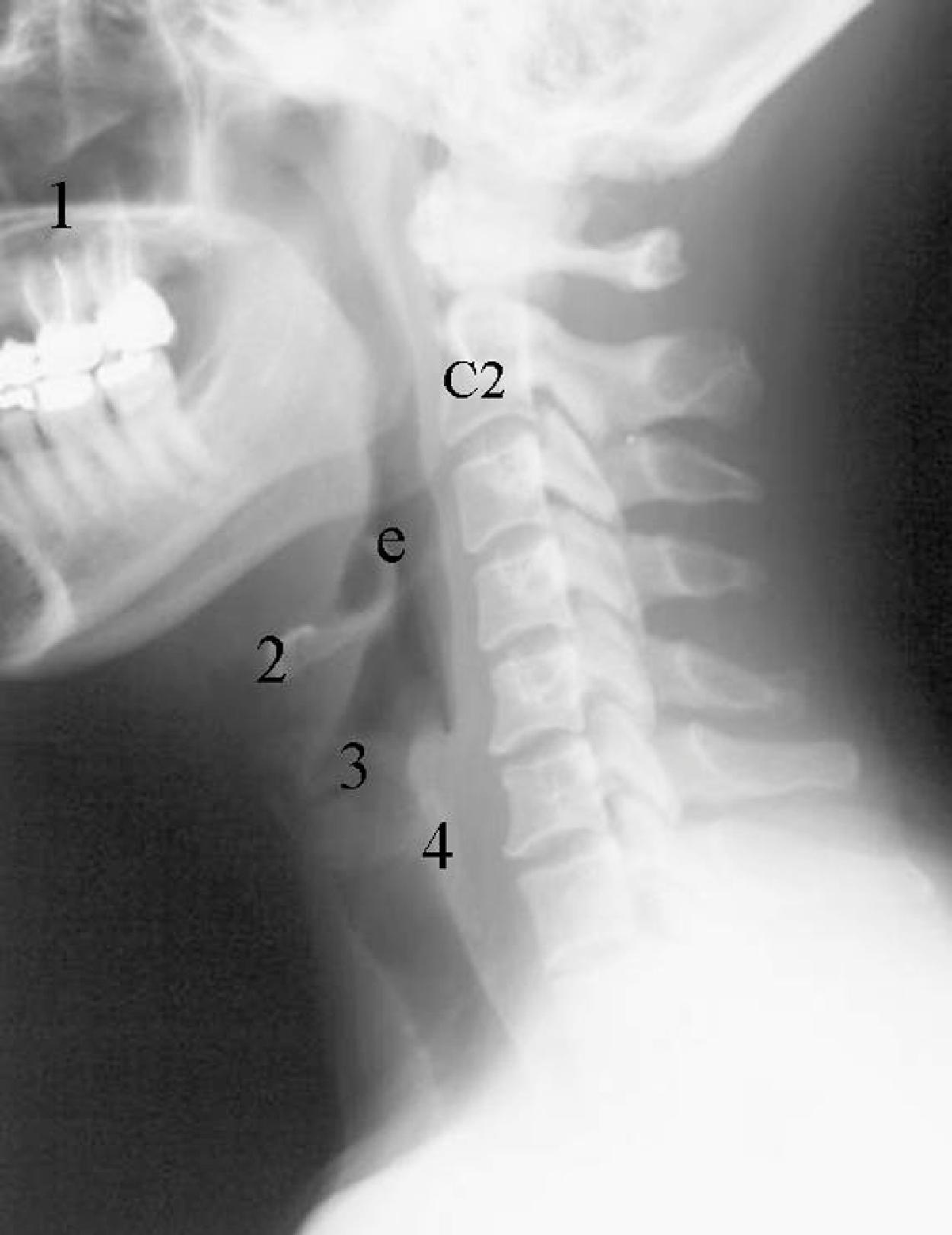
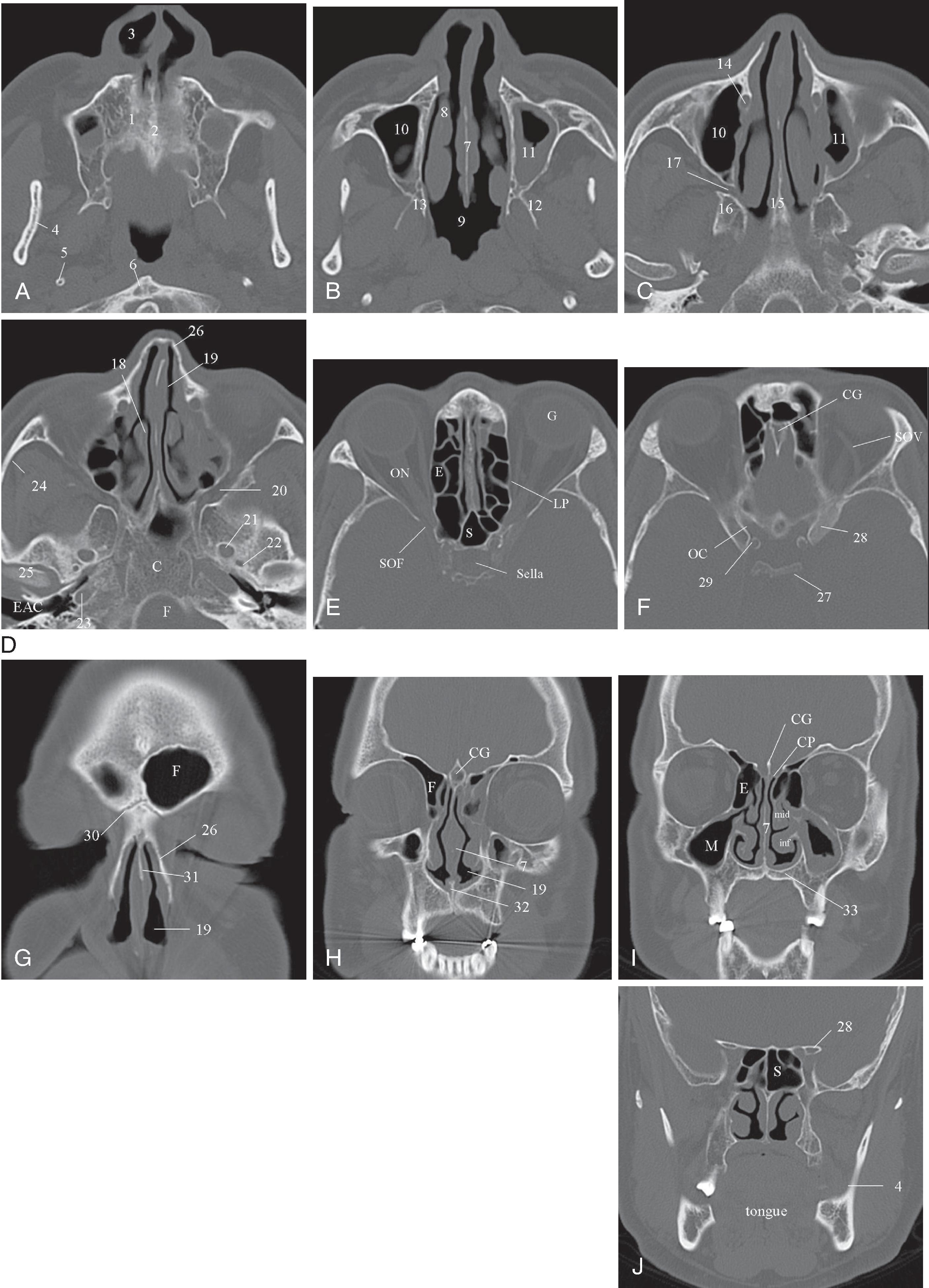
There are seven cervical vertebrae: C1 through C7. C1 and C2 are different from the other cervical vertebrae and are considered to be more a part of the cervico-cranium. The atlas (C1) is a ringlike vertebra characterized by the absence of a vertebral body. It does not contain pedicles or laminae, as do other vertebrae, and has no true spinous process. It consists of an anterior arch, the anterior tubercle, a lateral mass on each side, and a posterior arch. The anterior and posterior arches are relatively thin, and the lateral masses are heavy and thick structures. Rudimentary transverse processes extend laterally and contain the transverse foramina, through which pass the vertebral arteries. Ossification of the atlas begins with the lateral masses during intrauterine life. At birth, neither the anterior nor the posterior arches are fused. Fusion of the anterior arch is complete between the seventh and tenth years of life. During the second year of life, the center of the posterior tubercle appears, and by the end of the fourth year of life, the posterior arch becomes complete. Nonfusion of the anterior and/or posterior arch exists as a normal variant in adults and should not be mistaken as a fracture ( Fig. 2.1 ).
The second cervical vertebra, the axis (C2), is the largest and heaviest cervical segment. The odontoid process (dens) serves as the theoretical body of C1, around which the atlas rotates and bends laterally. In contrast to the other cervical vertebrae, C2 does not have a discrete pedicle. The dens is situated between the lateral masses of the atlas and is maintained in its normal sagittal relationship to the anterior arch of C1 by several ligaments, most important of which is the transverse atlantal ligament. Superiorly, the dentate (apical) ligament extends from the tip of the clivus to the tip of the dens. Alar ligaments secure the tip of the dens to the occipital condyles and to the lateral masses of the atlas. They are the second line of defense in maintaining proper position of the dens. The tectorial membrane is a continuum of the posterior longitudinal ligament from the body of C2 to the upper surface of the occipital bone, anterior to the foramen magnum.
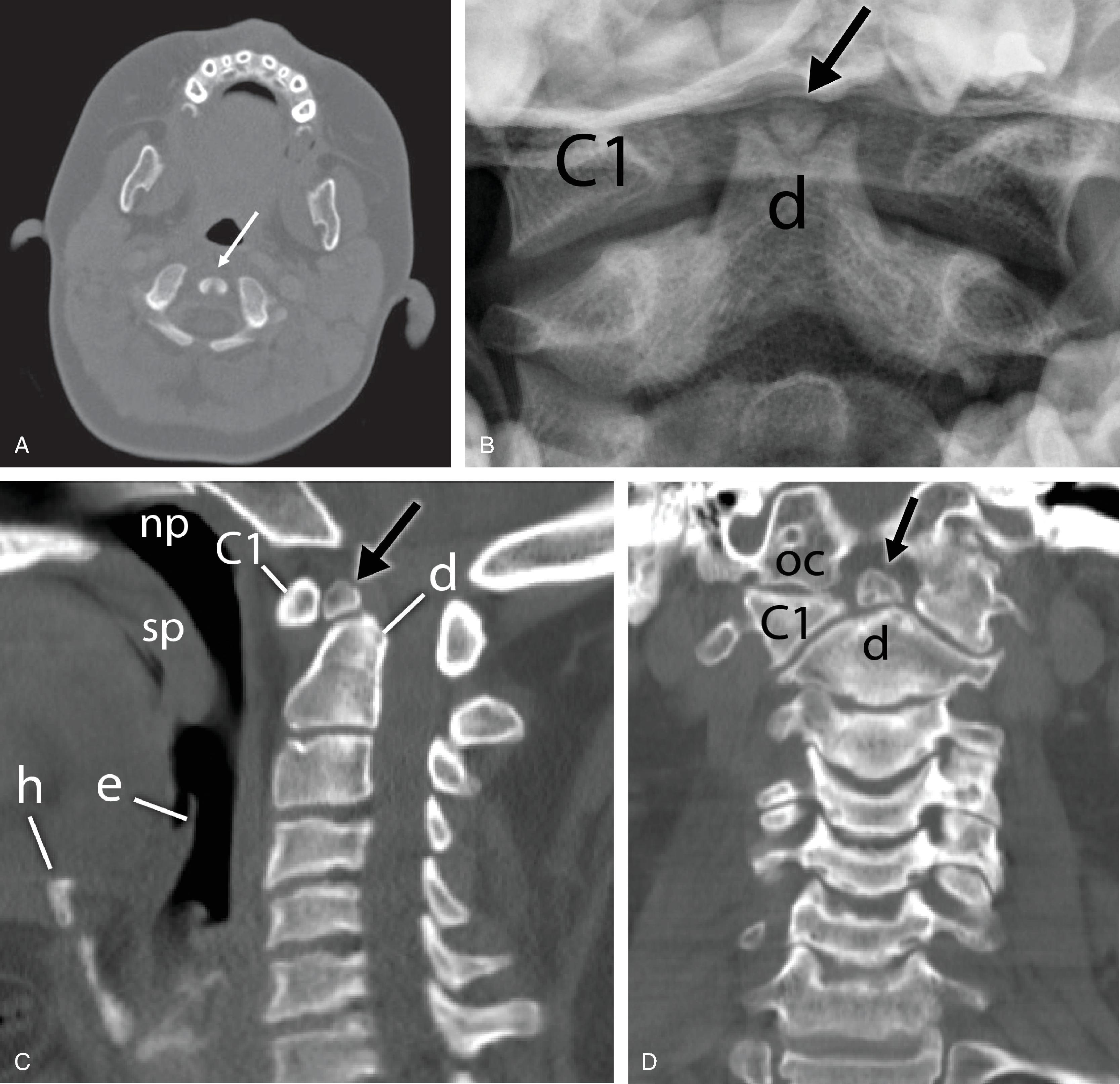
The C2 vertebra arises from five or six separate ossification centers, depending upon whether the vertebral body has one or two centers. The vertebral body is ossified at birth, and the posterior arch is partially ossified. They fuse posteriorly by the second or third year of life and unite with the body of the vertebrae by the seventh year. The dens ossifies from two vertically oriented centers that fuse by the seventh fetal month. Cranially, a central cleft separates the tips of these ossification centers ( Fig. 2.2A ), which can mimic a fracture when ossification is incomplete. The ossiculum terminale , the ossification center for the tip of the dens, may be visible on plain film, conventional tomograms, or CT scans and unites with the body by age 11 or 12 years. Failure of the ossiculum terminale to either develop or unite with the dens may result in a bulbous cleft dens tip. Incomplete fusion of dental ossification centers can result in persistent ossiculum terminale or os odontoideum, which can mimic fractures. ( Fig. 2.2B–D ).
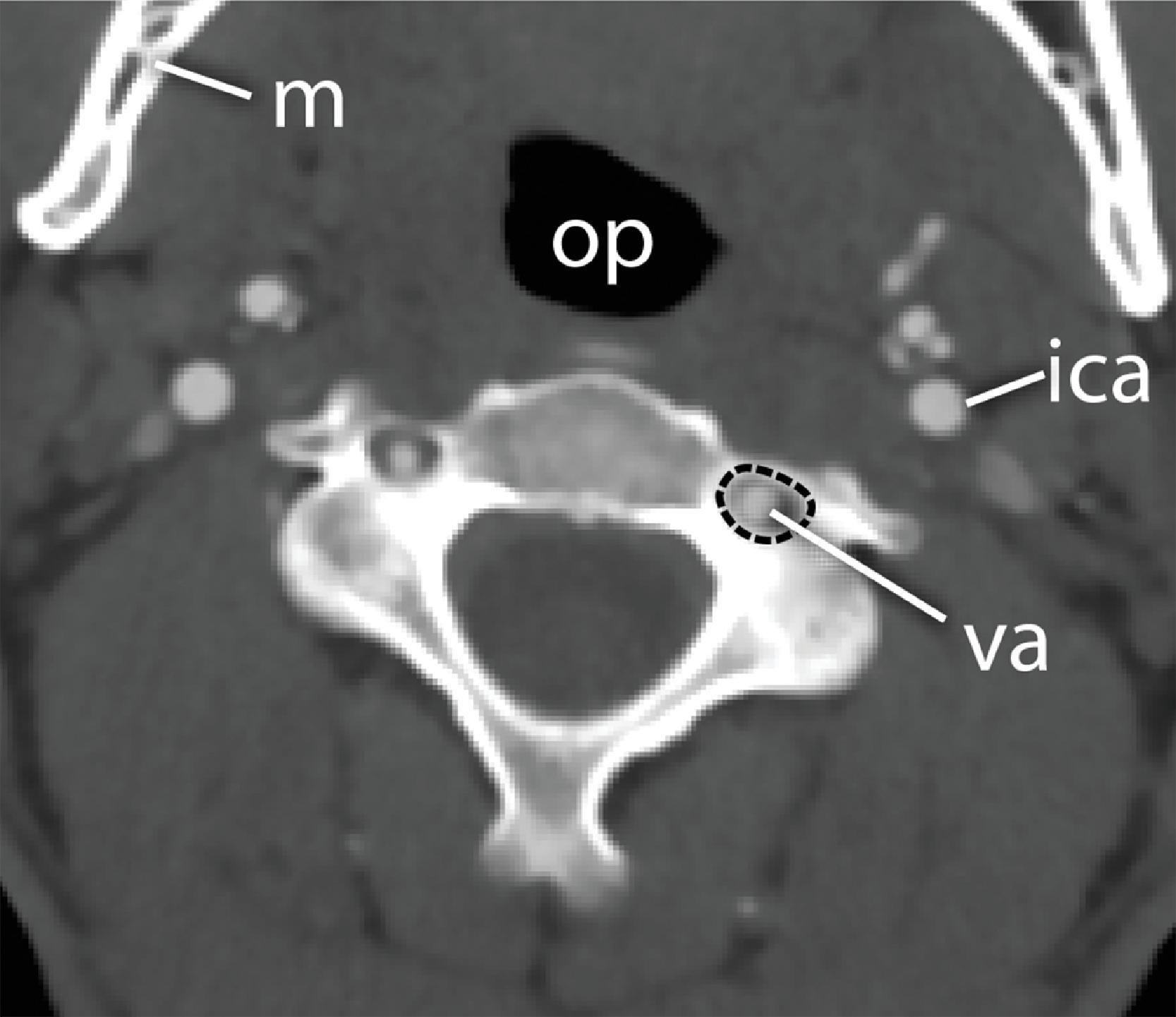
From C3 to C7, the cervical vertebrae are uniform in shape but increase in size, with the seventh vertebra being the largest and heaviest. All the vertebrae have transverse processes containing the foramen transversarium through which the vertebral arteries pass ( Fig. 2.3 ). The articular masses are dense, heavy, rhomboid-shaped structures bounded by articulating superior and inferior facets. The pedicles are short and posterolaterally oblique in orientation. ,
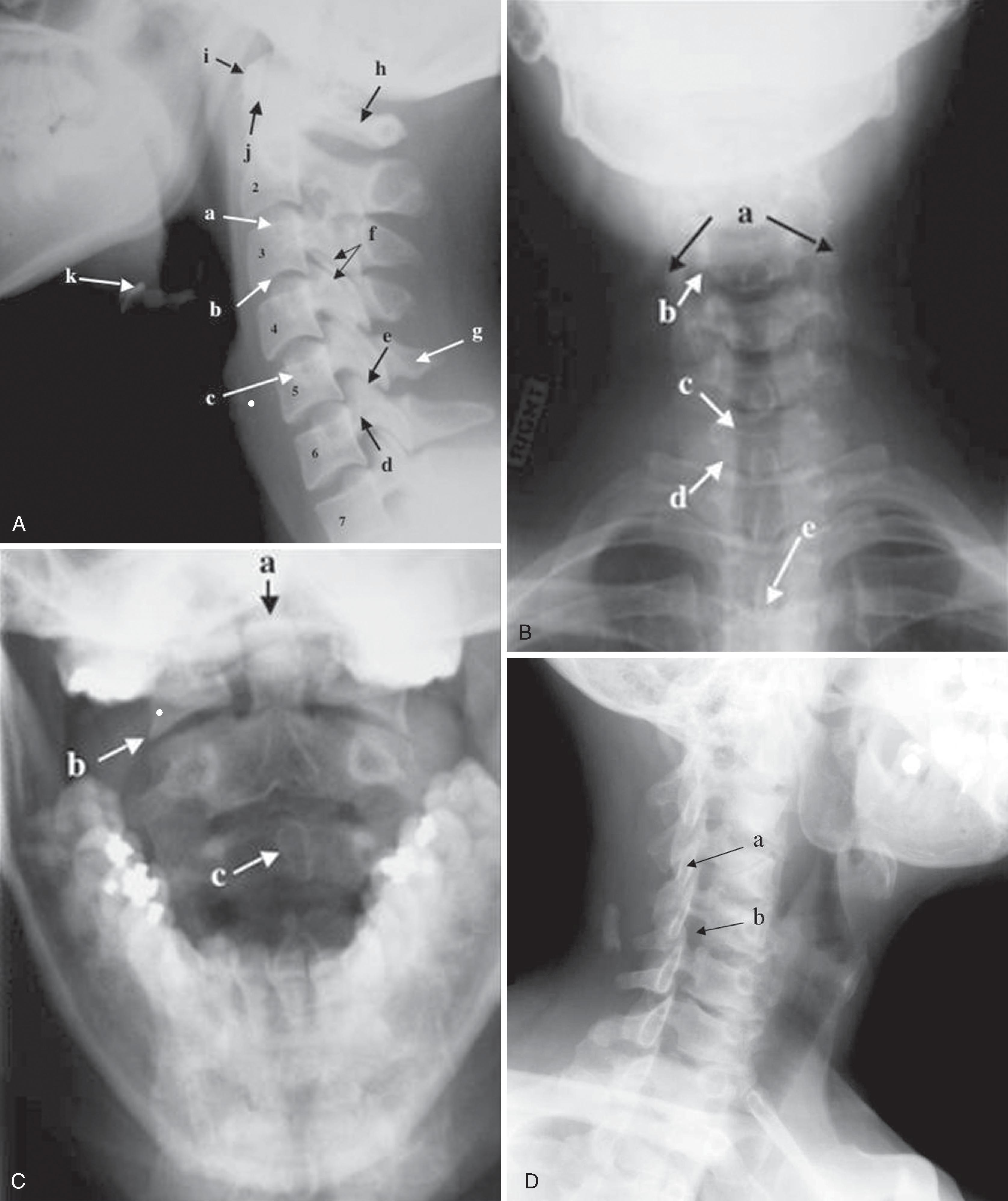
To answer a specific clinical question, different views of the cervical spine are frequently needed. The most common views are the lateral, anteroposterior (AP), open-mouth odontoid, oblique, and pillar views ( Fig. 2.4A–D ). A systematic approach is recommended to assess the integrity of the cervical spine. Examination of the cervical spine should include visualization of all seven cervical vertebrae and the first thoracic vertebra (T1). This is especially important for trauma victims because 7% to 14% of fractures are known to occur at the C7 or C7–T1 level. One must evaluate the spine for alignment, bone integrity, cartilage, joint space, and soft tissue abnormalities. The disadvantages of cervical spine x-rays are the limited range of tissue attenuation and the loss of spatial resolution caused by overlapping bone structures.
