Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Resection for curative intent is the gold standard for metastatic and primary hepatic tumors where feasible. Resectability, as previously discussed in other chapters, is the result of a coalescence of factors: technical/anatomic feasibility, adequacy of the predicted future liver remnant, and appropriate patient performance status to tolerate major surgery (see Chapter 101A ). Liver resections range widely in terms of technical difficulty and risks posed to the patient. This is governed largely by the disease burden and anatomic location of the lesion(s) in question. Though technically feasible in a suitable patient, some resections are prohibitive due to an insufficient future liver remnant (see Chapter 102 ). Cases of extensive multifocal or bilobar disease present a particular challenge to even the most skilled surgeon. Though techniques such as portal vein embolization and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) have arisen to optimize the future liver remnant, resectability remains limited by this. , In select cases, orthotopic liver transplantation for primary hepatic malignancies is an attractive option because it can be curative. Although a viable option that must be considered, transplantation is predicated on donor organ availability and exposes the patient to the potential toxicities of immunosuppressive medications.
Patients with unresectable disease may be candidates for ablative therapies that cause cancerous tissue necrosis by heating (radiofrequency ablation, microwave ablation, laser ablation, high-intensity focused ultrasound), irreversible electroporation (IRE), cooling (cryoablation), or injection of toxic agents (absolute alcohol) (see Chapters 96A , 96C , and 96D ).
Ablative techniques were initially reported as a treatment option for unresectable malignancies in the 1920s. The destructive potential of heat generated by passage of an electric current through tissue was originally reported by Clark et al., who described the application of a high-frequency current to cancerous tissues and the resultant thermic effect on that tissue.
During the last three decades, radiofrequency ablation (RFA) has been successfully used in the treatment of unresectable hepatic tumors. In 1990 McGahan et al. demonstrated the efficacy of ultrasound-monitored RFA in fresh bovine liver. Five years later, the first use of RFA in humans to treat small hepatocellular carcinoma (HCC) of the liver was reported. The US Food and Drug Administration (FDA) approved RFA for generic tissue ablation in 1996 and for ablation of unresectable hepatic metastases in 2000. The first randomized trial