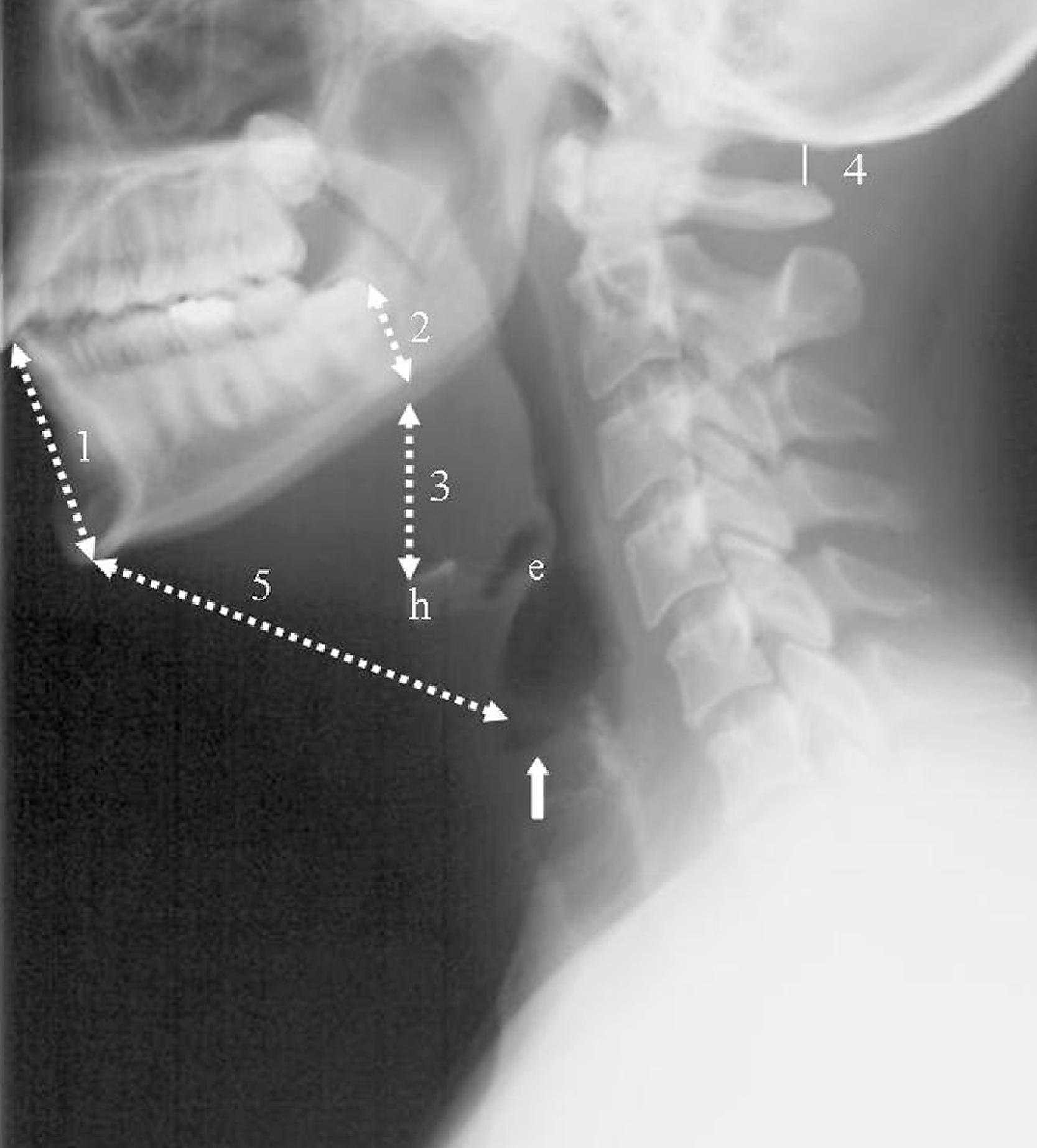
A normal lateral cervical radiograph should demonstrate normal alignment of the anterior and posterior aspects of the vertebral bodies and intact vertebrae ( Fig. 2.4A ). The posterior vertebral body line is more reliable than the anterior vertebral body line, which is often encumbered by the presence of anterior osteophytes and must be intact. The facet joints overlap in an orderly fashion, like shingles on a rooftop ( Fig. 2.4D ). The spinolaminar line is uninterrupted, and the interlaminar and interspinous distances are uniform. The spinolaminar line is the dense cortical line representing the junction of the posterior laminae with the posterior spinous process as seen on lateral radiographs. The posterior spinal line is an imaginary line extending from the spinolaminar line of the atlas to C3 ( Fig. 2.5A–D ). The anatomy and integrity of the craniocervical junction are crucial to the anesthesiologist. To achieve successful and safe endotracheal intubation, the anterior atlantodental interval (AADI), the vertical and anterior-posterior position of the dens, and the degree of extension of the head on the neck must be considered. The anterior arch of C1 bears a constant relationship to the dens (the AADI), or the predental space . It is defined as the space between the posterior surface of the anterior arch of C1 and the anterior surface of the dens. In flexion, because of the physiologic laxity of the cervicocranial ligaments, the anterior tubercle of the atlas assumes a more normal-appearing relationship to the dens, and the AADI increases in width, greater rostrally than caudally. In children and in flexion, the AADI is normally about 5 mm. In adults, it is generally accepted that the AADI should be 3 mm or less ( Figs. 2.6A and B ). If all the ligaments have been disrupted, AADI can measure 10 mm or more. In atlantoaxial subluxation, the dens is invariably displaced posteriorly ( Fig. 2.6C–E ), which causes narrowing of the spinal canal and potential impingement of the spinal cord. The space available for the spinal cord is defined as the diameter of the spinal canal as measured in the AP plane, at the C1 level, that is not occupied by the odontoid process. In the normal spine, this space is approximately 20 mm.
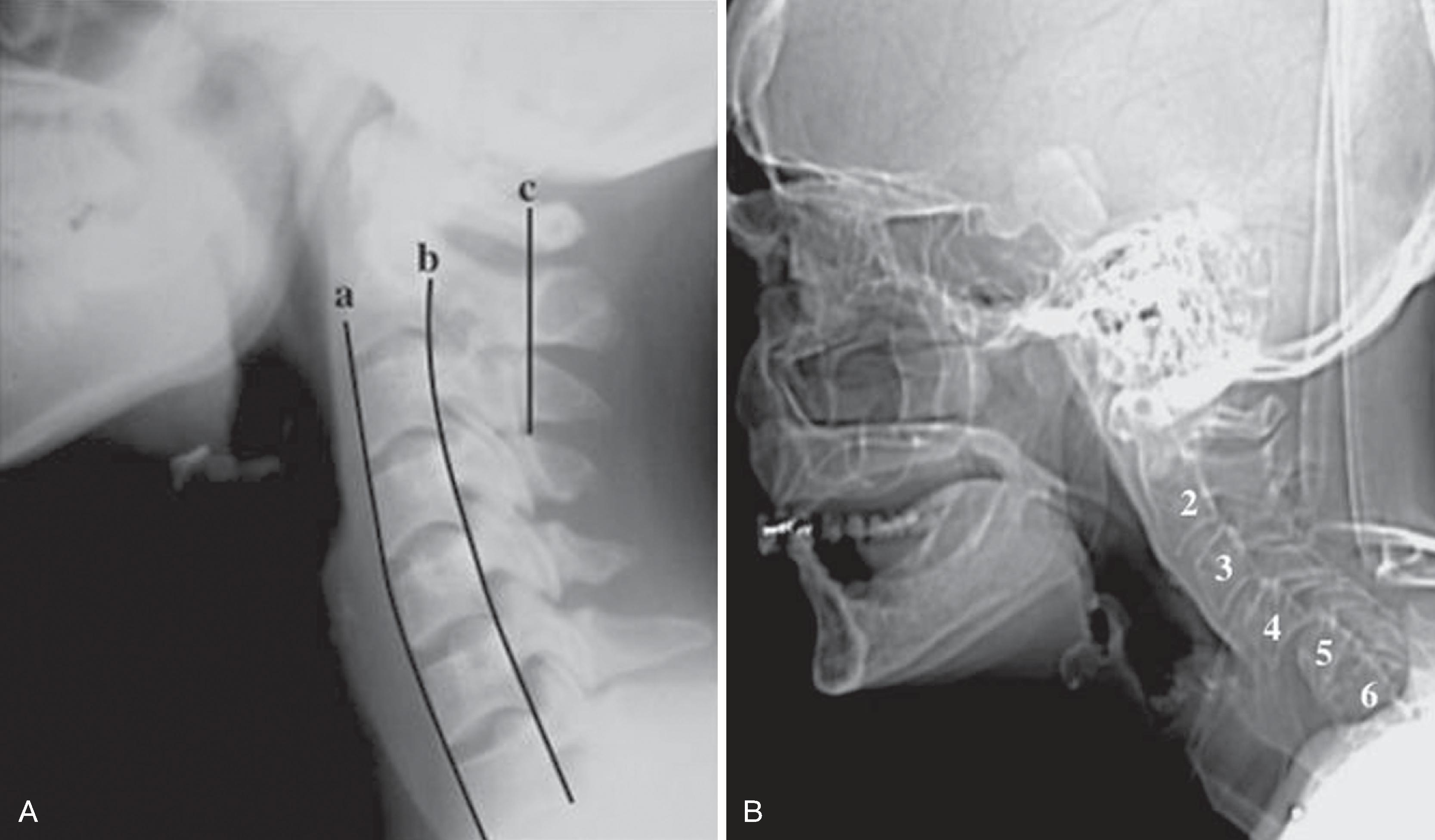
The distance between the occiput and the posterior tubercle of C1, the atlanto-occipital distance ( Fig. 2.7A,B ), is quite variable from individual to individual. Head extension is limited by the abutment of the occiput to the posterior tubercle of C1. Congenital occipitalization of C1 with the occiput not only limits head extension but also adds stress to the atlantoaxial joint. It has been proposed that a shorter atlanto-occipital distance decreases the effectiveness of head extension and contributes to difficult intubation. , Nichol and Zuck observed that in patients with limited or no extension possible at the atlanto-occipital joint, general extension of the head actually brings the larynx “anterior,” thus limiting the visibility of the larynx on laryngoscopy. Although the majority of head extension occurs at the atlanto-occipital joint, some extension can also occur at C1–C2.
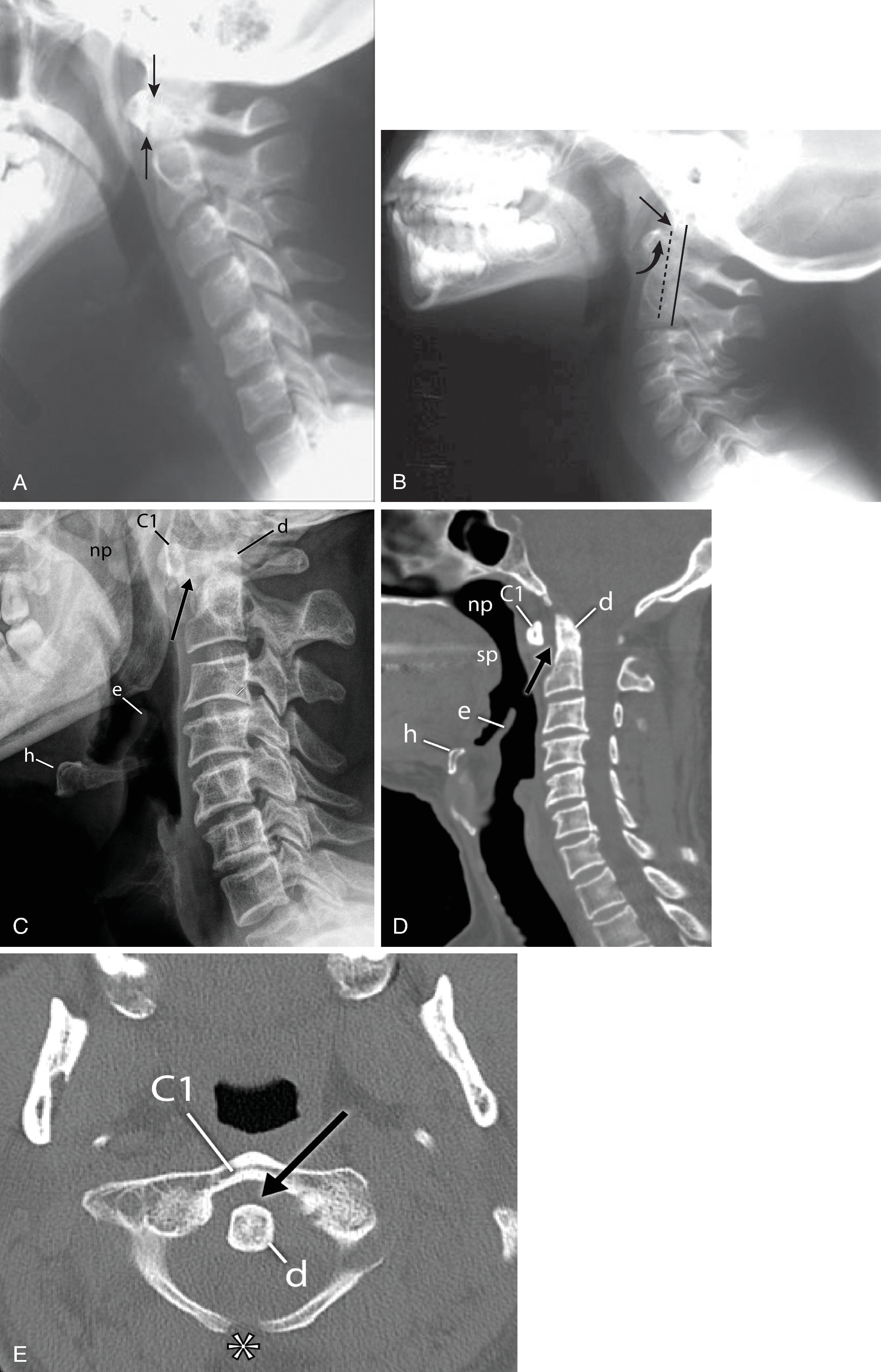
The position and anatomy of the dens with respect to the anterior arch of C1 and the foramen magnum are worthy of attention. Congenital anomalies of the dens, such as hypoplasia, can result in a loss of the buttressing action of the dens during extension and subsequent compression of neural elements. Conditions that are associated with odontoid hypoplasia are Morquio, Klippel-Feil, and Down syndromes; neurofibromatosis; dwarfism; spondyloepiphyseal dysplasia; osteogenesis imperfecta; and congenital scoliosis. , These patients are predisposed to atlantoaxial subluxation and craniocervical instability, and therefore hyperextension of the head for intubation should be avoided. In addition, congenital fusion of C2 and C3 ( Fig. 2.8A–D ), whether occurring as an isolated anomaly or as part of Klippel-Feil syndrome, places added stress at the C1–C2 junction.
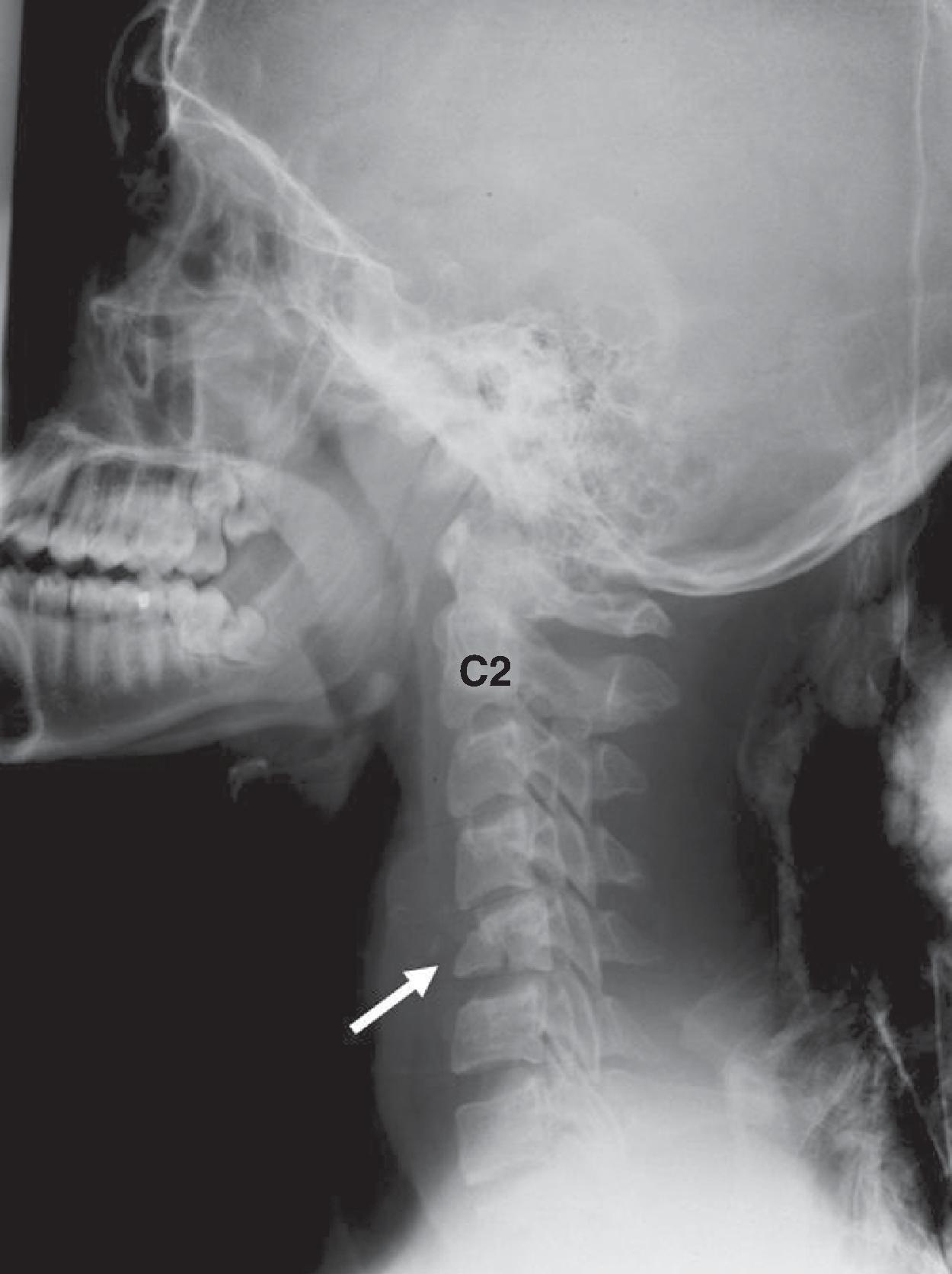
Pseudosubluxation and pseudodislocation are terms applied to the physiologic anterior displacement of C2 on C3 that is frequently seen in infants and young children ( Fig. 2.9 ). Physiologic anterior displacement of C2 on C3 and of C3 on C4 occurs in 24% and 14%, respectively, of children up to the age of 8 years. In pediatric trauma cases, if C2 is noted to be anteriorly displaced and there are no other signs of trauma, such as posterior arch fracture or prevertebral soft tissue hematoma, the spinolaminar lines of C1 through C3 should have a normal anatomic relationship. In a neutral position, the spinolaminar line of C2 lies upon, or up to 1 mm anterior or posterior to, the imaginary posterior spinal line. If the C2 vertebra is intact, the spinolaminar line of C2 moves 1 to 2 mm anterior to the posterior spinal line in flexion as the C2 body glides forward with respect to C3. Similarly, in extension the posterior translation of the C2 body is mirrored by similar posterior displacement of the spinolaminar line of C2 with respect to the posterior spinal line. In traumatic spondylolisthesis, which is rare in children and more common in adults, the C2 body would translate anteriorly in flexion and posteriorly in extension, and the posterior spinal line would be maintained because of intact ligaments. However, flexion and extension films are not advisable when traumatic spondylolisthesis is suspected.
Inflammatory arthropathies involving the atlantoaxial joint with subluxation are classically illustrated in rheumatoid arthritis and ankylosing spondylitis. The underlying cause of atlantoaxial subluxation is quite different in these two entities. Ankylosing spondylitis is characterized by progressive fibrosis and ossification of ligaments and joint capsules. In rheumatoid arthritis, there is bone erosion, synovial overgrowth, and destruction of the ligaments. Patients with rheumatoid arthritis are not only susceptible to AP subluxation at the C1–C2 junction but are also at risk for vertical subluxation of the dens. Whether this condition is referred to as “cranial settling,” superior migration of the odontoid process, or basilar invagination, the result is the same. The odontoid process protrudes above the foramen magnum, narrowing the available space for the spinal cord and potentially leading to cord compression with the slightest head extension ( Fig. 2.10A–C ).
In response to the effective foreshortening of the spine secondary to the superior migration of the dens, there is acquired rotational malalignment between the spine and larynx. The larynx and the trachea, because they are semirigid structures and as a result of the tethering effect of the arch of aorta as it passes posteriorly over the left main bronchus, are predictably displaced caudally, deviated laterally to the left, rotated to the right, and anteriorly angulated. The effective neck length can be affected by superior migration of the dens, severe spondylosis with loss of disc space, or iatrogenic causes secondary to surgery. The soft tissues of the pharynx become more redundant, which is attributable to the relative shortening of the neck and further obscures the view of the larynx. On laryngoscopy, the vocal cords are rotated clockwise. The presence of a rotated airway is suspected when the frontal view of the cervical spine demonstrates a deviated tracheal air column.
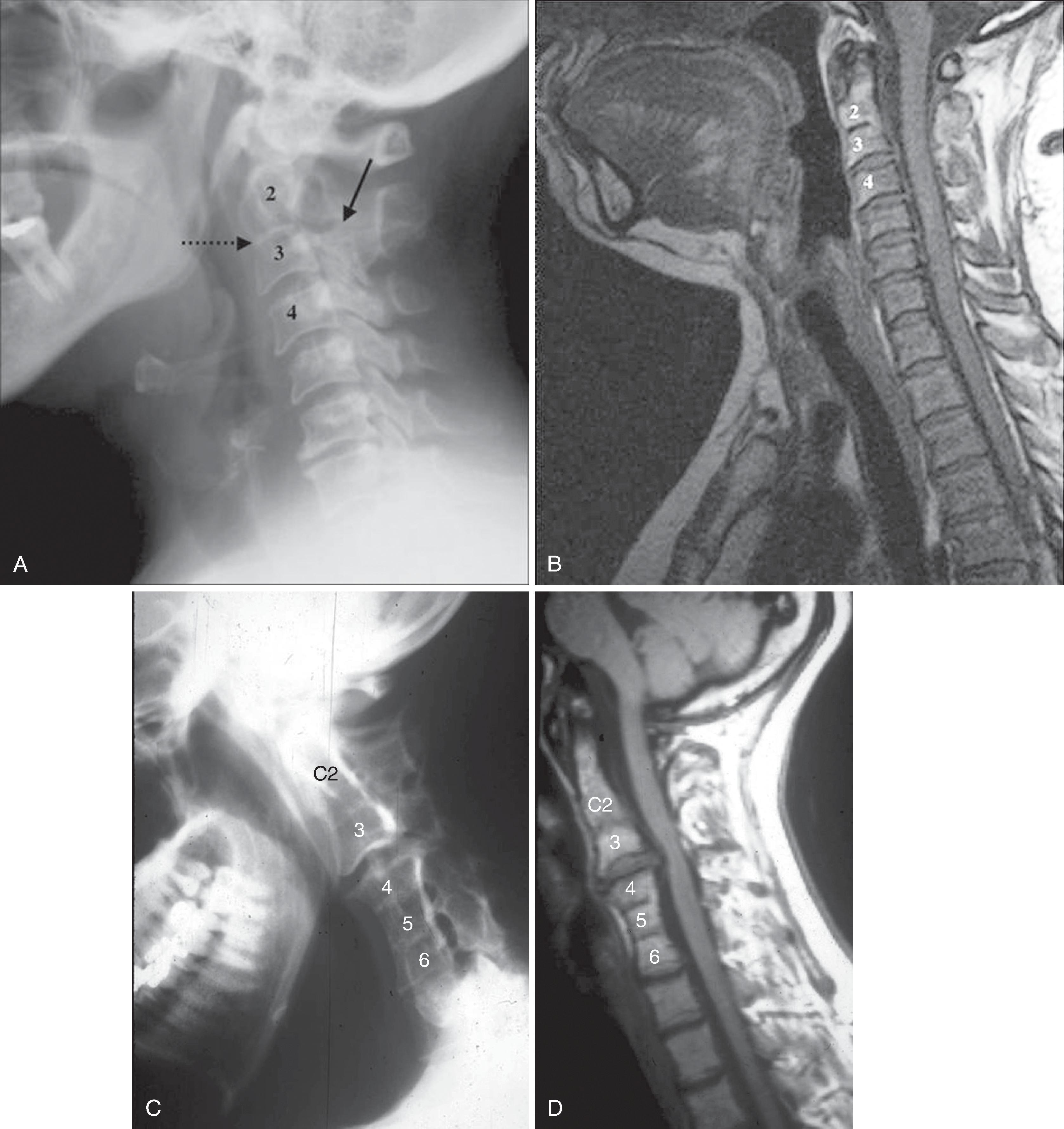
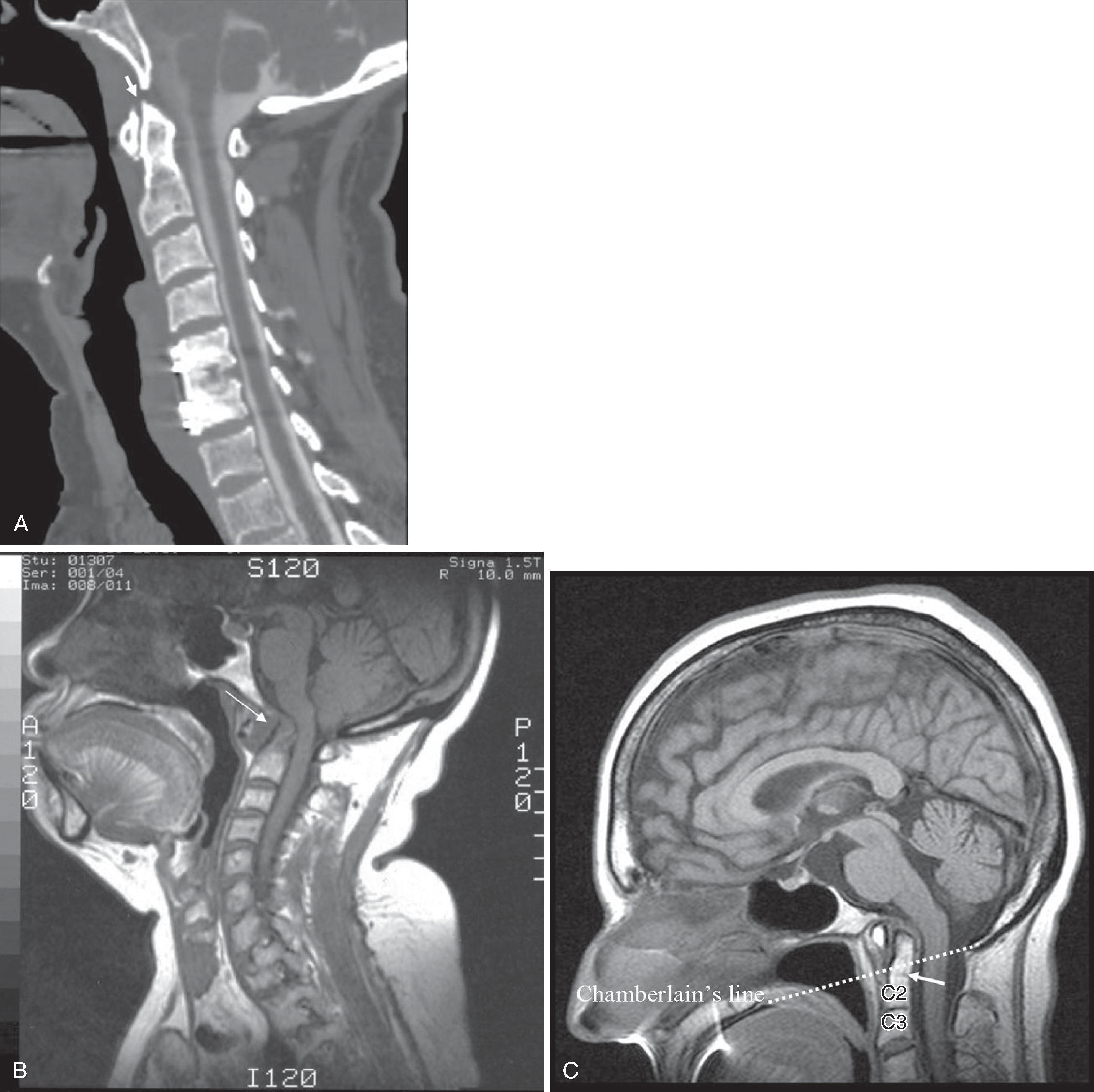
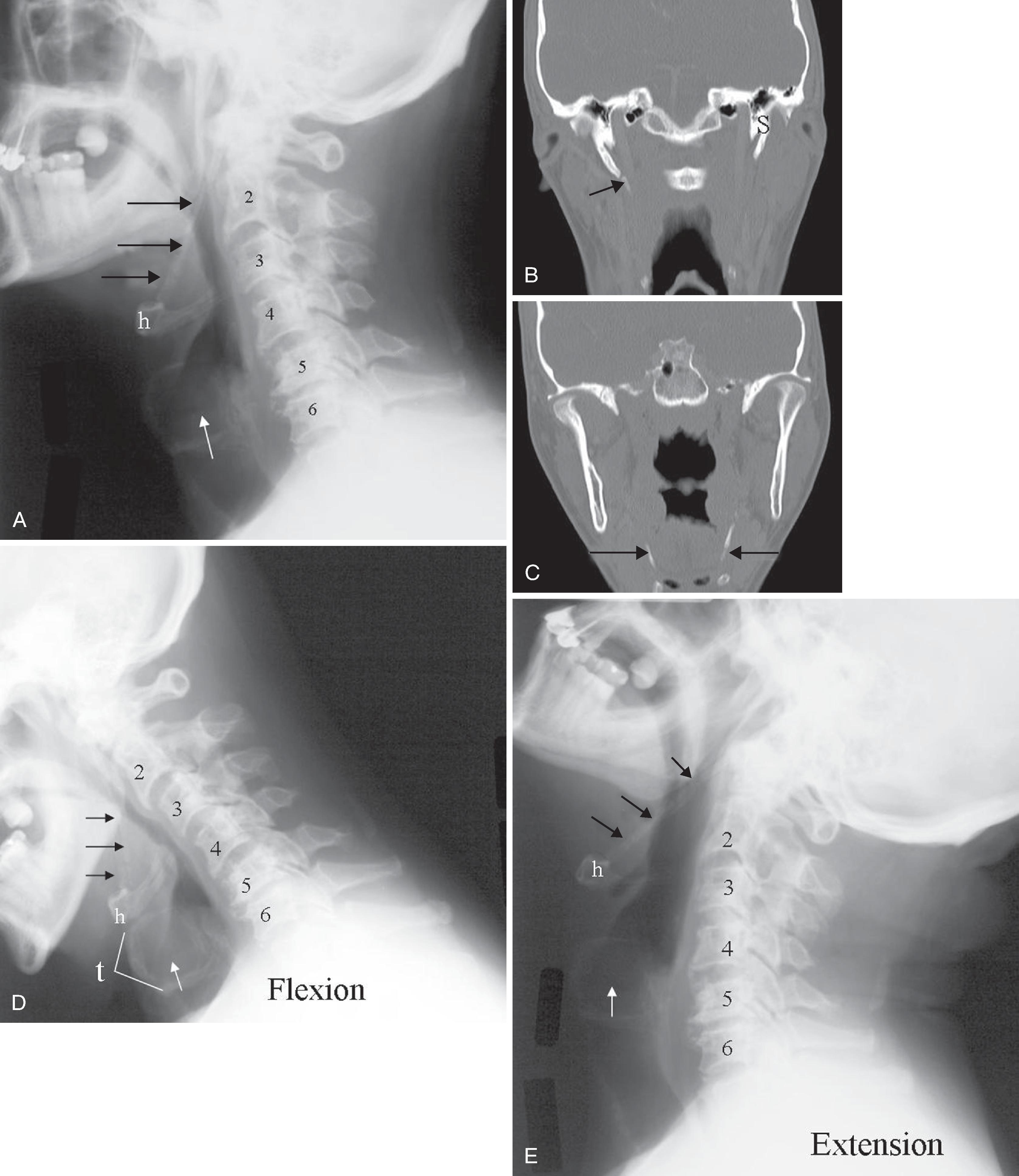
Historically, bone landmarks other than the spine, appreciated on a lateral cervical spine x-ray study, have been used to preoperatively predict difficult laryngoscopy and endotracheal intubation based on anatomic factors. Mandibular size and the ratios of the various measurements and their relationship to the hyoid bone have been proposed as predictors of difficult laryngoscopy ( Fig. 2.11 ). These measurements are meant to reflect the oral capacity, degree of mouth opening, and the level of larynx. , It is apparent that the causes of difficult laryngoscopy and endotracheal intubation are multifactorial. Combined with a clinical examination, anatomic measurements and findings assessed by radiography can help to alert the anesthesiologist to a potentially difficult airway. Acute cervical spine injury is often the indication for ordering a cervical spine examination. Although CT and MRI are exceptional in detailing bone and soft tissue abnormalities, plain film of the cervical spine remains a good initial screening study. It is useful to tailor and focus further imaging of the spine. For evaluation of trauma, cross-table lateral, AP, and open-mouth odontoid views are recommended. A lateral view reveals most injuries ( Fig. 2.12 ); however, patients who are rendered quadriplegic by severe ligamentous injuries may demonstrate a normal lateral cervical spine x-ray. By adding the AP and open-mouth odontoid views to the cross-table lateral view of the cervical spine, the sensitivity of detecting significant injury is increased from 82% to 93%. Cross-sectional imaging (i.e., CT and MRI of the spine) has become a mainstay in the evaluation of the cervical spine, especially in the setting of acute trauma ( Fig. 2.13A–B ).
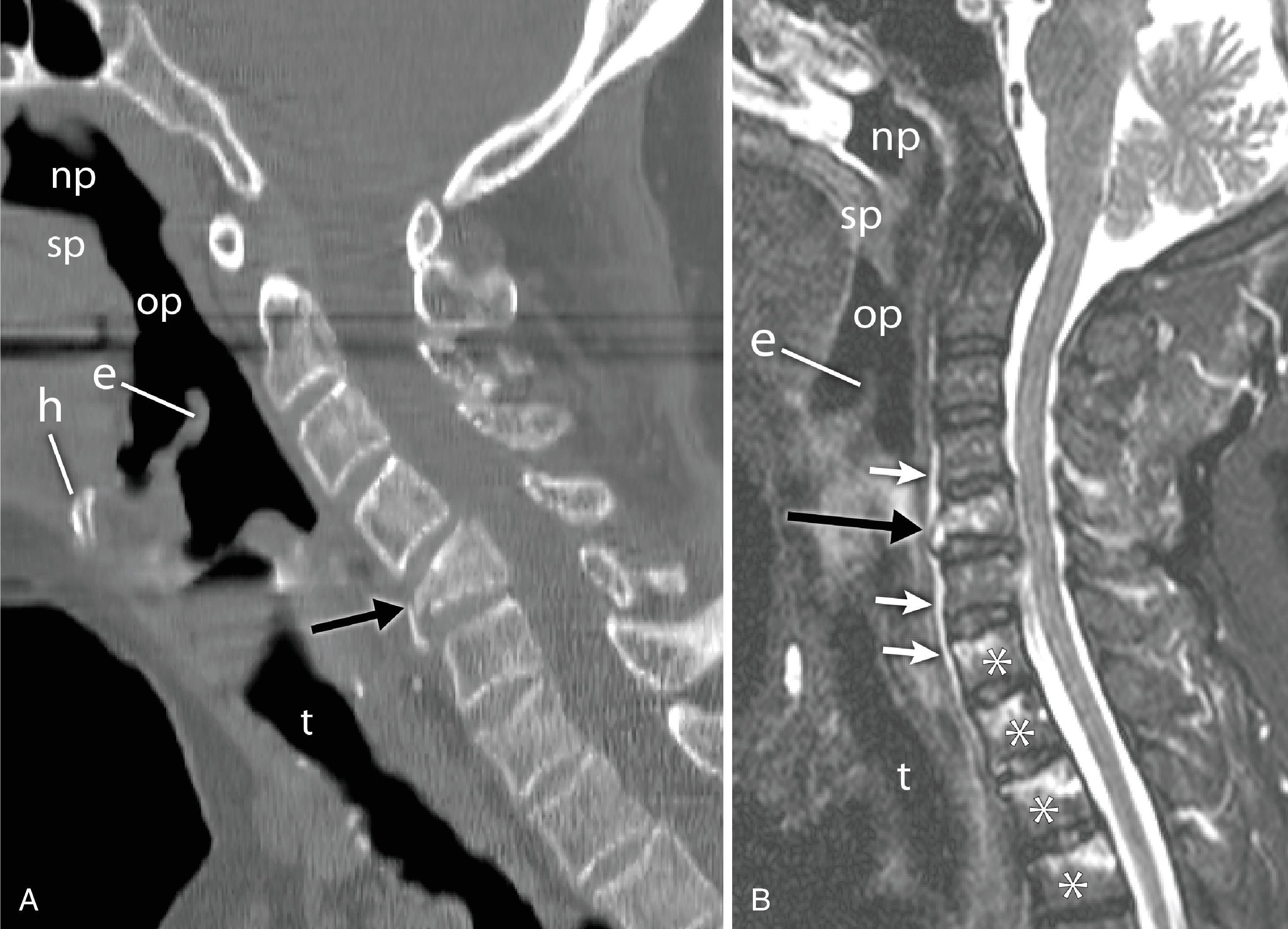
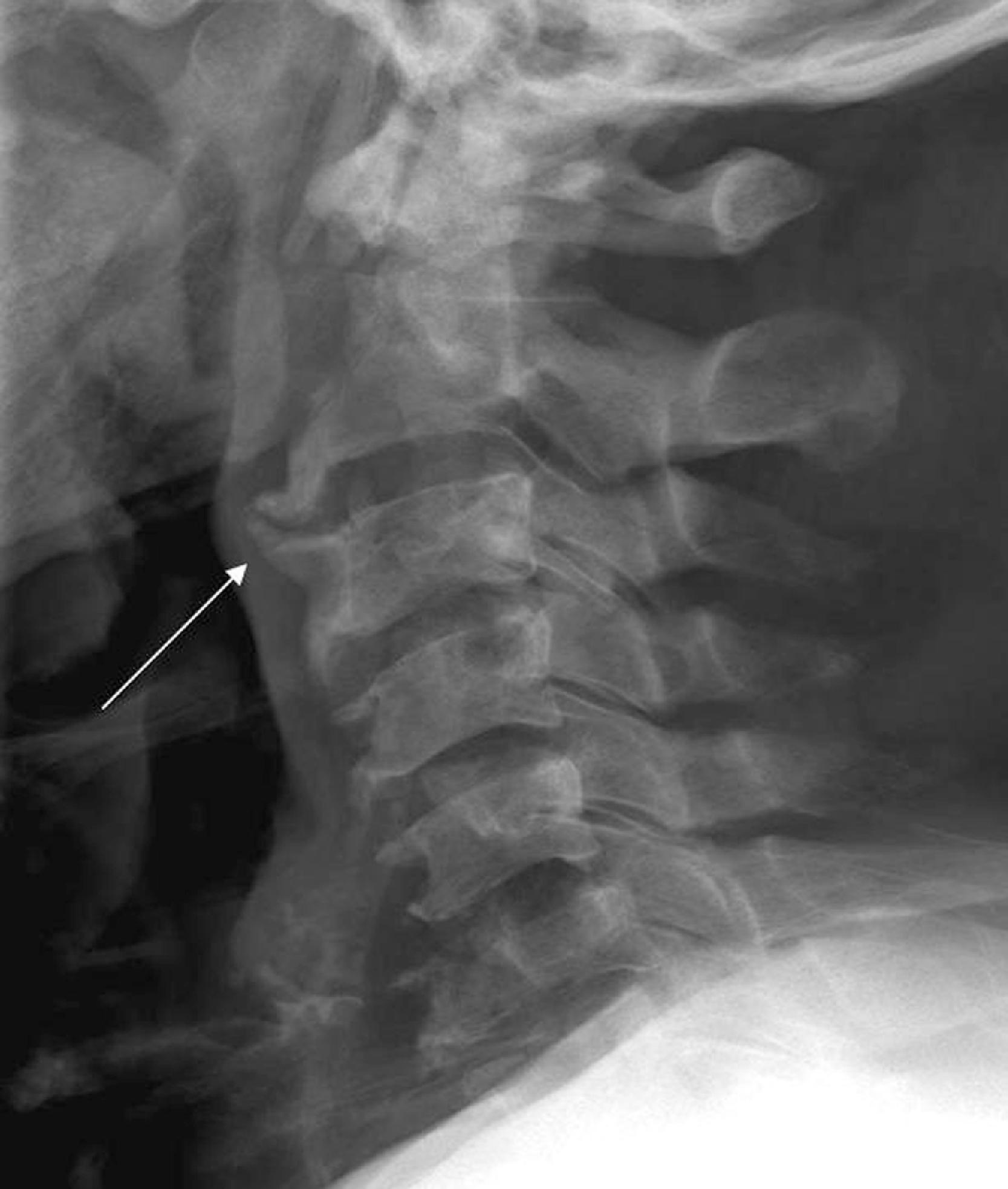
Cervical spine x-rays are also ordered for the evaluation of cervical spondylosis ( Fig. 2.14 ). The hypertrophic bone changes associated with this condition are well depicted on x-ray studies. Large anterior osteophytes that project forward may cause dysphagia and difficult intubation. The spinal canal and neural foramina are assessed for stenosis, and, when present, precautions can be taken when hyperextending the neck and positioning the patient to avoid exacerbation of baseline neurologic symptomatology. Calcification and ossification are well depicted on x-ray examination. Ossification of the anterior longitudinal ligament and diffuse idiopathic skeletal hyperostosis can also lead to large anterior osteophytes that narrow the airway and have been reported as causes of difficult intubation ( Fig. 2.15A–C ). This can be readily appreciated on plain films. Another condition that also may signal difficult intubation is calcification of the stylohyoid ligament ( Fig. 2.16A–E ).