investigating the efficacy of RFA versus resection in early HCC demonstrated comparable overall survival, although further trials have uncovered discrepancies. , Table 96B.1 details the long-term survival in recent nonrandomized trials comparing RFA with surgical resection of early HCC. RFA treatment of colorectal liver metastases and intrahepatic cholangiocarcinoma has also been studied, but no data exist from randomized controlled trials (RCTs). ,
| STUDY | TREATMENT | RFA TYPE | NO. PATIENTS | TUMOR SIZE (MEAN ± SD, CM) | DISEASE-FREE SURVIVAL (%) | OVERALL SURVIVAL (%) |
|---|---|---|---|---|---|---|
| Wang et al., 2012 | LR | 208 | ≤3 | 50.8 | 77.2 | |
| RFA | Perc | 254 | ≤3 | 14.1 | 57.4 | |
| Peng et al., 2012 | LR | 74 | 1.1 ± 0.5 | 40 | 62.1 | |
| RFA | Perc | 71 | 1.2 ± 0.6 | 67 | 71.9 | |
| Imai et al., 2013 | LR | 101 | 2.14 ± 0.55 | 46.8 | 87.5 | |
| RFA | — | 82 | 1.87 ± 0.50 | 23.9 | 59.4 | |
| Tohme et al., 2013 | LR | 50 | 3.07 ± 1.17 | 34 | 47 | |
| RFA | Open/lap | 60 | 2.36 ± 0.94 | 28 | 35 | |
| Desiderio et al., 2013 | LR | 52 | ≤3 | 26.9 | 46.2 | |
| RFA | Perc | 44 | ≤3 | 22.7 | 36.4 | |
| Hasegawa et al., 2013 | LR | 5361 | ≤3 | 36.2 | 71.1 | |
| RFA | Perc | 5548 | ≤3 | 28.3 | 61.1 | |
| Lai & Tang, 2013 | LR | 80 | 2.9 ± 1.1 | 60 | 71 | |
| RFA | Lap | 31 | 1.8 ± 0.6 | 40 | 84 | |
| Wong KM et al., 2013 | LR | 46 | 2.1 ± 0.6 | 53.7 | 84.6 | |
| RFA | Perc | 36 | 1.9 ± 0.6 | 14.9 | 72.8 | |
| Peng et al., 2013 | LR | 91 | ≤5 | 33.1 | 51.9 | |
| RFA | Perc | 89 | ≤5 | 35.5 | 55.2 | |
| Zhou et al., 2014 | LR | 21 | 1.7 ± 0.3 | 76.2 | 81 | |
| RFA | Lap/perc | 31 | 1.7 ± 0.4 | 71 | 80.6 | |
| Iida et al., 2014 | LR | 15 | 2.5 ± 0.4 | 48.6 | 68.7 | |
| RFA | Perc | 33 | 2.0 ± 1.1 | 7.2 | 32.7 | |
| Park et al., 2014 | LR | 129 | 3.0 ± 1.0 | 20.6 | 64.9 | |
| RFA | Open/perc | 57 | 2.3 ± 1.0 | — | 74 | |
| Lei et al., 2014 | LR | 133 | 3.9 ± 0.6 | 58.6 | 66.2 | |
| RFA | Open/perc | 156 | 3.7 ± 0.5 | 54.5 | 66 | |
| Gory et al., 2015 | LR | 52 | ≤5 | 51 | 62 | |
| RFA | Lap/Perc | 96 | ≤5 | 24 | 37 | |
| Lee et al, 2015 | LRLR | 330330 | — | — | 7676 | |
| RFARFA | — | 369369 | ≤5 | — | 6666 | |
| Santambrogio et al., 2018 | LR | Lap | 59 | 2.09 ± 6.7 | 45 | 56 |
| Lap | 205 | 1.91 ± 5.8 | 20 | 40 | ||
| Uhlig et al., 2019 | LR | 10085 | 5.1 (3.1–8.1) b | — | 45.6 | |
| RFA | Mixed | 8211 | 2.8 (2.1-3.9) b | — | 30.7 | |
| Jianyong et al., 2019 | LR | 72 | 3.7 ± 0.5 | 47 | 48 | |
| RFA | Open | 59 | 3.8 ± 0.5 | 30 | 33 | |
| Yamashita et al., 2019 | LR | Lap | 40 | 2.4 ± 0.9 | 57 a | 76 |
| RFA | Mixed | 62 | 2.0 ± 0.6 | 55 a | 85 |