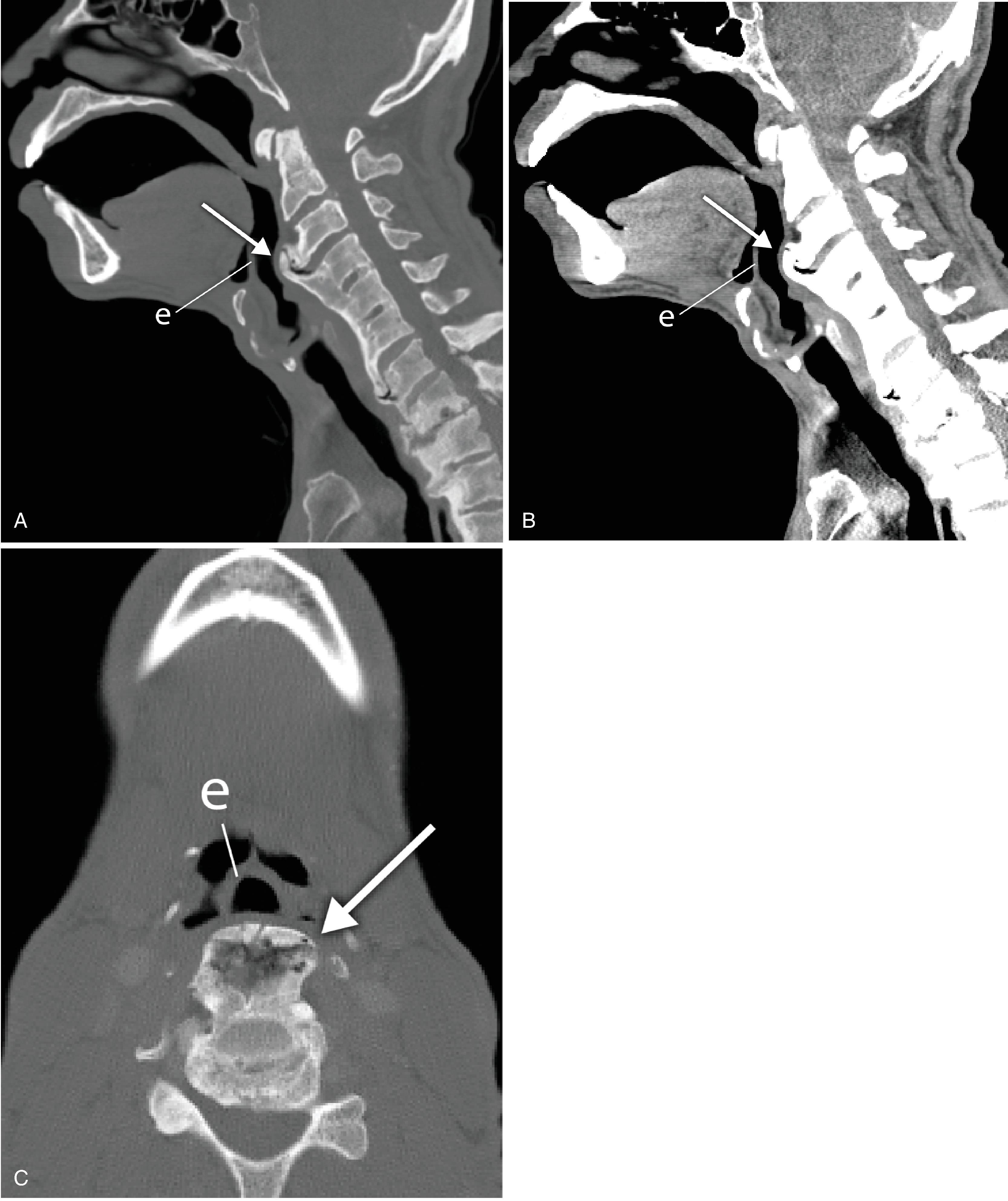
The lateral cervical spine radiograph, obtained to evaluate the integrity and alignment of the bony cervical spine, allows an incidental view of the aerodigestive tract and a gross assessment of the overall patency of the airway. Useful bony landmarks of the pharynx and larynx that can be appreciated on the lateral neck x-ray are the hard palate, hyoid bone, thyroid, and cricoid cartilages ( Fig. 2.17 ). The hard palate separates the nasopharynx from the oropharynx. The larynx can be thought of as being suspended from the hyoid bone. Muscles acting on the hyoid bone elevate the larynx and provide the primary protection from aspiration. The largest cartilage in the neck is the thyroid cartilage, which, along with the cricoid cartilage, acts as a protective shield for the inner larynx. The cricoid cartilage is the only complete cartilaginous ring in the respiratory system. It is located at the level where the larynx ends and the trachea begins.
Normal air-filled structures seen on lateral plain film are the nasopharynx, oropharynx, and hypopharynx. Air in the pharynx outlines the soft palate, uvula, base of the tongue, and nasopharyngeal airway ( Fig. 2.18 ). Any sizable soft tissue pathology results in deviation or effacement of the airway. The tongue constitutes the bulk of the soft tissue in the oral cavity and the oropharynx. In children, and sometimes in adults, prominent lymphatic tissues, such as adenoids and palatine tonsils, may encroach on the nasopharyngeal and oropharyngeal airway. Lingual tonsils are located at the base of the tongue above the valleculae, which are air-filled pouches between the tongue base and the free margin of the epiglottis.
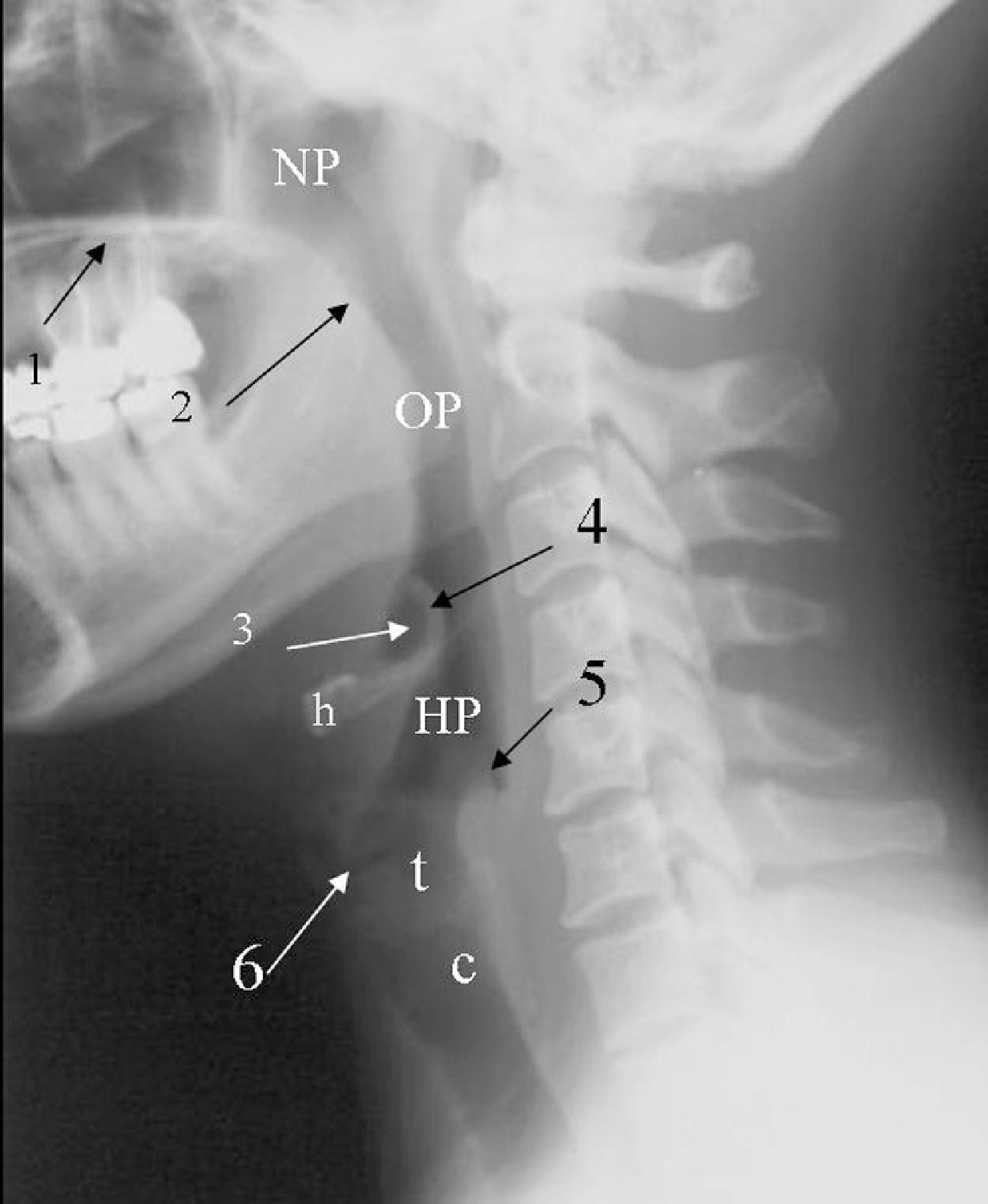
The epiglottis is an elastic fibrocartilage in the shape of a flattened teardrop or leaf that tapers inferiorly and attaches to the thyroid cartilage. The epiglottis tends to be more angular in infants than in adults. During the first several years of life, the larynx changes its position in the neck. , The free edge of the epiglottis in neonates is found at or near the C1 level, and the cricoid cartilage, representing the most caudal portion of the larynx, is at the C4–C5 level. By adolescence, the epiglottis is found at the C2–C3 level, and the cricoid is at the C6 level. The adult epiglottis is usually seen at the C3 level, with the cricoid at C6–C7. However, the position of these structures in the normal population varies by at least one vertebral body level. A thin, lucent, air-filled stripe oriented in the AP direction on a lateral view demarcates the position of the laryngeal ventricle at the base of the aryepiglottic folds, just above the true vocal cords ( Fig. 2.19A and B ). Lateral to the aryepiglottic fold is the pyriform sinus of the hypopharynx. This anterior mucosal recess is between the posterior third of the thyroid cartilage and the aryepiglottic fold. The extreme lower aspect of the pyriform sinus is situated between the mucosa-covered arytenoids and the mucosa-covered thyroid cartilage at the level of the true vocal cords. The air column caudally represents the cervical trachea. On the AP view, the false and true vocal cords above and below the laryngeal ventricles, as well as the subglottic region and the trachea, may be identified. Calcified thyroid cartilage can sometimes be visualized.
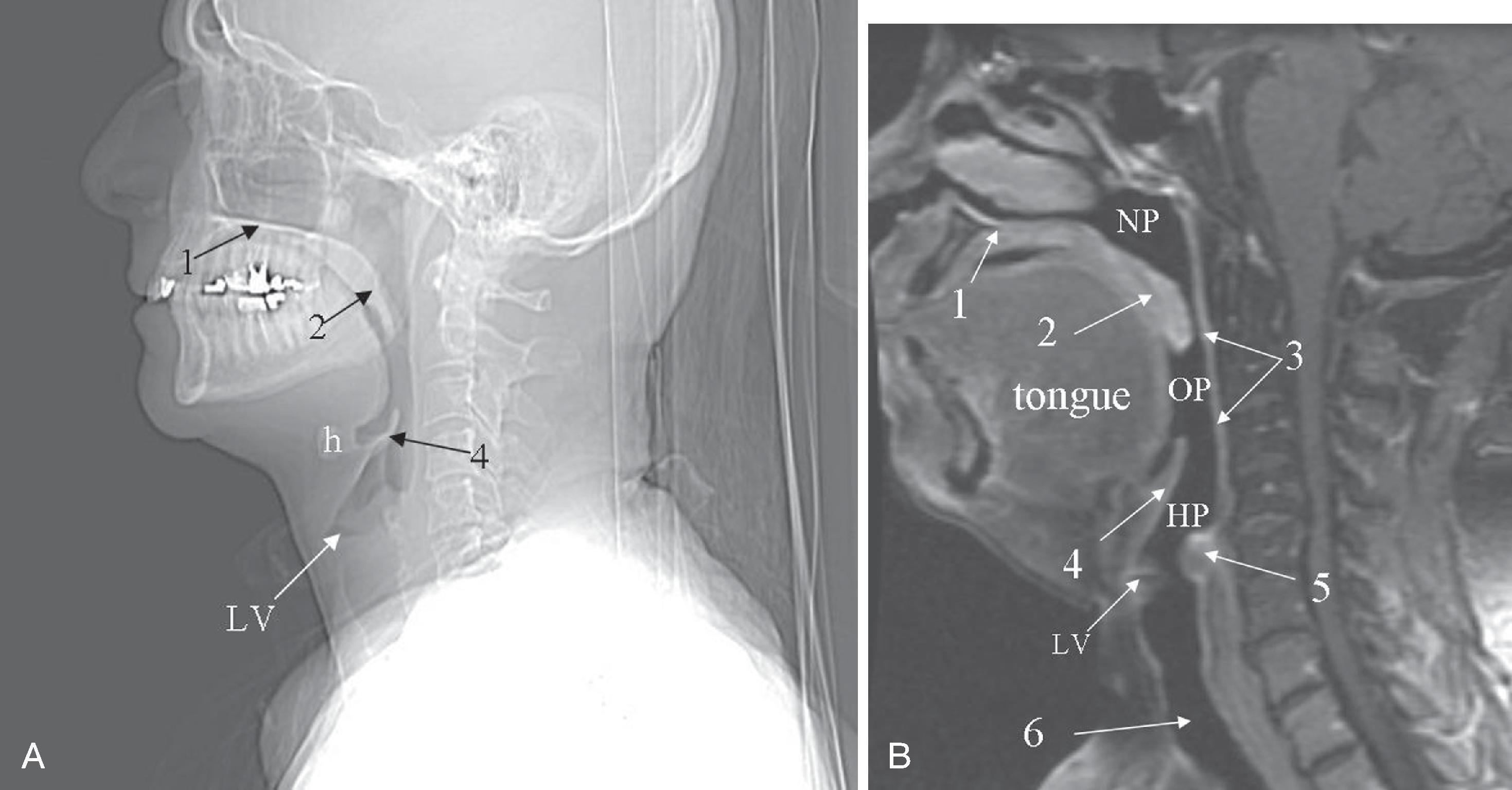
The soft tissues dorsal to the airway, the prevertebral soft tissue, are adherent to the anterior surface of the atlas and the axis and are the normal soft tissue structures of the posterior pharynx extending from the clivus to the nasopharynx and hypopharynx. The ligaments of the cervicocranium, critical to maintaining stability throughout this region, are directly involved in the range of motion of the cervicocranium and anteriorly contribute to the prevertebral soft tissue shadow. Superimposed on these deep structures are the pharyngeal constrictor muscles and the mucosa of the posterior pharyngeal wall. The cervicocranial prevertebral soft tissue contour should normally be slightly posteriorly concave rostral to the anterior tubercle of C1, anteriorly convex in front of the anterior tubercle, and posteriorly concave caudal to the anterior tubercle, depending on the amount of adenoidal tissue and on the amount of air in the pharynx. Adenoidal tissue appears as a homogeneous, smoothly lobulated mass of varying size and configuration. The anterior surface of the adenoid is demarcated by air anteriorly and inferiorly. The air inferior to the adenoids allows differentiation between adenoids and the presence of a nasopharyngeal hematoma commonly associated with major midface fractures. In infants and young children, the soft tissues of the cervicocranium are lax and redundant. Depending on the phase of respiration and position, the thickness of the prevertebral soft tissues may appear to increase and simulate a retropharyngeal hematoma. This finding may extend to the lower cervical spine. This anomaly becomes normal if imaging is repeated with the neck extended and during inspiration. By 8 years of age, the contour of the soft tissues should resemble that seen in adults. Of note, in pediatric patients, sedation may result in a decrease in the AP diameter of the pharynx at the levels of the palatine tonsils, the soft palate, and the epiglottis.
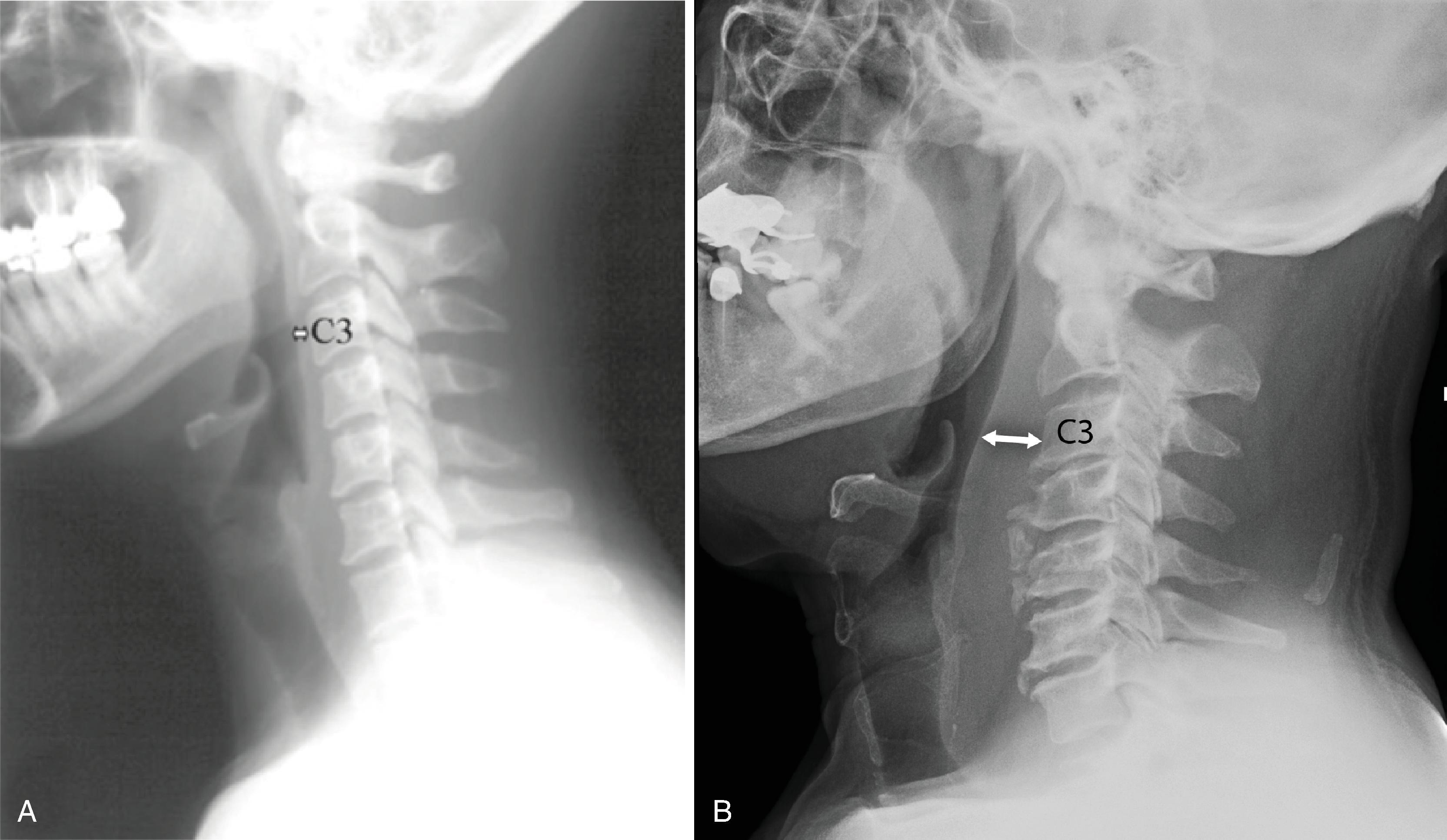
In the lower neck, from C3 to C7, the prevertebral soft tissue shadow differs from that in the cervicocranium because of the beginning of the esophagus and the prevertebral fascial space, which are recognized on the lateral radiograph as a fat stripe. By standard anatomic description, the esophagus begins at the level of C4; however, the esophageal ostium in vivo may normally be found as high as C3 and as low as C6 and varies with the phase of swallowing and the flexion or extension of the cervical spine. The prevertebral soft tissue thickness—the distance between the posterior pharyngeal air column and the anterior portion of the third or fourth vertebra—should not exceed one-half to three-quarters of the diameter of the vertebral body. In the opinion of Harris and Mirvis, only the measurement at C3 is valid, and it should not exceed 4 mm ( Fig. 2.20A and B ). More caudally, at the cervicothoracic junction, assessment of the prevertebral soft tissues is based on contour rather than actual measurement. This contour should parallel the arch formed by the anterior cortices of the lower cervical and upper thoracic vertebral bodies.