Radiofrequency ablation can be performed in the operating room by laparotomy or laparoscopy or in the radiology suite through a percutaneous approach. RFA may be combined with hepatic resection (see Chapters 101 and 102 ), hepatic artery perfusion (see Chapter 100 ), or transarterial chemoembolization (TACE) (see Chapter 94 ) along with systemic therapy, if there are other sites of metastatic disease. , , The Society of Interventional Radiology described preferred candidates for RFA as patients with inadequate liver function or comorbid conditions, patients whose tumors have an anatomic distribution not compatible with resection, and those in whom local control of tumor is necessary before transplantation.
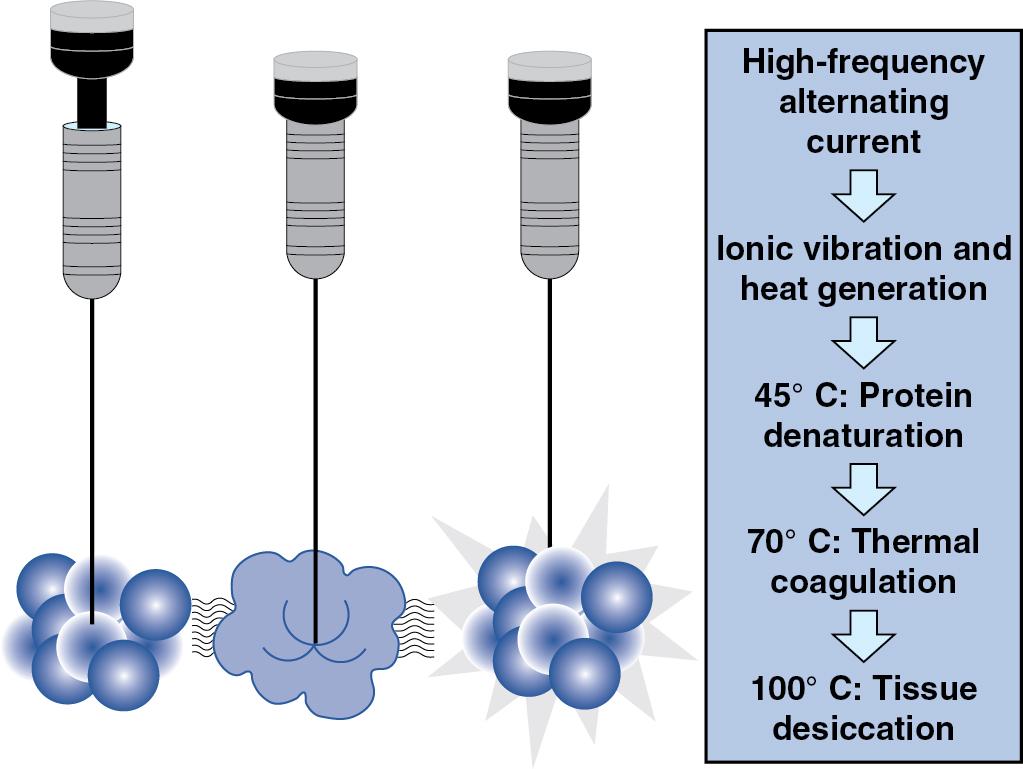
Radiofrequency ablation destroys tissues by denaturing cell proteins and causing cell death with heat ( Fig. 96B.1 ). Early work in ablation technology demonstrated that the rate of necrosis is exponentially related to the temperature generated within a tissue. Instantaneous cell death occurs at 60°C, a temperature readily achieved with commercially available RFA devices. During RFA, an electric generator propagates a rapidly alternating radiofrequency (RF) current that ranges from 300 to 500 kilohertz (kHz) across an electric circuit formed between the needle electrode and a grounding pad placed on the patient’s skin. Resistive heating is generated through intense friction by the Joule effect. The current around the ablation electrode creates a uniform zone of heat that radiates out from the electrode through conduction. The size of the ablation zone is directly related to the impedance of the tissue being ablated. If tissue impedance is low, an expanding spherical zone of ablated tissue is created. The spherical size of the ablated tissue is proportional to the square of the RF current (radiofrequency power density). Between 45° and 100°C, an exponential rise in irreversible coagulative necrosis destroys the tumor without disrupting tissue architecture. Necrosis may be limited by carbonization and charring at ablative temperatures above 100°C (because of increased tissue impedance), convective cooling from nearby blood vessels (the heat-sink effect), and reduced RF power density at longer distances from the heat source. ,
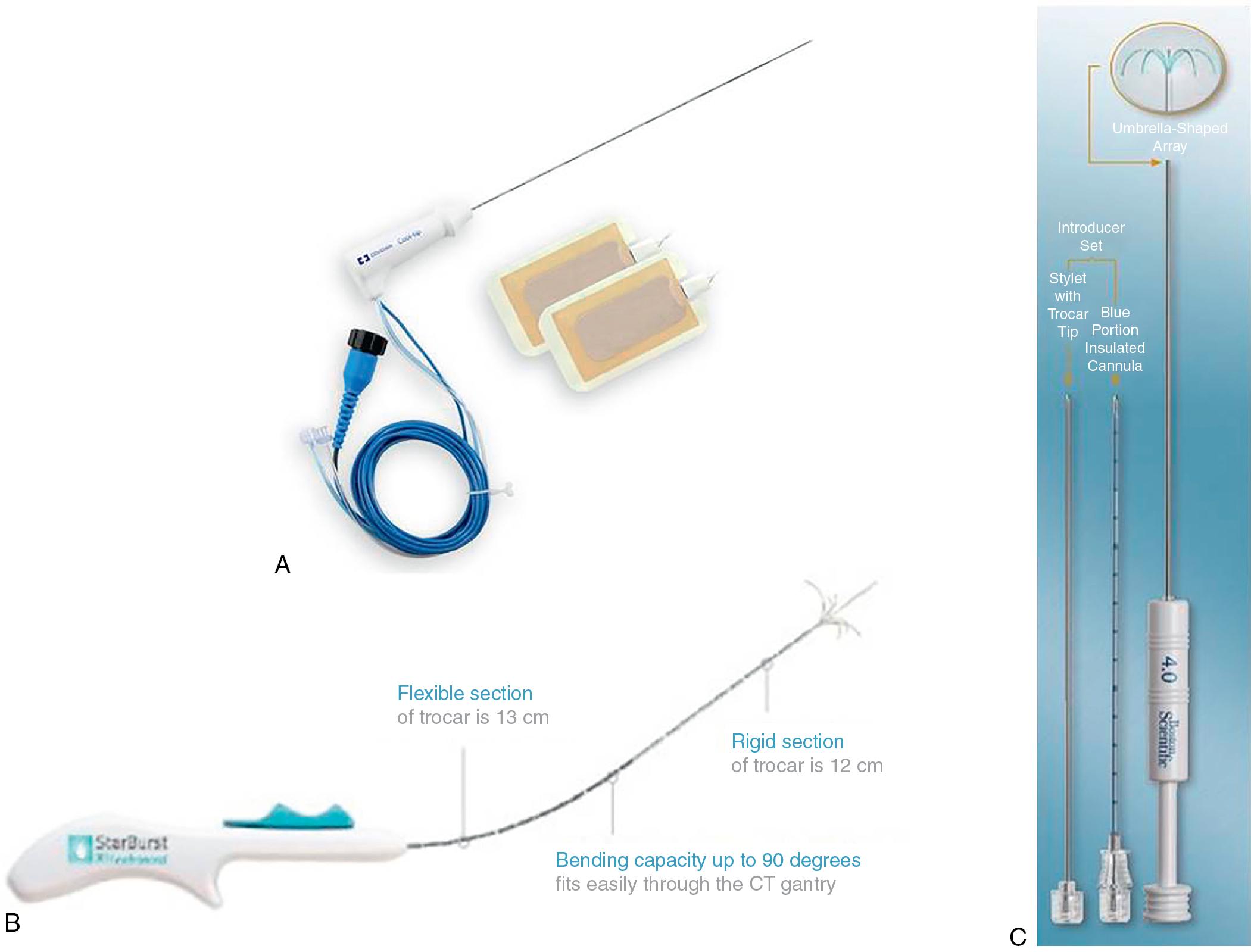
Several companies are currently producing RF electrodes for commercial use in liver ablation. AngioDynamics and Boston Scientific both offer multiarray electrodes and are commonly used. AngioDynamics StarBurst Radiofrequency Ablation System offers real-time temperature monitoring at ablative margins. A variety of percutaneous RFA devices are compatible with multiple imaging modalities ( Fig. 96B.2 ). Flexible trocars allow 90-degree