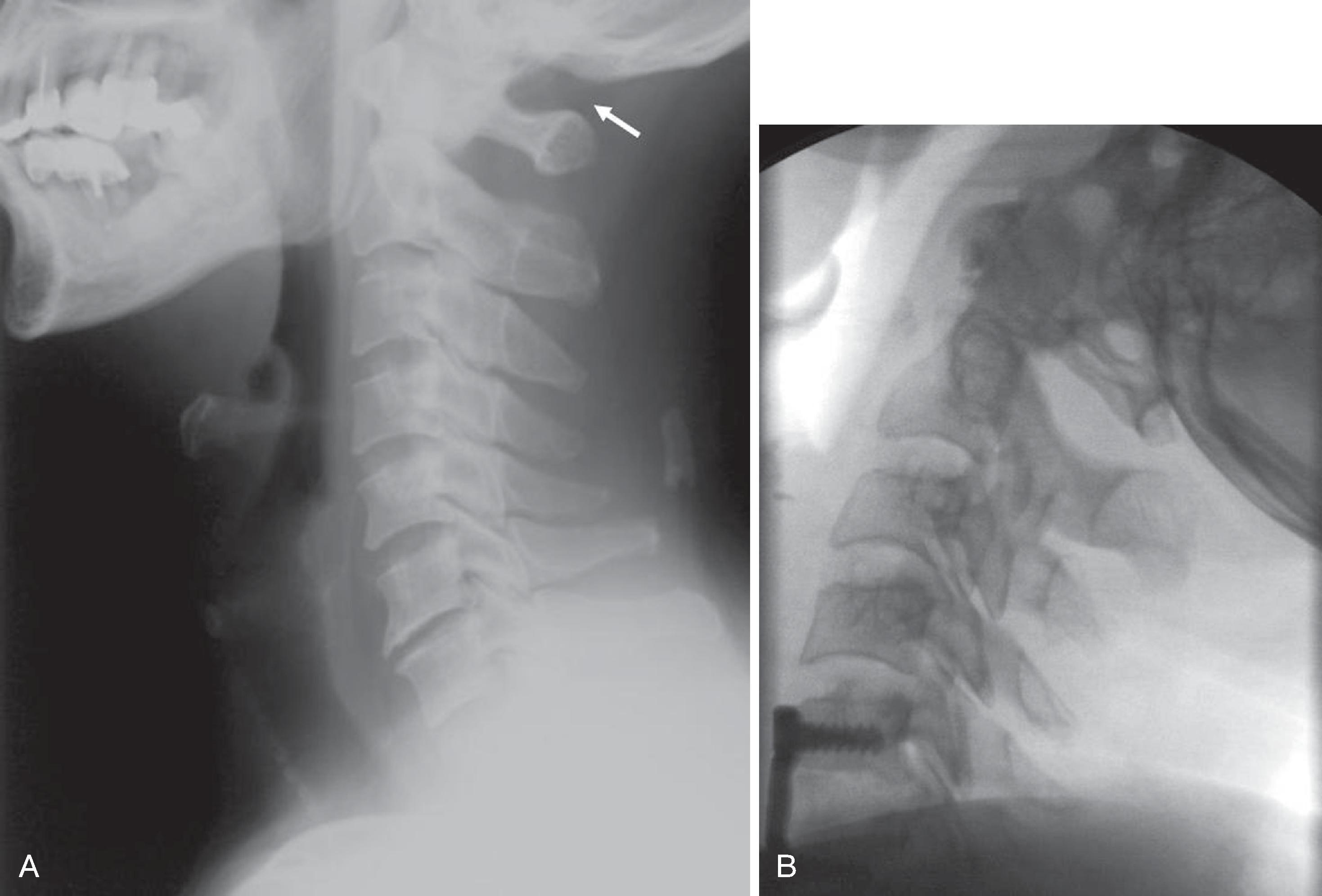
In truth, plain film or digital radiograph diagnosis of upper airway diseases has been supplanted by cross-sectional imaging, except in a few situations in which plain x-ray findings are pathognomonic of the disease. Two classic examples of plain film or digital radiologic diagnosis are acute epiglottitis and croup. In acute epiglottitis, or supraglottitis , a more encompassing term, edema and swelling of the epiglottis are present, with or without involvement of the aryepiglottic folds and arytenoids. Historically, the causative organism was usually Haemophilus influenza ; with the introduction of the Haemophilus vaccine, group A β-hemolytic streptococcus is more commonly responsible for such infections. Airway compromise with a rapidly progressive course requiring emergency tracheostomy is a possibility if the entity goes unrecognized and untreated. In general, the infection is milder in adults than in children. The radiographic findings are swelling or enlargement of the epiglottis. On a lateral radiograph of the neck, thickening of the free edge of the epiglottis can be appreciated and is referred to as the “thumb sign” ( Fig. 2.21 ). The width of the adult epiglottis should be less than one-third of the AP width of the C4 body. Cross-sectional imaging is superfluous; however, theoretically, the degree of airway compromise can be quantified by three-dimensional reconstruction.
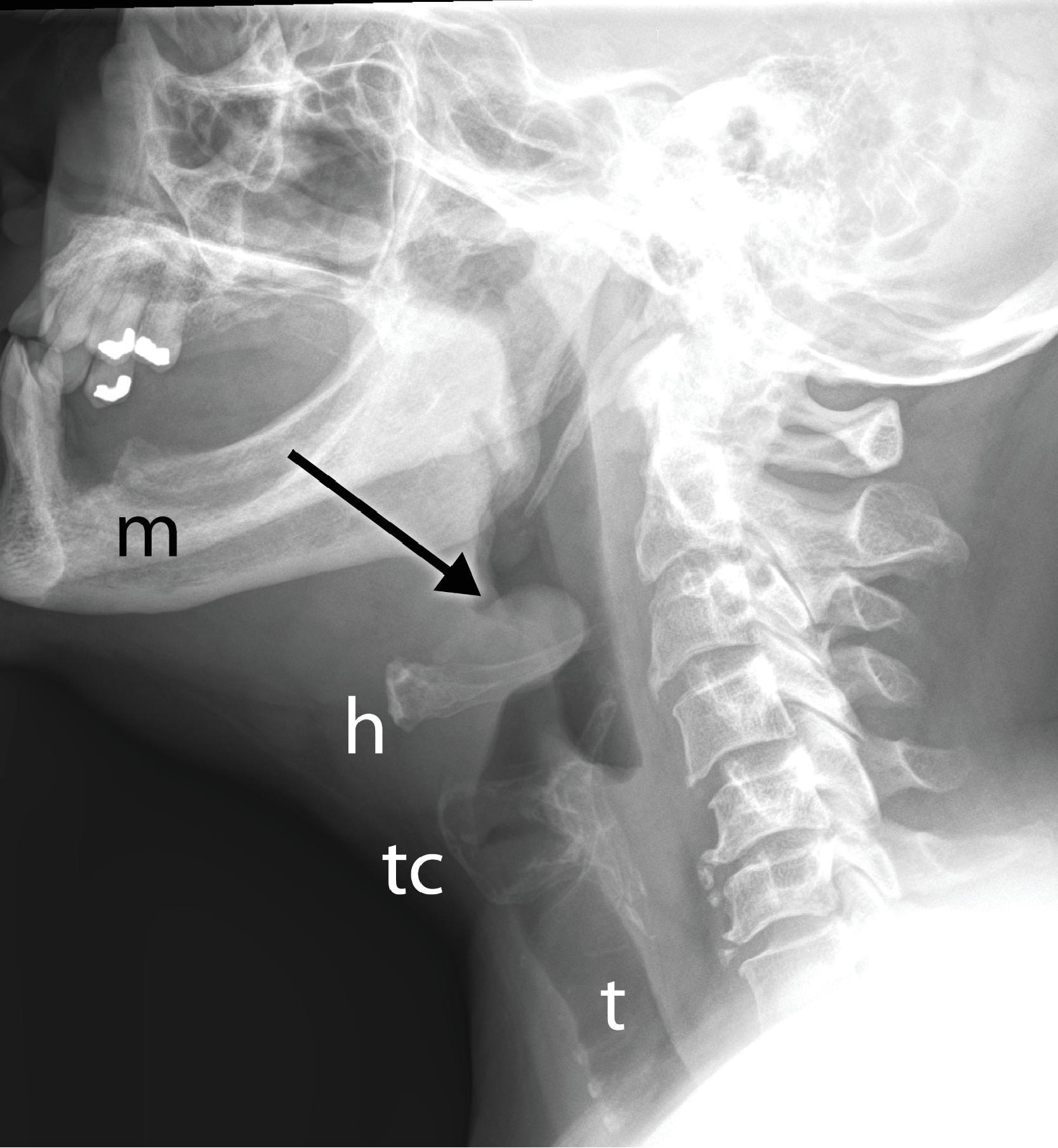
In laryngotracheobronchitis, or croup, the subglottic larynx is involved. It affects younger children and has a less fulminant course than acute epiglottitis. The swelling of the soft tissues in the subglottic neck can be appreciated on an AP view of the neck ( Fig. 2.22 ). There is usually a long, segmental narrowing of the glottis and subglottic airway with loss of the normal angle between the vocal cords and the subglottic airway. The narrowing of the upper trachea has been referred to as the “steeple” sign. The hypopharynx is usually dilated because of the airway obstruction distally.
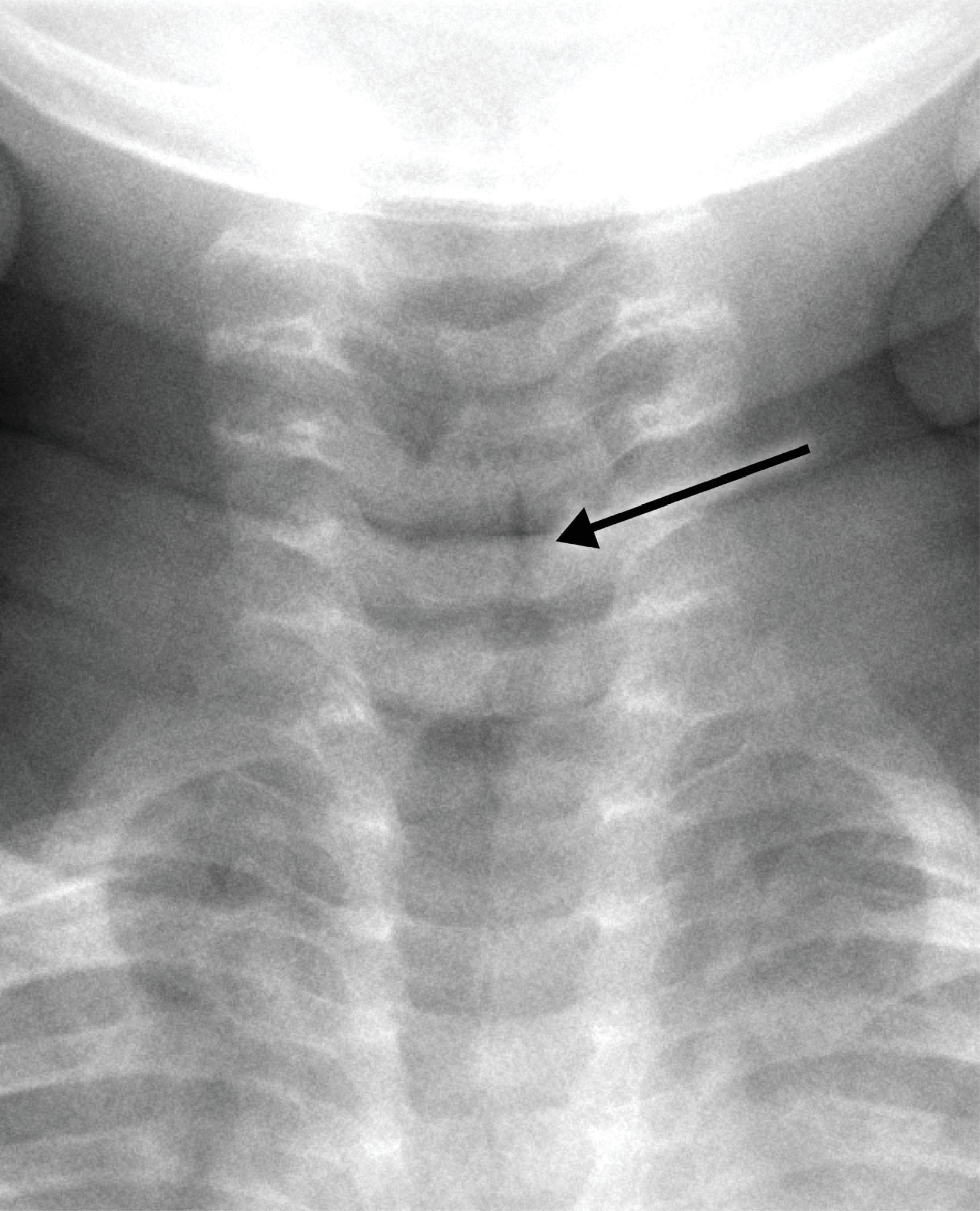
Cervical spine examination using a soft tissue technique is also useful for the evaluation for the presence of radiopaque foreign bodies, such as a fish bone. Ingested foreign bodies most often lodge at the level of the pyriform sinus ( Fig. 2.23A–D ).
Before the advent of CT, chest x-ray (CXR) was routinely ordered to assess pulmonary and cardiovascular status. Today, a CXR or digital chest radiograph is still a cost-efficient examination that yields a great deal of general information. The most common views of the chest are the posteroanterior (PA), AP, and lateral projections ( Fig. 2.24A–D ). Film cassette-based PA CXR is obtained with the patient’s anterior chest closest to the film cassette and the x-ray beam directed from a posterior to anterior direction. Alternatively, the AP chest view is done with the patient’s back closest to the film cassette and the x-ray beam directed in the anterior to posterior direction. The part of the chest closest to the film cassette is the least magnified; therefore, the cardiac silhouette is larger on the AP projection. The lateral projection is most often performed with the patient’s left chest closest to the film cassette for better delineation of the structures in the left hemithorax, which is more obscured by the heart on a PA projection. Other common projections obtained include oblique, decubitus, and lordotic views. The oblique view is useful for assessing a lesion with respect to other structures in the chest. The decubitus view is helpful to assess whether an apparent elevated hemidiaphragm is due to a large subpulmonic pleural effusion. The lordotic view is useful to look for a suspected small apical pneumothorax, which can also be accentuated on an expiratory phase view.
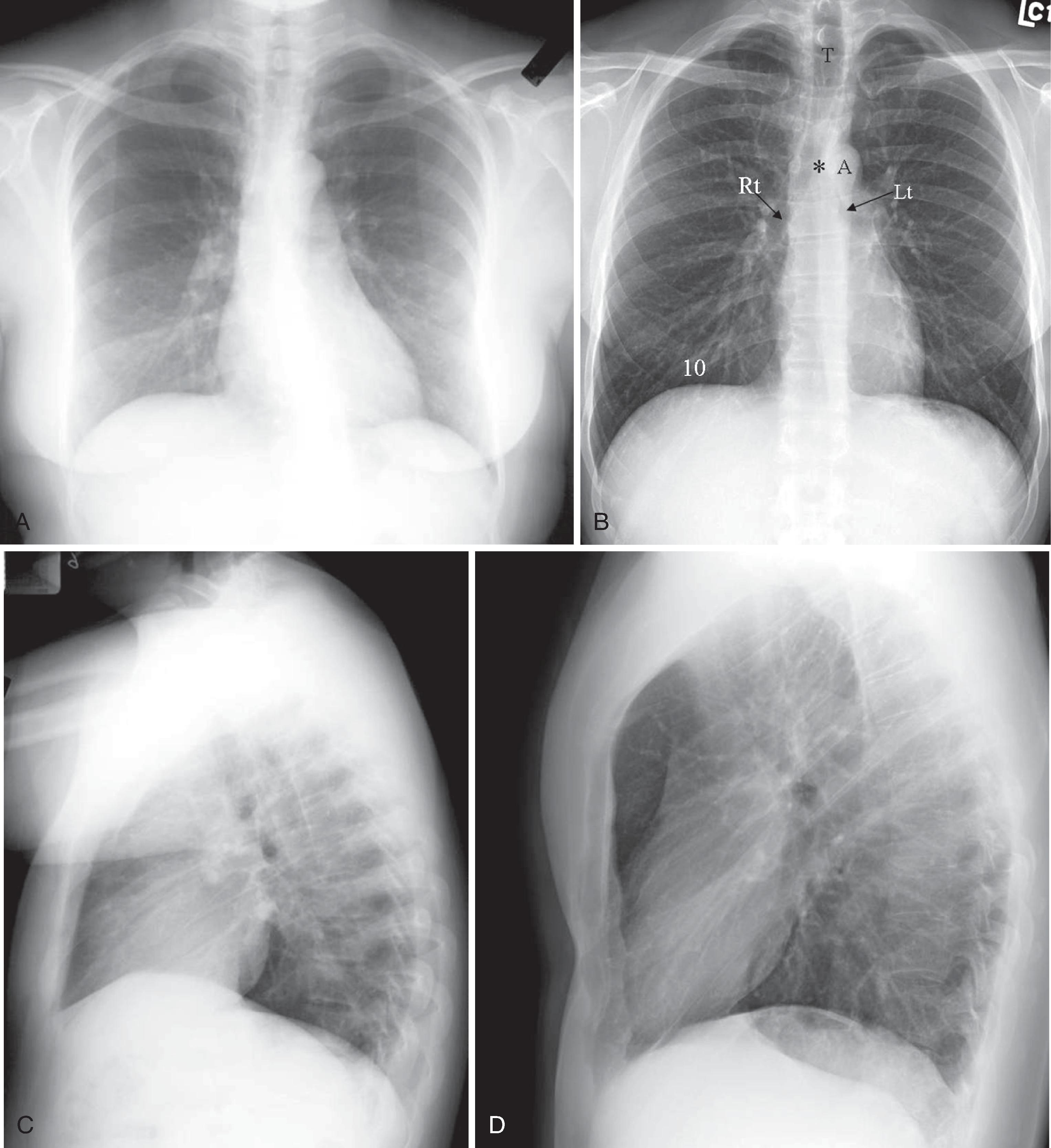
It is helpful to train one’s eyes to analyze the CXR systematically to cover the details of the chest wall, including the ribs, lungs, and mediastinal structures that include the heart and the outline of the tracheobronchial tree. On an adequate inspiratory film, the hemidiaphragms are below the anterior end of the 6th rib, or at least below the 10th posterior rib, and the lung expansion should be symmetrical. The right hemidiaphragm is usually half an interspace higher than the left, which is depressed by the heart (see Fig. 2.24 ). Without a doubt, the art of CXR interpretation has been supplanted by the advent of CT, which demonstrates chest pathology with unparalleled clarity. However, CXR can still afford a composite survey of the chest at one quick glance. One can easily compare lung volumes, the position of the mediastinum, the presence or absence of major airspace disease, and a gross assessment of cardiac status.
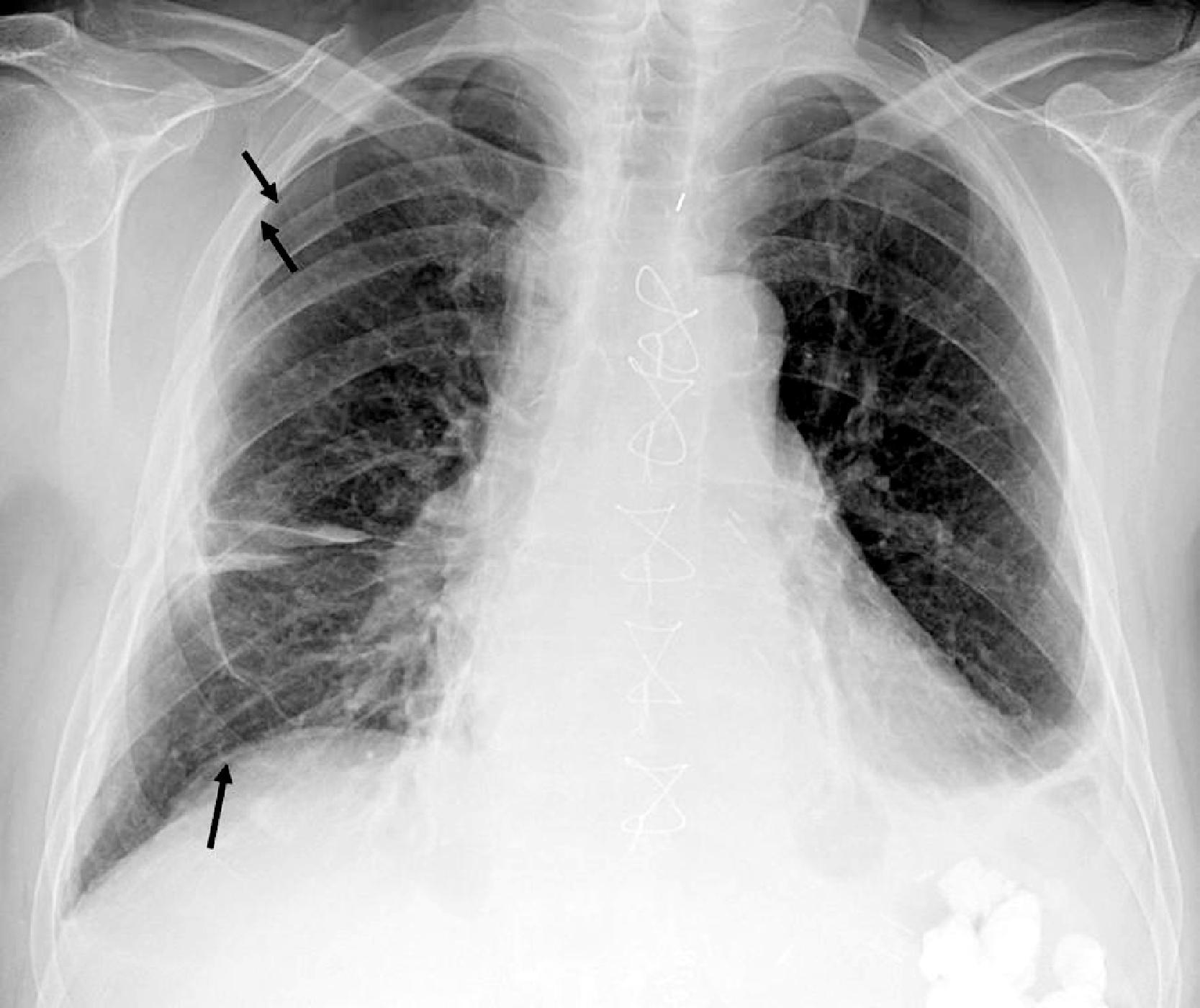
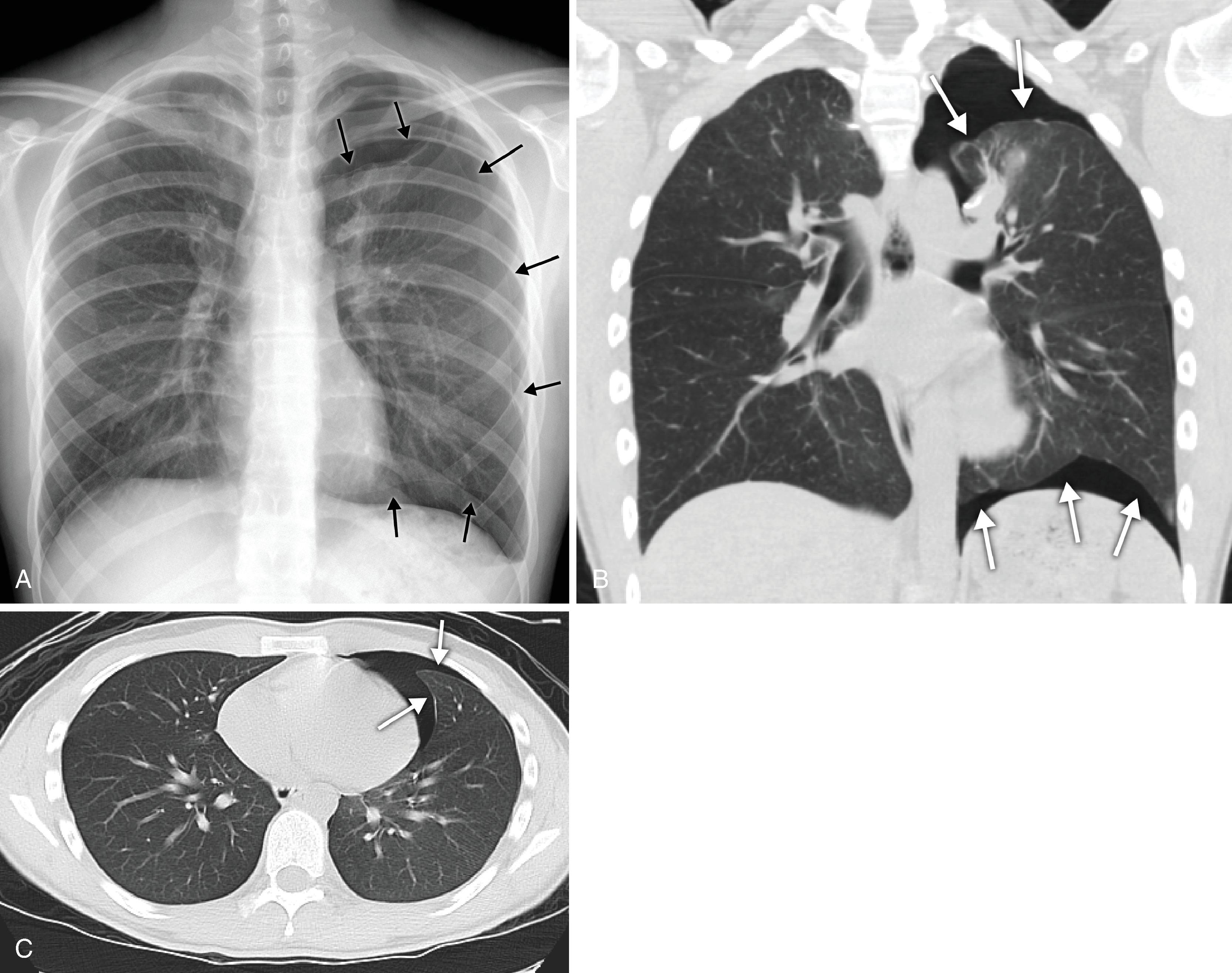
A high hemidiaphragm implies reduced lung volume, which can be caused by phrenic nerve paralysis, thoracic conditions causing chest pain that results in splinting, or extrapulmonic processes such as an enlarged spleen or liver, pancreatitis, or subphrenic abscess. The presumed level of the hemidiaphragm is seen as the edge or transition between aerated lungs and the opacity of the organs in the abdomen. If the thin leaves of the hemidiaphragm are outlined by air, a pneumoperitoneum should be considered ( Fig. 2.25 ). A well-expanded lung should appear radiographically lucent but be traversed by “lung markings”—thin threads of interstitium consisting of septa and arterial, venous, and lymphatic vessels. In most normal individuals, the lungs appear less lucent at the bottom, owing to the distribution of the pulmonary vasculature, the effect of gravity, and overlying soft tissues such as breast tissues. In congestive heart failure or pulmonary venous hypertension, this pattern is reversed, with “cephalization” and engorgement of the pulmonary veins in the upper lung zones ( Fig. 2.26 ; see Fig. 2.24 ). In general, any process that replaces the airspaces of the lungs with a substance such as fluid, pus, or cells causes the x-ray beam to be more attenuated, allowing less of the beam to be transmitted through the patient to the film, resulting in an image that is less dark or more opaque (white) in the areas affected. A whole host of diseases could be responsible, depending on the clinical picture, including pleural effusion, pulmonary edema, pneumonia, lung mass, lung collapse (atelectasis), lung infarct or contusion, and metastatic disease ( Fig. 2.27A–J ). The key, from the anesthesiologist’s point of view, is not to make the correct pathologic diagnosis but to note the abnormality, which may affect ventilation, and to adjust the anesthetic practice accordingly. In contrast to the increased opacity of the lung caused by the preceding conditions is a hemithorax that appears too lucent and devoid of the expected lung markings. Two entities should be considered. Foremost is a pneumothorax ( Fig. 2.28A–C ); if large, the collapsed lung is medially positioned against the mediastinum. If the mediastinum is shifted away from the midline, a tension pneumothorax may be present, and emergent management is required. Occasionally, the cause of a unilateral lucent lung is the presence of large emphysematous blebs in patients with chronic obstructive pulmonary disease, which are sometimes difficult to differentiate from a moderate to large pneumothorax. More rare causes include pulmonary oligemia (decreased pulmonary flow) from a thromboembolism of the right or left pulmonary artery, pulmonary neoplasm, or obstructive hyperinflation. Bilateral lucent lungs are more difficult to appreciate. These are usually seen in patients with pulmonary stenosis secondary to cyanotic heart disease and right-to-left shunts. A discussion of the pediatric chest and congenital heart and lung diseases is beyond the scope of this chapter.
Moving centrally in the chest, one encounters the mediastinum, which contains the hila, tracheobronchial tree, heart and great vessels, lymph nodes, esophagus, and thymus. The mediastinum is extrapleural and outlined by air in the adjacent lungs. Except for the air within the trachea and the mainstem bronchi, on conventional chest radiographs the remainder of the mediastinal structures are soft tissues or water density. Therefore, it is extremely difficult to localize a mediastinal lesion. Traditional pleural reflections or vertical lines are described for a frontal CXR that, if deviated, would suggest the presence of mediastinal pathology. Felson has proposed a radiologic approach to subdividing the mediastinum on a lateral radiograph into three compartments: anterior, middle, and posterior. The anterior and middle mediastinum are divided by the line extending along the back of the heart and the front of the trachea. The middle and posterior mediastinal compartments are separated by the line connecting a point on each thoracic vertebra about a centimeter behind its anterior margin ( Fig. 2.29 ).
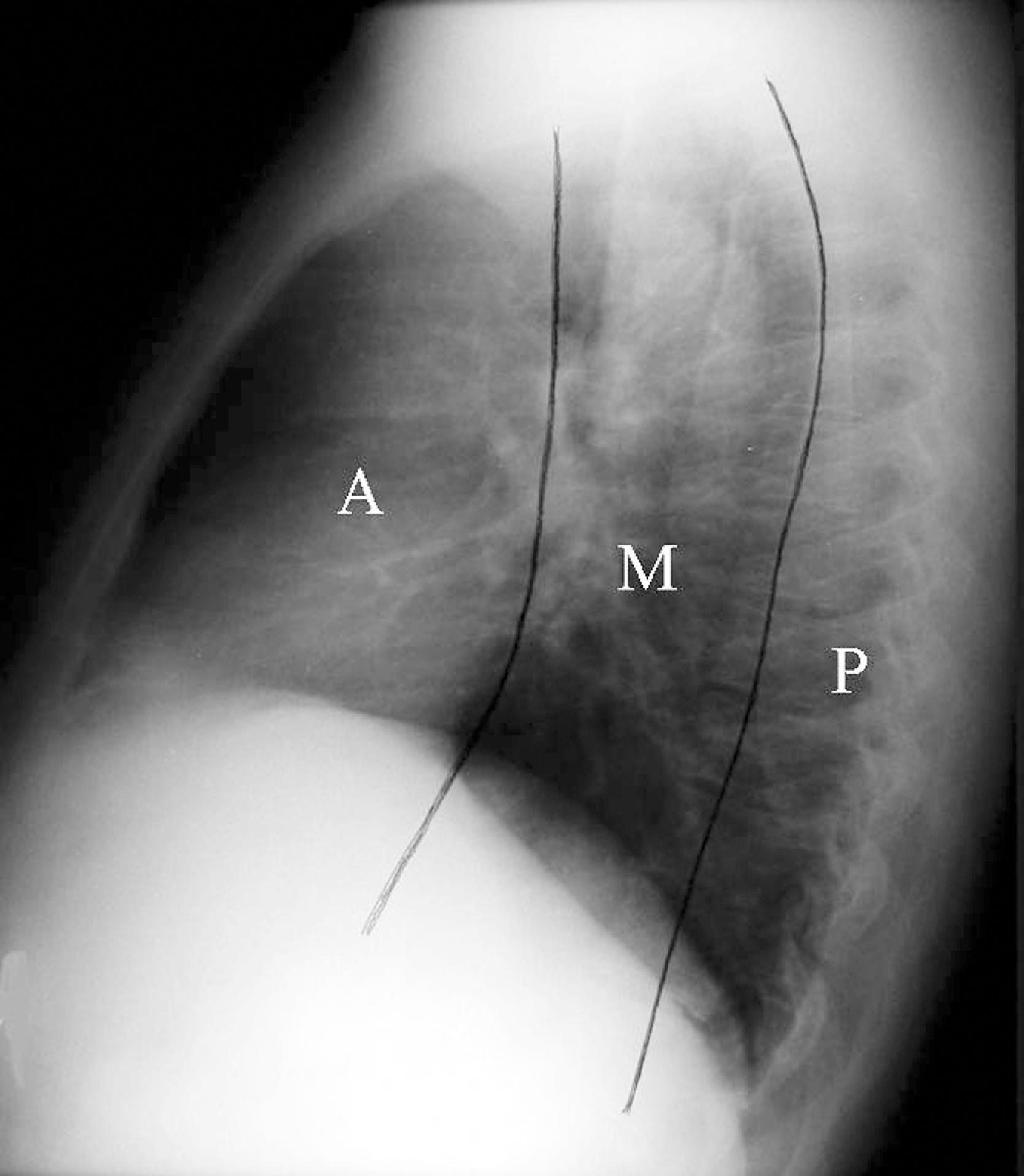
Conditions that can be found in each of the compartments of the mediastinum are logically based on the anatomic structures found within the compartments. For example, tracheal, esophageal, and thyroid lesions would lie in the middle mediastinum. Neurogenic tumors and spinal problems would be in the posterior mediastinum. Cardiac and thymic lesions would occupy the anterior mediastinum. Certain diseases, such as lymph node disorders, lymphoma, and aortic aneurysms, may arise in any or all three compartments. Many modifications to the divisions of the mediastinum have been proposed.
The great vessels and the heart should be centrally located on the AP view of the mediastinum. The aortic knob is usually on the left, and the cardiothoracic ratio on the AP view should be roughly less than 50%. The hila are composed of the pulmonary arteries and their main branches, the upper lobe pulmonary veins, the major bronchi, and the lymph glands ( Fig. 2.30A and B ). The positions of the trachea, carina, and mainstem bronchi are outlined by air. The carinal bifurcation angle is typically 60 to 75 degrees. The right mainstem bronchus has a steeper angle than the left (see Fig. 2.24 ); it usually branches off the trachea at 25 to 30 degrees, whereas the left mainstem bronchus leaves the trachea at a 45-degree to 50-degree angle. The trachea is a tubular structure extending from the cricoid cartilage to the carina, which is located at approximately the T5 level. C-shaped hyaline cartilage rings, which can calcify with age, outline the trachea anteriorly; the posterior trachea is membranous. The mean transverse diameter of the trachea is approximately 15 mm for women and 18 mm for men. The trachea in the cervical region is midline, but it is deviated to the right in the thorax.
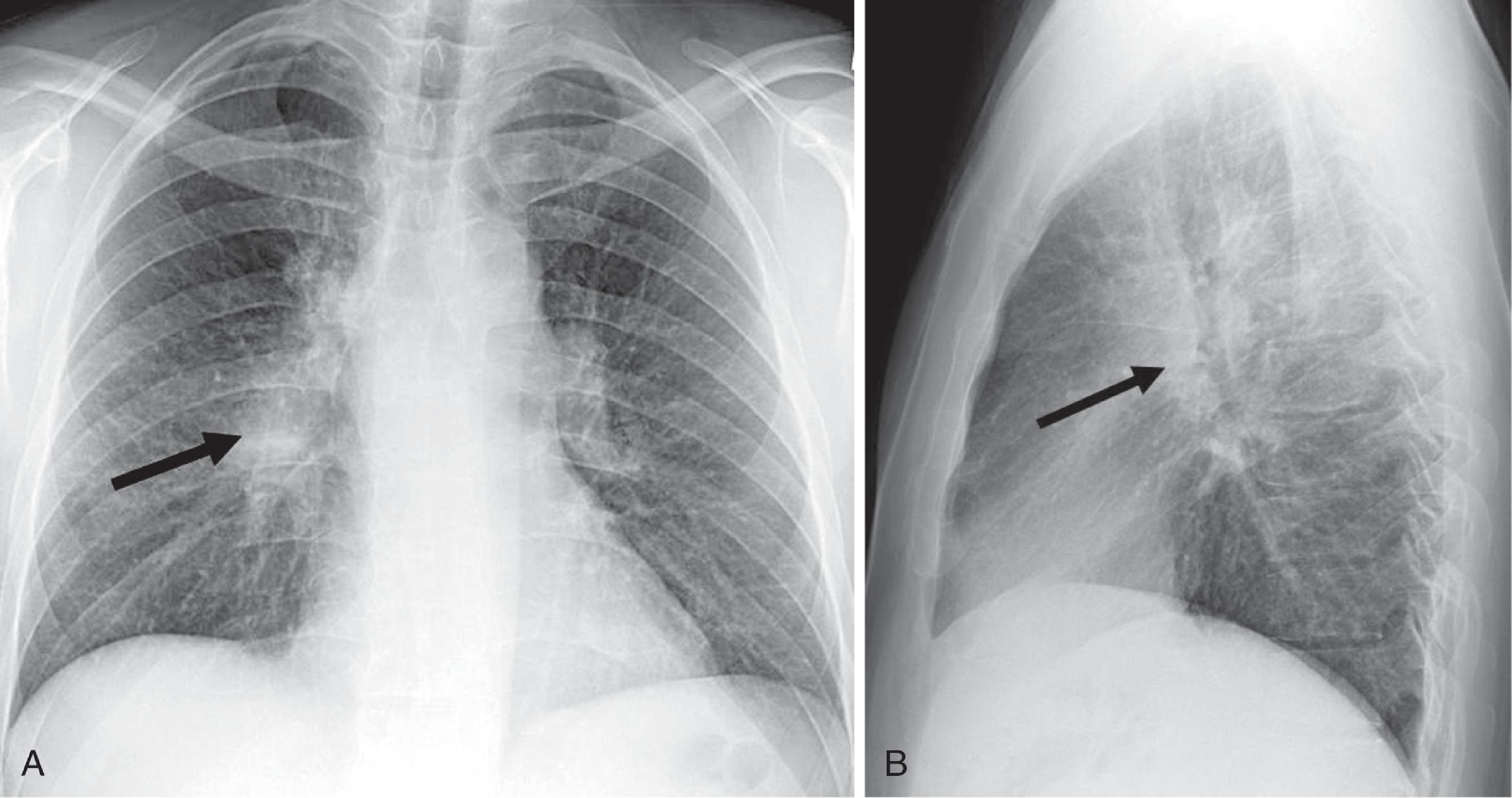
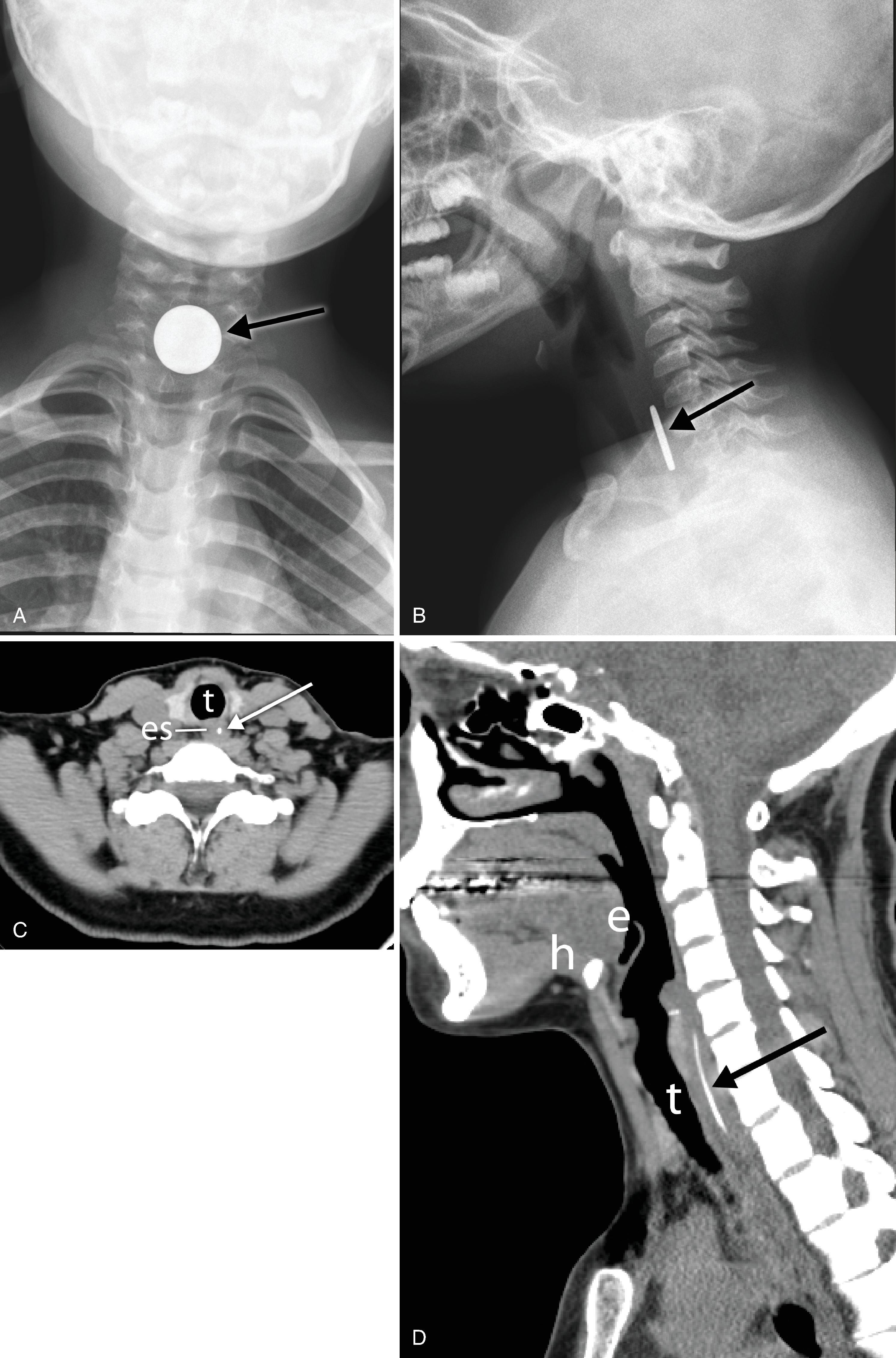
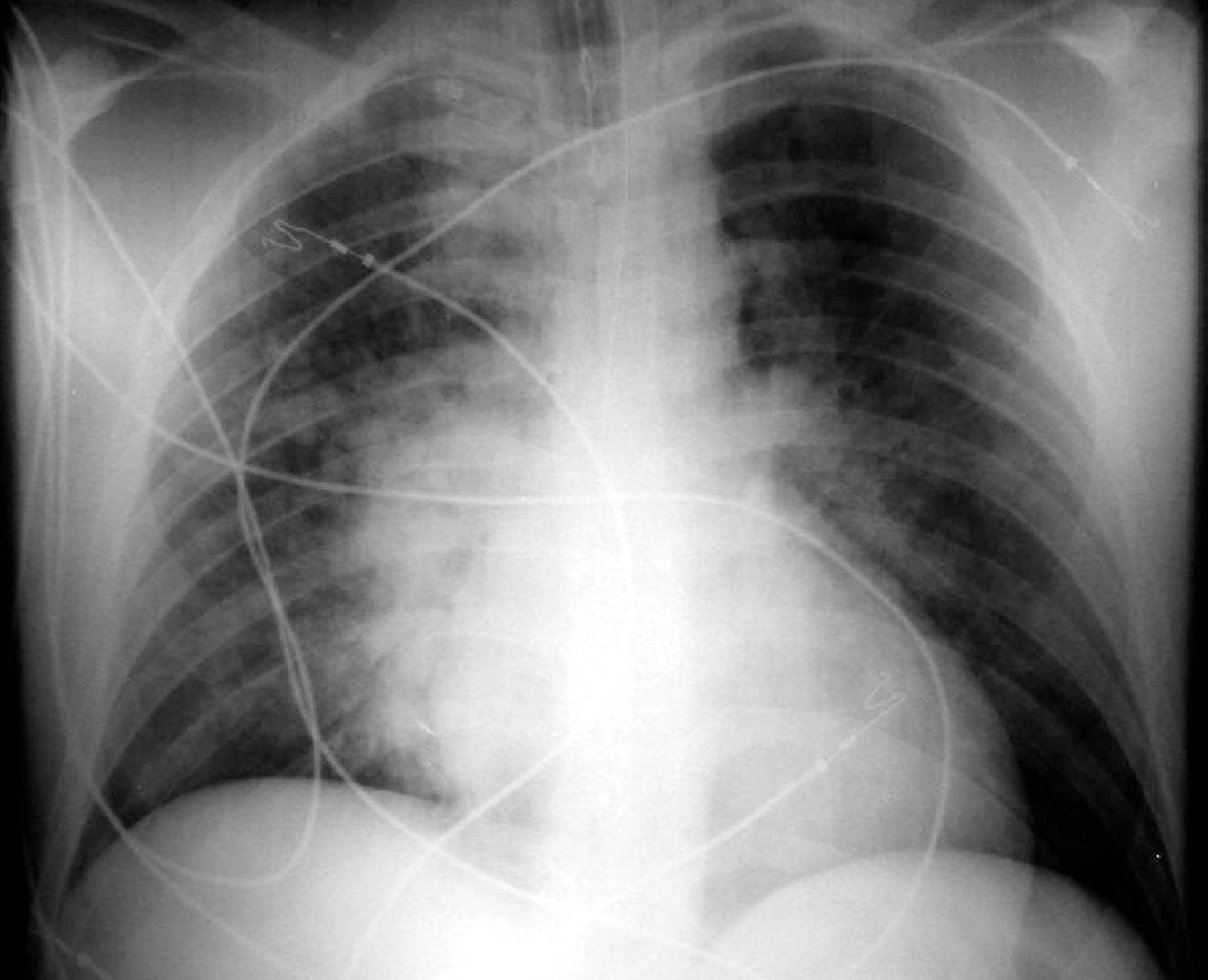
Adequate positioning of an ETT in an intubated patient is usually documented by obtaining a chest radiograph. The tip should be intrathoracic and at a distance above the carina that ensures equal ventilation to both lungs. One should evaluate the ETT position with the patient’s head and neck in a neutral position; however, in an intensive care unit setting, this may not be possible. The tip of the ETT may move up or down by 1 to 2 cm with extension or flexion of the neck, respectively. Rotation of the head and neck usually results in ascent of the tip. The optimum position of the tip of the ETT is approximately 3 to 5 cm above the carina; this allows enough latitude for movement of the patient’s head such that the tip does not enter the right main bronchus and the inflated cuff remains below the vocal cords ( Fig. 2.31A and B ). Malpositioning of the cuff at the level of the vocal cords or pharynx increases the risk of aspiration. Overinflation of the cuff at the level of the vocal cords may lead to necrosis. The inflated cuff of the ETT should fill the tracheal air column without changing its contour. Overall, the ETT size should be about two-thirds the diameter of the tracheal lumen. If the tip of the ETT extends beyond the carina, intubation of the right main bronchus occurs, which can be detected by asymmetric breath sounds or on CXR. If unrecognized, atelectasis in the underventilated lung may result.