articulation, and ablative fields can reach as large as 7 cm in diameter. The RF3000 Radiofrequency Generator (Boston Scientific) uses impedance to assess end points. The LeVeen Needle electrode (Boston Scientific) has multiple talons in an umbrella-shaped array that allows spherical ablation. An RCT showed that two stepwise hook extension techniques that use the 17 gauge LeVeen SuperSlim 30 mm electrode could tailor the ablation field to tumor size and shape. The Soloist single-needle electrode (Boston Scientific) is also available for treating lesions as large as 1 cm in diameter. Covidien offers the Cool-tip RF Ablation System E Series. This system has a touch-screen interface and single or three-pronged electrodes. Internal probe perfusion with cold saline reduces charring and maximizes ablative potential. Concurrent use of three separate electrodes can extend the ablative diameter to 6.5 cm. A temperature probe can be placed in adjacent liver parenchyma to monitor temperature.
Patients who present with primary liver tumors should be evaluated for curative resection or transplantation and discussed in a multidisciplinary conference. Computed tomography (CT), magnetic resonance imaging (MRI), and in some circumstances fluorodeoxyglucose (FDG) positron emission tomography (PET) (see Chapter 18 ) should be used to verify extent of disease before consideration of resection or ablation. MRI with gadoxetate disodium (Eovist), a gadolinium-based contrast agent with extracellular and hepatocyte-specific properties, is highly sensitive and specific for characterizing metastatic colorectal disease (see Chapter 15 ). If whole-body imaging techniques reveal extrahepatic sites of metastatic disease, systemic therapy should be considered. Baseline hepatic function should be assessed through laboratory data and the patient’s Model for End-Stage Liver Disease (MELD) score, and the Child-Pugh classification should be calculated to determine the severity of disease (see Chapter 4 ). A thorough history and physical examination are essential to determine patient performance and prior treatment history. If a patient’s comorbidities, performance status, tumor location, and either hepatic or systemic burden of disease preclude resection, ablative therapies should be considered.
Radiofrequency ablation can be performed via laparotomy, laparoscopy, or a percutaneous approach. The choice of technique depends on the patient’s condition, the number and location of liver tumors, and the skill of the physician performing the ablation. Each approach offers certain advantages and disadvantages that must be weighed to determine the best approach for the individual patient.