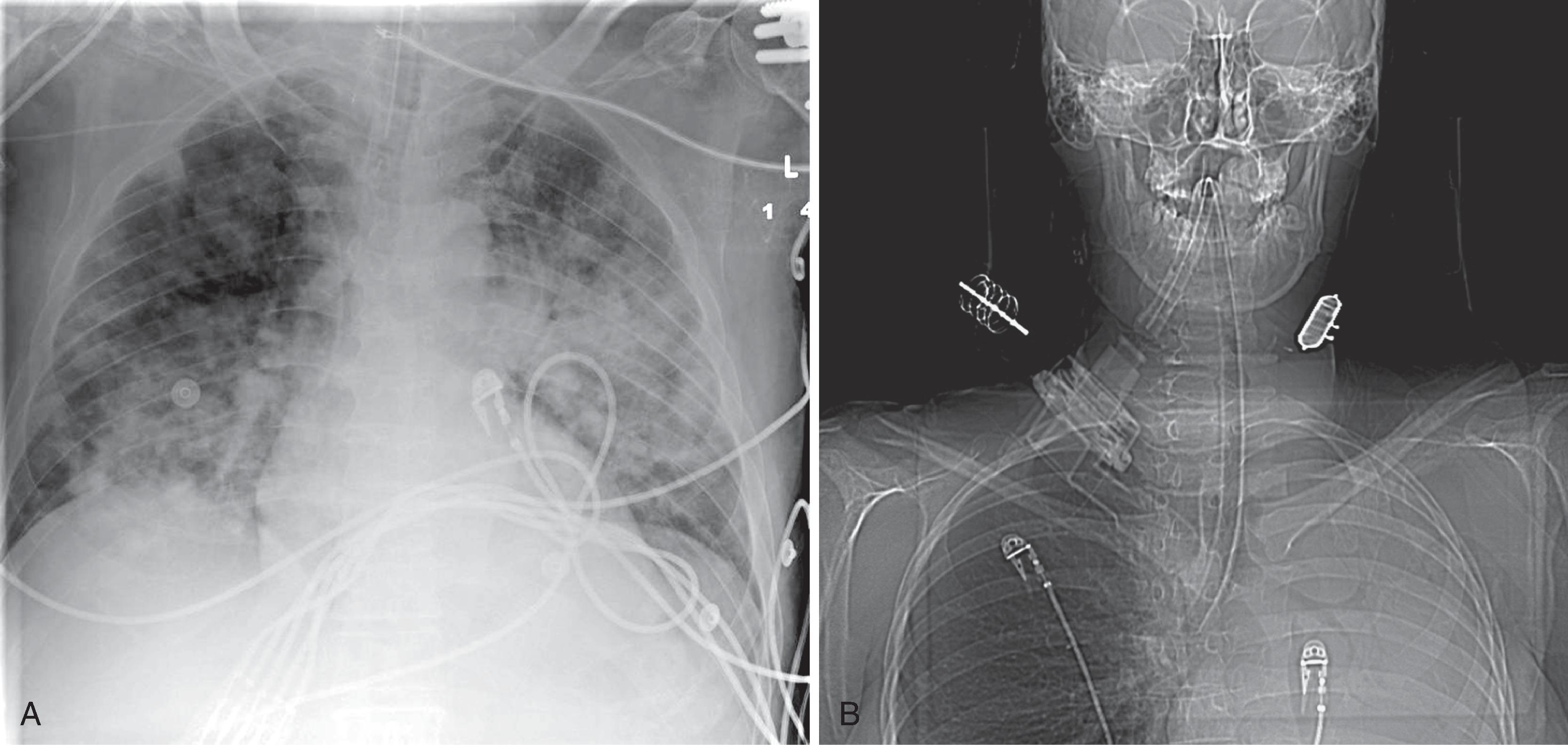
If a nasogastric or orogastric tube is in place, it should be seen on CXR to course inferiorly and to the left, toward the fundus of the stomach in the left upper quadrant, except in the unusual case of situs inversus. Inadvertently, a gastric tube may achieve bronchial intubation; the errant course of the tube would be evident.
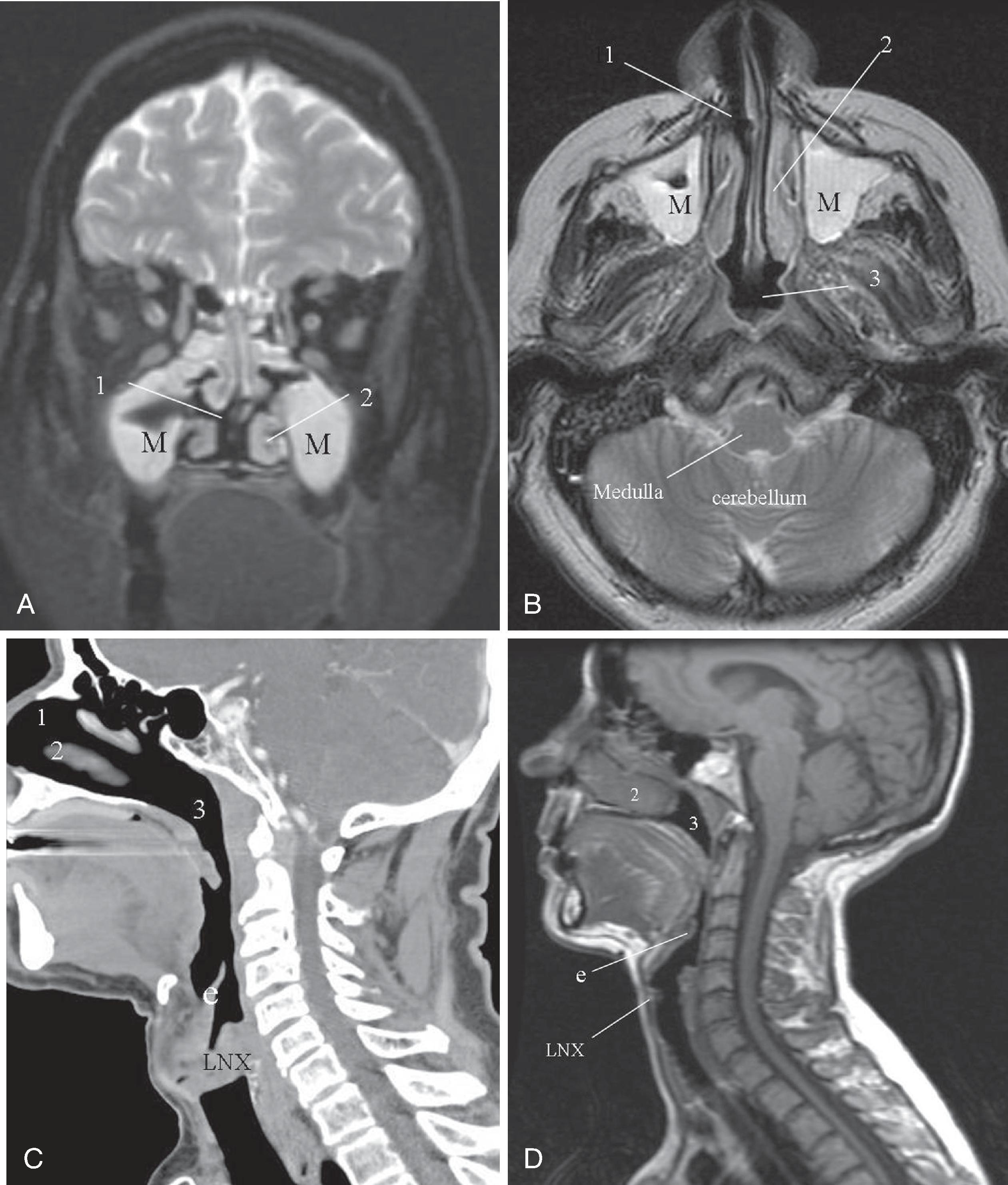
The cross-sectional anatomy of the airway from the nasal cavity to the lungs is exceptionally well depicted by CT; MRI can be a useful complement in the evaluation of these regions. MRI is superior to CT in evaluating tumor infiltration of soft tissues but lacks the ability to depict bone erosions secondary to tumor because cortical bone gives no MRI signal. MRI is susceptible to motion artifact, including breathing and vascular pulsation artifacts, whereas spiral CT technology allows the entire neck or thorax to be scanned in a single breath-hold. Both techniques allow either direct scanning or three-dimensional volume acquisition with multiplanar postprocessing and reformation capabilities.
The following sections describe airway anatomy applicable to both MRI and CT. Recognizing that the pharynx is a continuous structure, for ease of discussion, the nasopharynx, oropharynx, and hypopharynx are discussed separately. Within each section, the anatomy and pertinent pathology with relevance to anesthetic practice are elaborated.
The development of the face, nose, and sinuses is complex but systematic. Thus, the occurrence of congenital lesions and malformations in these areas is quite logical and predictable, dependent on the time of prenatal insult. Face development, nose development, and sinus development are temporally and spatially related to the development of the optic nerve, globe, and corpus callosum, which accounts for the frequency of concurrent anomalies.
The major features of the face develop in the fourth to eighth week of gestation, owing to the growth, migration, and merging of several processes adjoining the stomodeum, which is a slitlike invagination of the ectoderm that marks the location of the mouth. At the fourth week of gestation, two sets of paired and one set of unpaired prominences, derivatives of the first branchial arch, can be identified bordering the stomodeum. The unpaired median frontonasal prominence is located superiorly, the paired maxillary processes are lateral, and the paired mandibular processes are inferior. The various cleft lip, palate, and face syndromes ( Figs. 2.32A–C and 2.33A–G ) can be explained by the failure of different processes to grow, migrate, and/or merge properly. ,
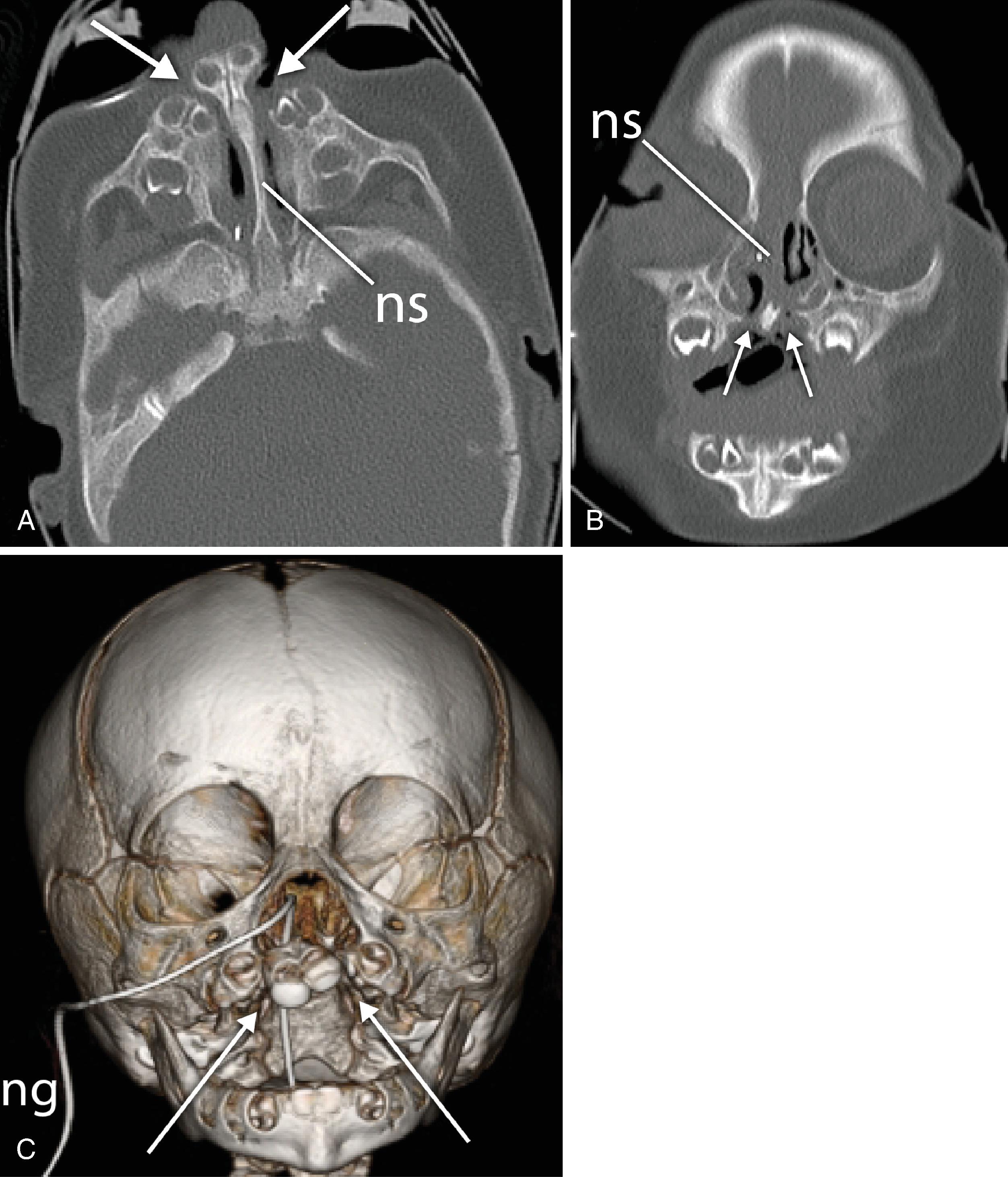
The development of the nasal cavity is complete by the second month of fetal life. From the second to the sixth month of prenatal life, the nostrils are closed by epithelial plugs that recanalize to establish a patent nasal cavity. Failure to do so could account for the congenital stenoses and atresias that cause nasal airway obstruction, which are often seen in conjunction with craniofacial anomalies.
The nose is pyramidal in shape and refers to both the external feature and the nasal cavity. It is one of the two gateways to the aerodigestive tract. Most of the airflow to the lungs occurs through the nasal cavity. Mouth breathing is not physiologic; it is a learned action. The three physiologic functions of the nose are respiration, defense, and olfaction. In respiration, airflow is modified by nasal resistance at the level of the nares and the nasal valves to allow efficient pulmonary ventilation. A major portion of the nasal airflow passes through the middle meatus. The passage of inspired air through the nasal cavity allows humidification and warming.
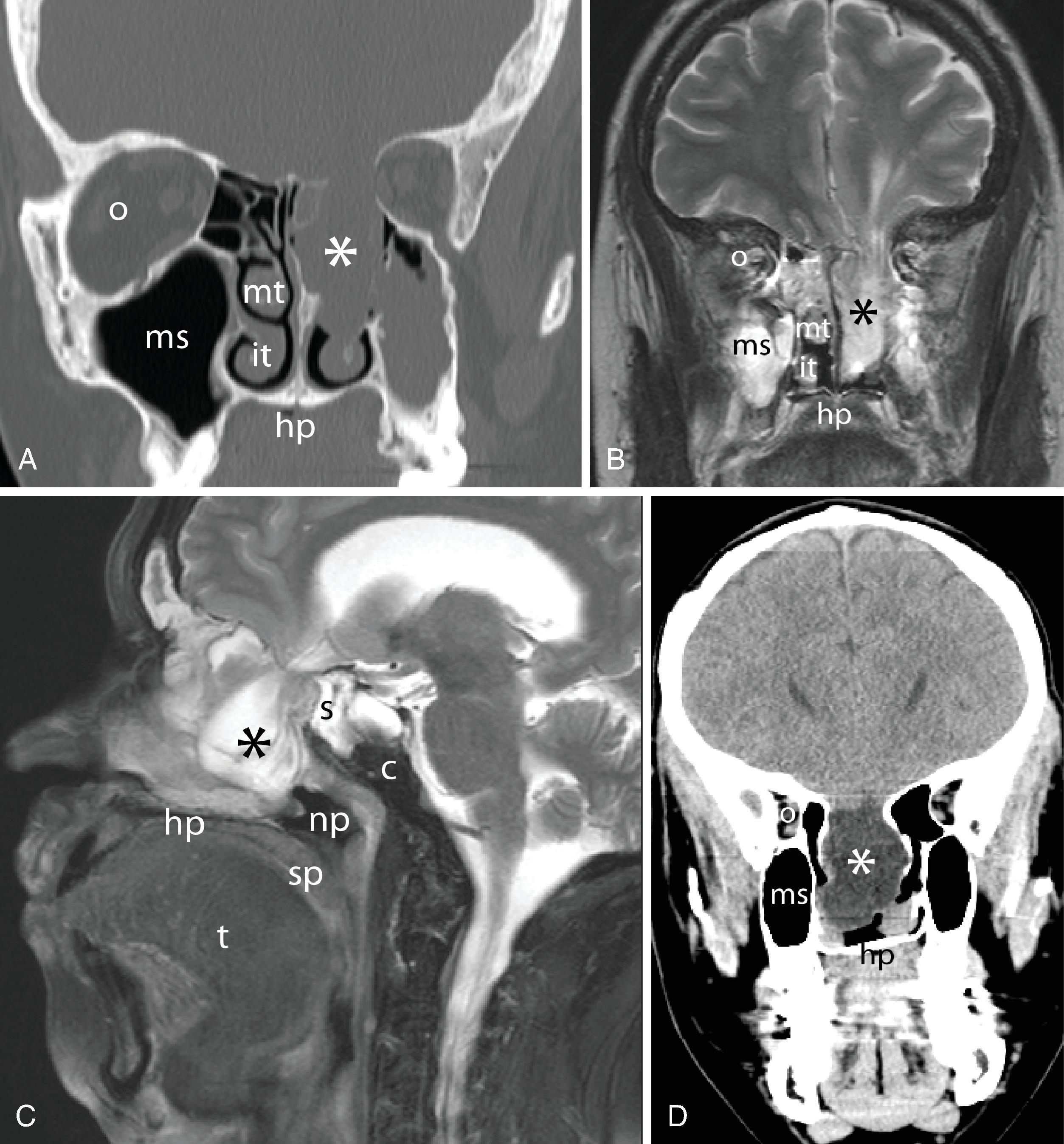
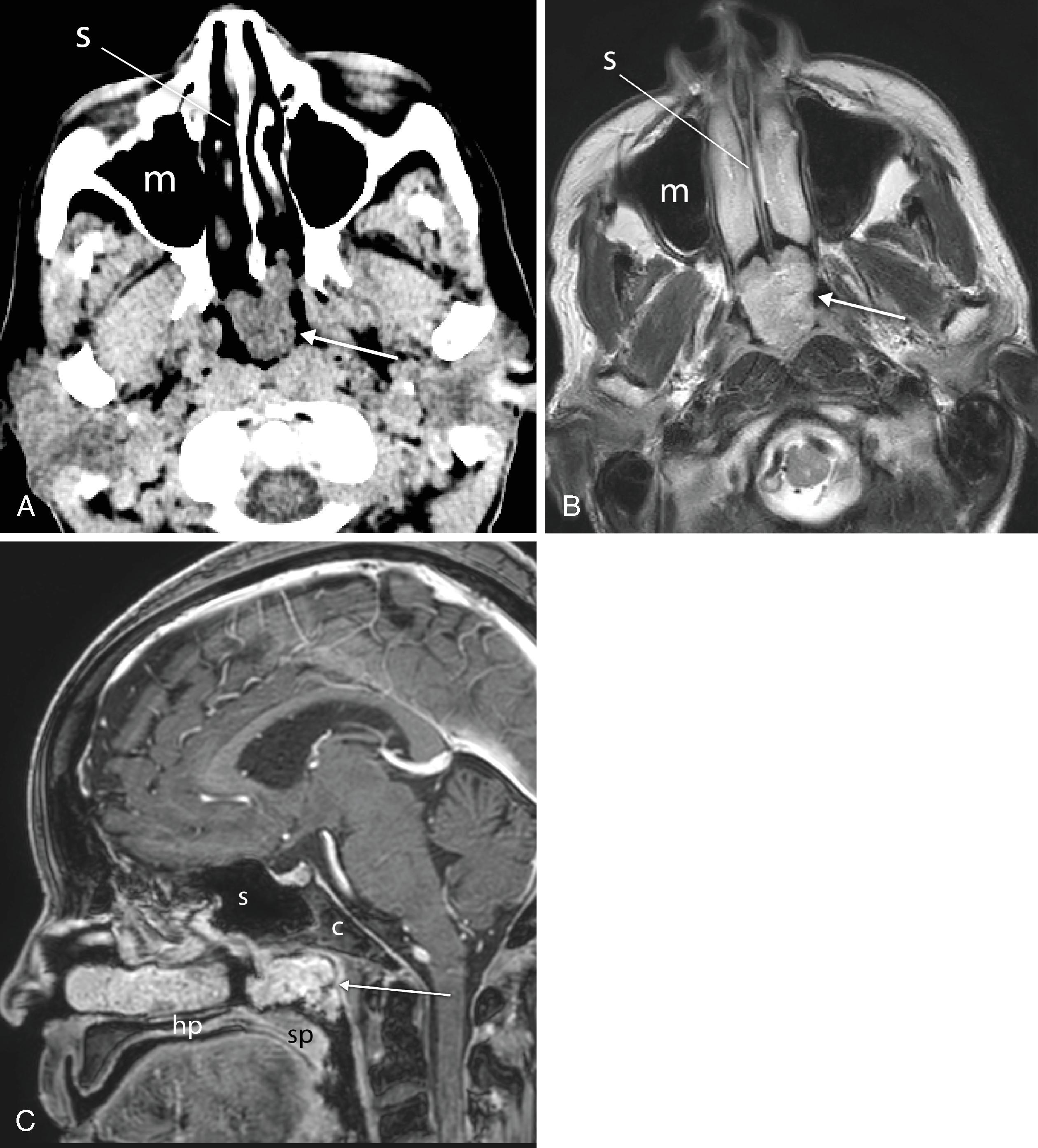
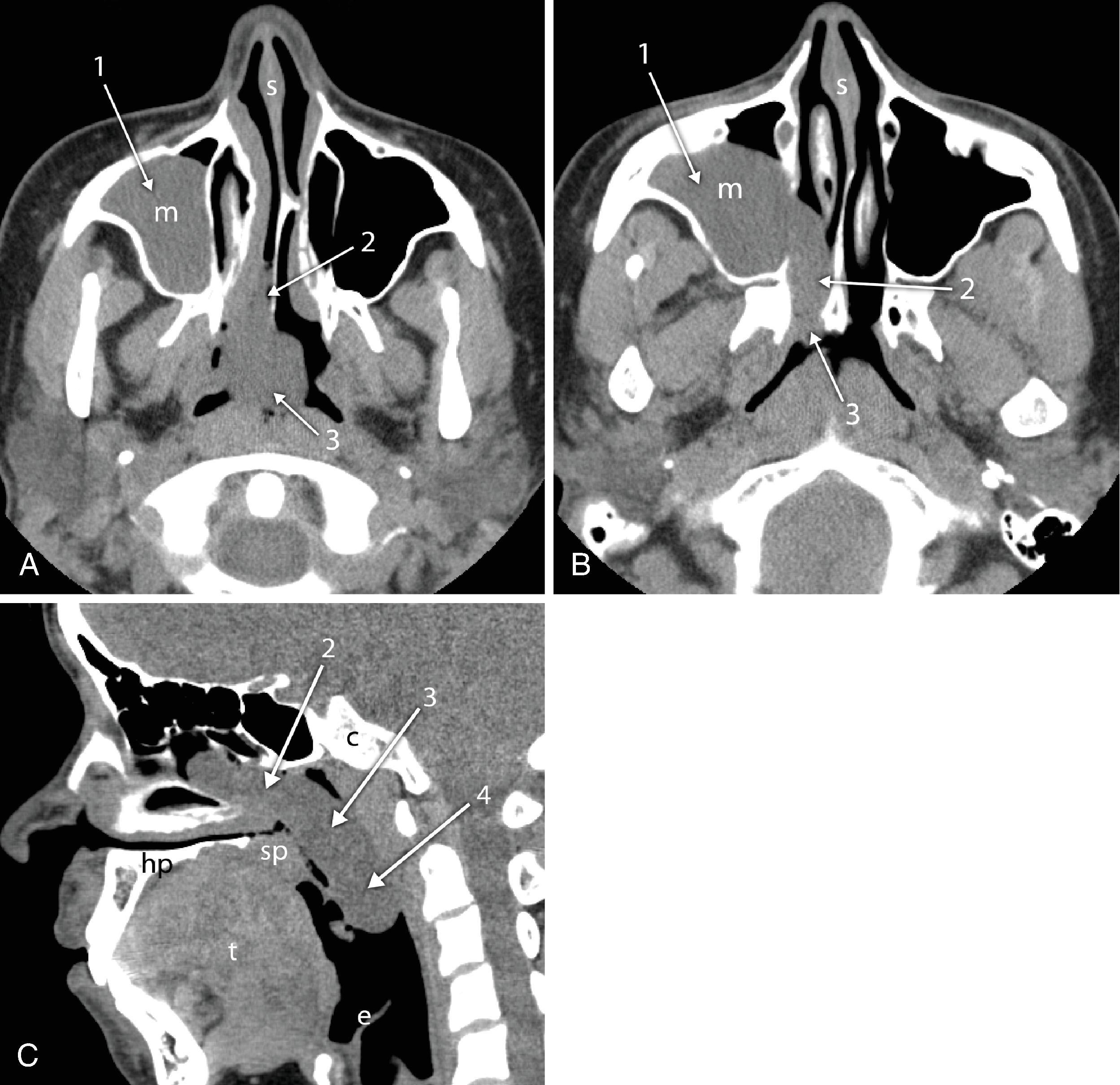
Cross-sectional imaging of the nose and paranasal sinuses allows examination of the airway from the nares to the nasopharynx. A dedicated examination of the nose and sinuses yields detailed information about this region ( Fig. 2.34A–J ). Incidental imaging of the sinuses and airway on a routine brain or spine study often allows general assessment of the airway that may be useful in the overall preoperative assessment of a patient ( Fig. 2.35A–D ). Not only is anatomy well defined by cross-sectional imaging, but it can also be a window to viewing physiologic function, in particular the nasal cycle (the cyclic variation in the thickness of the mucosa of the nasal cavity), which occurs every 20 minutes to 6 hours. , This physiologic change is manifested as an alternating side-to-side swelling of the turbinates.
The bony housing of the nose and nasal cavities is well depicted by CT; by changing the viewing windows and level, the soft tissue component can be better appreciated. The nasal cavity is divided into two cavities separated by the nasal septum. The roof of the nasal cavity is formed by the cribriform plate of the ethmoid bone; the hard palate serves as the floor. Protruding into the nasal cavity along the lateral wall of each nasal cavity are mucosa-covered scroll-like projections of bone called turbinates or conchae . They are named the inferior, middle, superior, and supreme turbinates; the latter is seen only in 60% of people. The airspace beneath and lateral to each turbinate is referred to as the meatus, into which the paranasal sinuses drain.
Relevant to anesthetic practice is an awareness that the midline craniofacial dysraphisms can be categorized into two groups: an inferior group, in which the clefting primarily affects the upper lip, with or without the nose; and a superior group, in which the clefting primarily affects the nose, with or without involvement of the forehead and upper lip (see Fig. 2.32 ). It is the inferior group that is associated with basal encephalocele (i.e., sphenoidal, sphenoethmoid, and ethmoid encephaloceles), callosal agenesis, and optic nerve dysplasia. The superior group is characterized by hypertelorism, a broad nasal root, and a median cleft nose, with or without a median cleft upper lip. The superior group is also associated with an increased incidence of frontonasal and intraorbital encephaloceles (see Fig. 2.33 ). The presence of these phenotypic features should alert the anesthesiologist to the possibility of an encephalocele intruding into the nasal cavity, and caution can be exercised when inserting a nasogastric tube or nasal airway.
Congenital nasal airway obstruction most commonly occurs in the posterior nasal cavity, secondary to choanal atresia ( Fig. 2.36 ). The atresia may be bony, membranous, or both. At birth, severe respiratory difficulty and the inability to insert a nasogastric tube more than 3 to 4 cm into the nose despite the presence of air in the trachea and the lungs suggest the diagnosis of bilateral choanal atresia. Most atresias, however, are unilateral and may remain undetected until late in life. Stenosis of the posterior nasal passages (choanae) is more common than true atresia. Approximately 75% of children with bilateral choanal stenosis or atresia have other congenital abnormalities, including Apert, Treacher Collins, and fetal alcohol syndromes. Because the pathology is usually manifested as bony overgrowth, CT is the imaging modality of choice. The major feature of choanal atresia is an abnormal widening of the vomer ( Fig. 2.37A and B ).
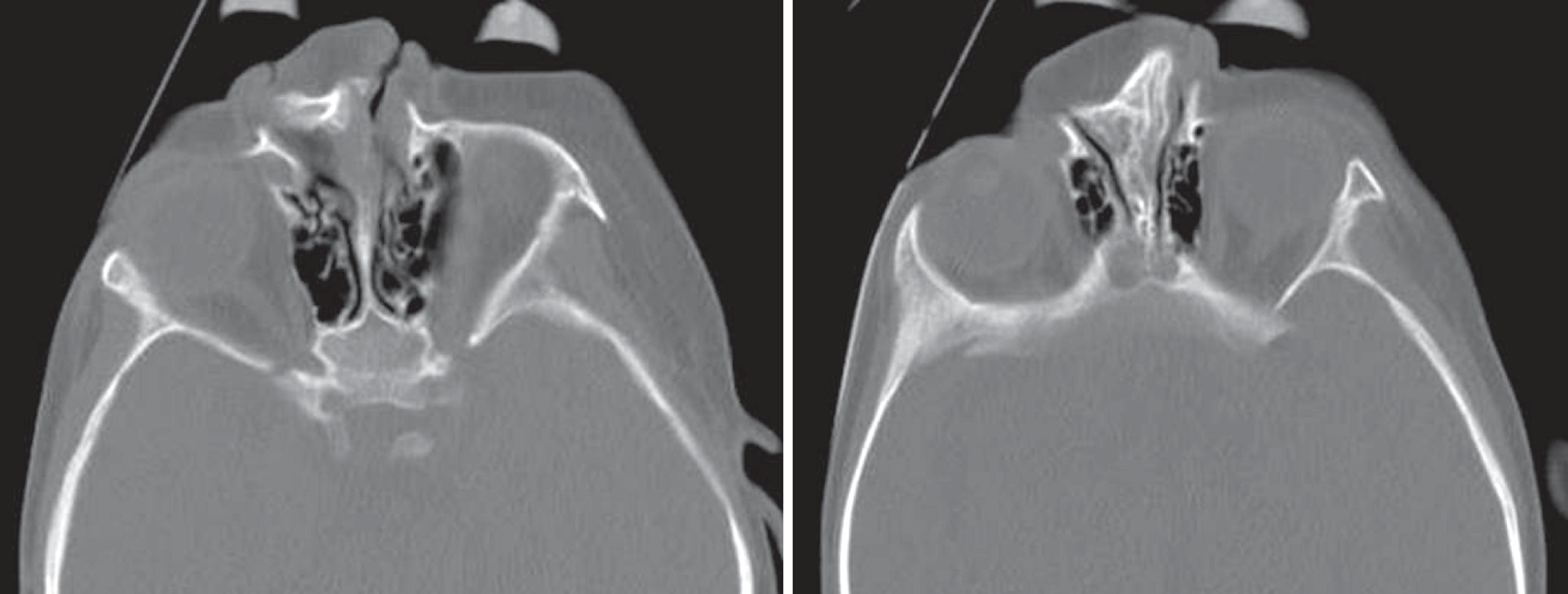
The nasal capsule, which is the cartilage around the developing nasal cavity of the embryo, serves as the foundation of the upper part of the face. During development, this cartilage eventually becomes ossified or atrophied; all that remains in adults is the anterior part of the nasal septum and the alar cartilages that surround the nostrils. The midline septal cartilage is continuous with the cartilaginous skull base. At birth, the lateral masses of the ethmoid are ossified, but the septal cartilage and the cribriform plates are still cartilaginous. Another ossification center appears in the septal cartilage anterior to the cranial base and becomes the perpendicular plate of the ethmoid. In about the third to sixth year, the lateral masses of the ethmoid and perpendicular plates become united across the roof of the nasal cavity by ossification of the cribriform plate, which unites somewhat later with the vomer below. Growth of the septal cartilage continues for a short time after craniofacial union is complete, which probably accounts for what is commonly seen as a deviated nasal septum. There are acquired etiologies of a deviated septum, such as secondary to trauma, and there are varying degrees of septal deviation. In most cases, septal deviation is not problematic for nasogastric tube or nasal airway insertion, but having prior knowledge of anatomy of the nasal cavity allows one to choose the path of least resistance.
In general, no imaging is required for work-up of uncomplicated rhinosinusitis. Views of the sinus are included in a routine CT study of the brain. The air within the sinuses abuts the bony sinus wall and appears black on both CT and MRI. To assess the bony structures, CT is preferred; however, MRI is extremely useful to differentiate inflammatory disease from tumor. Inflammatory sinus disease is characterized by increased water content; therefore, an increased T2 signal (i.e., a bright signal) is produced on T2-weighted imaging. Common inflammatory sinus disease is most often seen as T2 hyperintense lining of the walls of the sinus representing thickened mucosa. In contrast, with increased cellularity, tumor masses generally exhibit an isointense signal on T2-weighted imaging. The most common local complications of inflammatory sinusitis are swollen turbinates, polyps, and retention cysts.
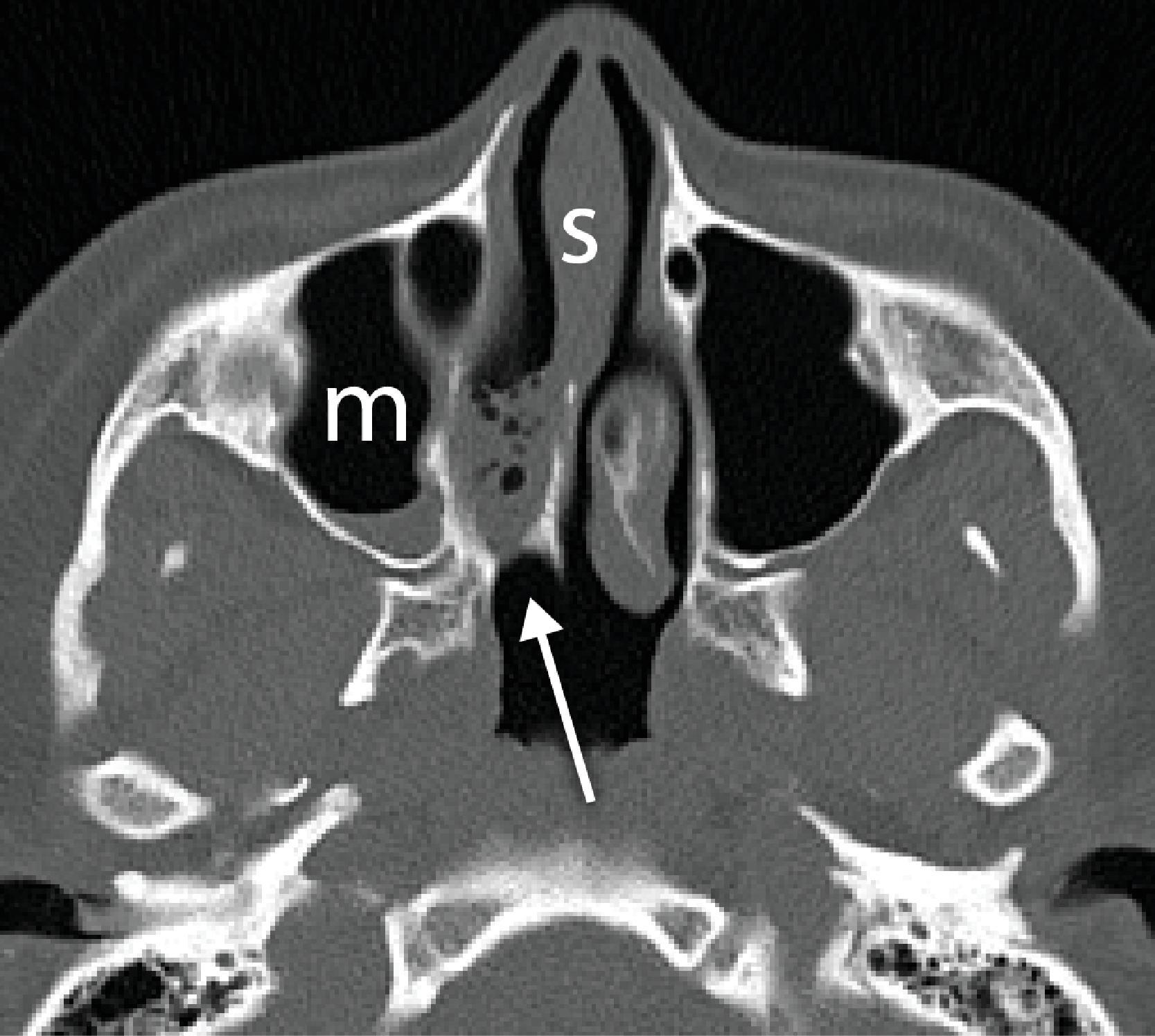
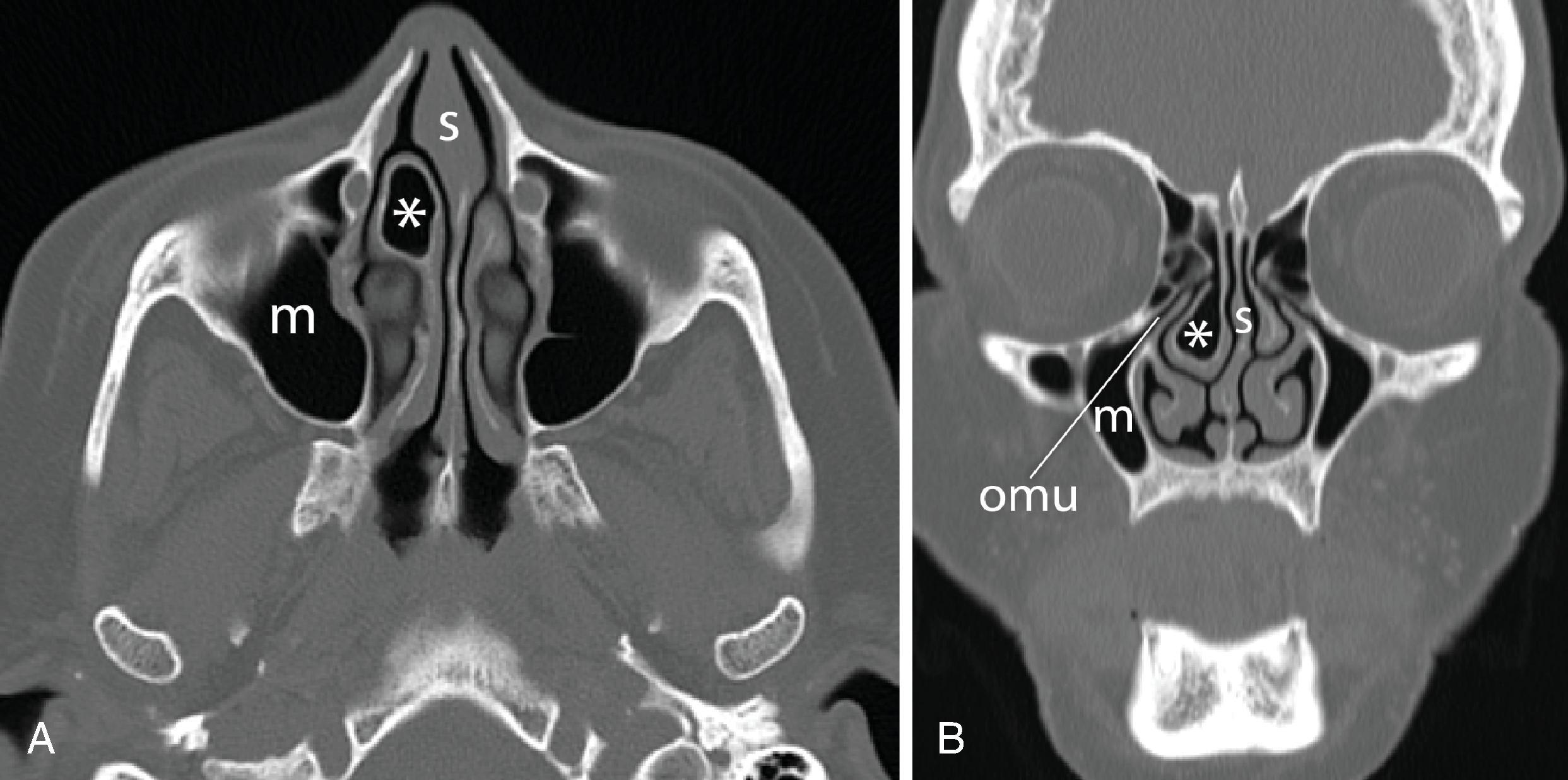
Nasal airway obstruction may result from rhinitis and turbinate hypertrophy. Common sinonasal pathologies that can affect the airway are easily recognizable on cross-sectional imaging. Examples include nasal polyps or a concha bullosa, which is an enlarged and aerated turbinate, most often the middle turbinate. Sensibly, the presence of nasal polyps or polyposis may discourage an anesthesiologist from attempting nasal intubation ( Figs. 2.38A–C and 2.39A–C ). Knowing which nasal cavity is narrowed by the presence of a concha bullosa helps to guide the selection of which nasal cavity to cannulate ( Fig. 2.40A and B ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here