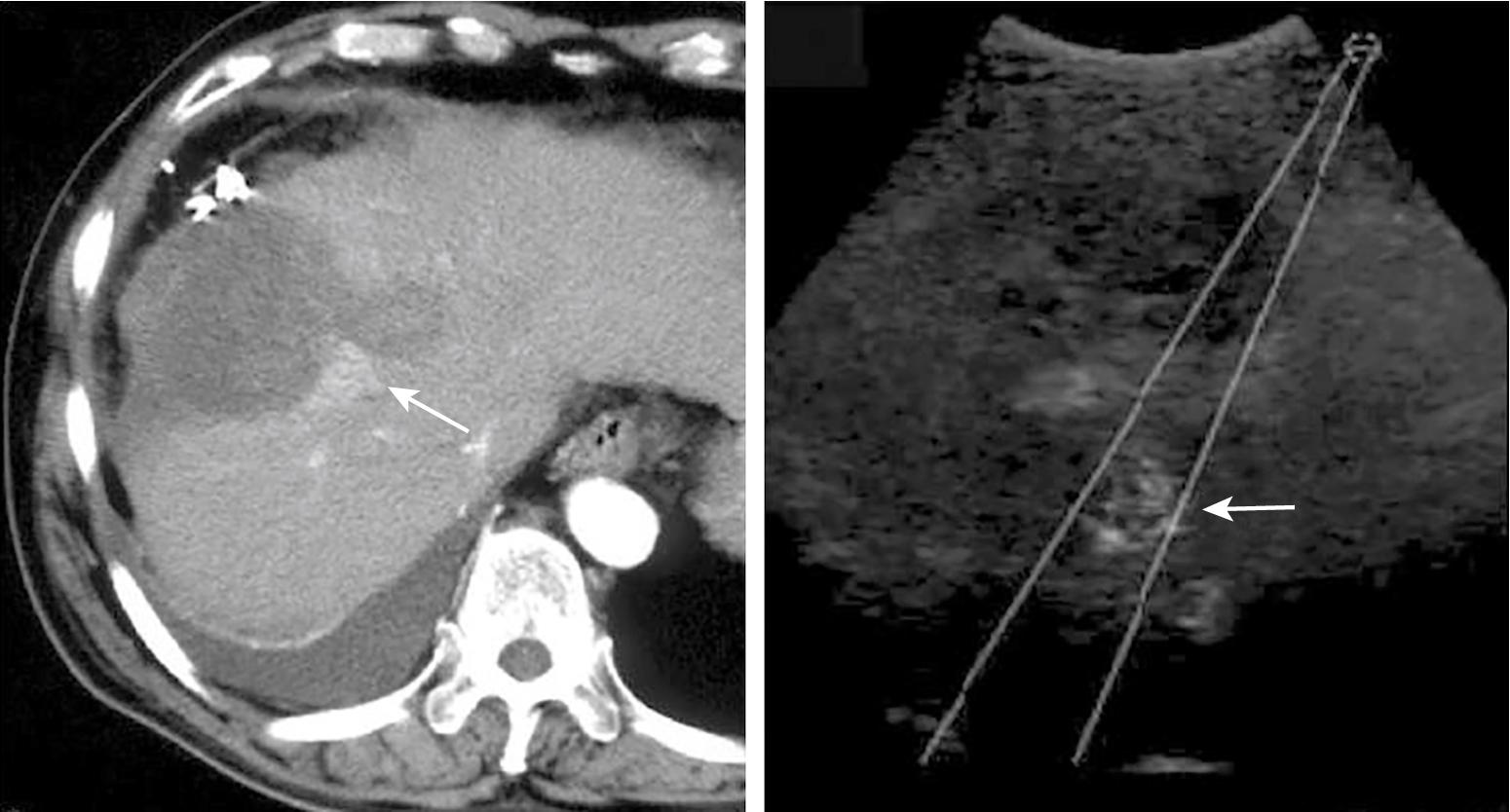
Ultrasonography, MRI, or CT imaging is used during RFA to guide the probe into a lesion and monitor the expanding hyperechogenic zone of ablation. Compared with B-mode sonography RFA, contrast-enhanced harmonic sonographic guided RFA has a higher rate of complete ablation and a lower number of treatment sessions ( Fig. 96B.3 ). Contrast-enhanced ultrasound-guided RFA has also been compared with contrast-enhanced CT-guided RFA and found to outperform CT-guided RFA in terms of obtaining a complete ablation. Each ablation should create at least a 5 mm margin of treated normal parenchyma to ensure complete tumor destruction and reduce the risk of local recurrence. Multiple overlapping ablations may be required to completely treat larger lesions and produce the necessary peripheral rim of necrosis ( Fig. 96B.4 ). In such cases, it is prudent to begin with ablation at the most posterior aspect of the lesion as it is not possible to reliably interpret this location with intervening hyperechogenic tissue from ablation. By starting at the posterior aspect of the lesion and moving anteriorly, the treating physician is able to visualize adequate ablation of the entire lesion. Between ablations, the probe should be slowly withdrawn in 2 cm increments to create sequentially overlapping zones of ablated tissue. If target temperatures are not reached, the tines should be withdrawn slightly or rotated approximately 45 degrees to increase temperature within the region of ablation. After ablation, the probe tract is cauterized as the RFA needle is slowly withdrawn, to prevent hemorrhage and tumor seeding of the needle tract.
Interventional radiologists perform percutaneous RFA under ultrasound or CT guidance or with a combination of ultrasound, CT, or MRI. The patient is given a local anesthetic, mild sedation is used, and the procedure can be performed in the outpatient setting. Intraprocedural use of contrast-enhanced CT or contrast-enhanced ultrasonographic imaging allows for identification of any areas of inadequate ablation so that additional targeted ablations can be carried out to ensure completeness of ablation.
A percutaneous approach may not be appropriate for hilar tumors proximal to vessels larger than 4 mm given the heat-sink effect and potential for inadequate ablation or for tumors that are near major bile ducts due to complications. Lesions at the periphery of the liver also may be problematic because thermal ablation can injure other visceral structures such as the small bowel, stomach, gallbladder, or transverse colon. Ablation of liver lesions located at the superior aspect of segments II, IVa, VII, and VIII may result in thermal injury to the diaphragm. Techniques to avoid diaphragmatic injury include preablation use of “artificial ascites,” or introduction of carbon dioxide between the dome of the liver and the diaphragm. ,
Percutaneous RFA has the advantage of being less invasive and may be preferable in patients unable to undergo surgery. Compared with resection in the setting of very early HCC (<2 cm) and with two or three nodules (≤3 cm), percutaneous RFA has also been found to be more cost-effective.
Radiofrequency ablation via laparotomy or laparoscopy is performed in the operating room by a surgeon while the patient is under general anesthesia. The initial surgical approach may use diagnostic laparoscopy with intraoperative ultrasound to identify any extrahepatic or intrahepatic disease not detected by preoperative imaging (see Chapter 24 ). This avoids committing a patient to a larger operation, as the long-term benefit of ablation in the setting of widely metastatic disease is questionable. A thorough intraabdominal survey examining all parietal and visceral peritoneal surfaces, the lesser sac, omentum, and viscera should be undertaken. Careful sonographic examination of the hepatic parenchyma using an articulating laparoscopic ultrasonic probe can identify liver lesions and their proximity to major vascular and biliary structures. To assist in visualizing difficult lesions in the superior or posterior aspects of segments VII and VIII, the patient can be placed in the Trendelenburg position and normal saline instilled in the right upper quadrant; this allows for more clear visualization of the parenchyma where the probe cannot contact the liver due to anatomic constraints. In a study of 308 patients undergoing laparoscopic RFA with intraoperative hepatic ultrasonography, preoperative imaging failed to identify extrahepatic disease in 12% of patients and additional hepatic lesions in 33% of patients. Another advantage of the operative approach is that a Pringle maneuver may be performed to reduce the heat-sink effect associated with ablating lesions near large vessels. RFA may also be combined with formal surgical resection to manage bilateral liver metastases. Typically, larger lesions will be targeted for resection, whereas smaller lesions in the opposite lobe would be candidates for RFA.
Laparoscopy is less invasive than laparotomy and patients often require slightly shorter hospital stays. In some cases, however, a laparotomy is unavoidable due to the location of lesions within the liver and physical limitations of the ultrasound and RFA probe under laparoscopy. Laparoscopy may not be possible if there are multiple adhesions in the abdomen from prior operations or if the tumor cannot be approached safely. In this case, a laparotomy is prudent.
After laparoscopic or percutaneous RFA, patients are either discharged home the same day or admitted to the hospital for a 23-hour observation period. RFA by laparotomy usually requires hospitalization for 3 to 5 days. Postablation hemoglobin levels, leukocyte counts, liver function tests (LFTs), and body temperature are closely monitored. Transient mild elevations in LFTs, core temperature, and leukocyte count are not uncommon and typically normalize within 3 days. Persistent elevation for 5 or more days after RFA may indicate an underlying complication and should prompt further clinical investigation.
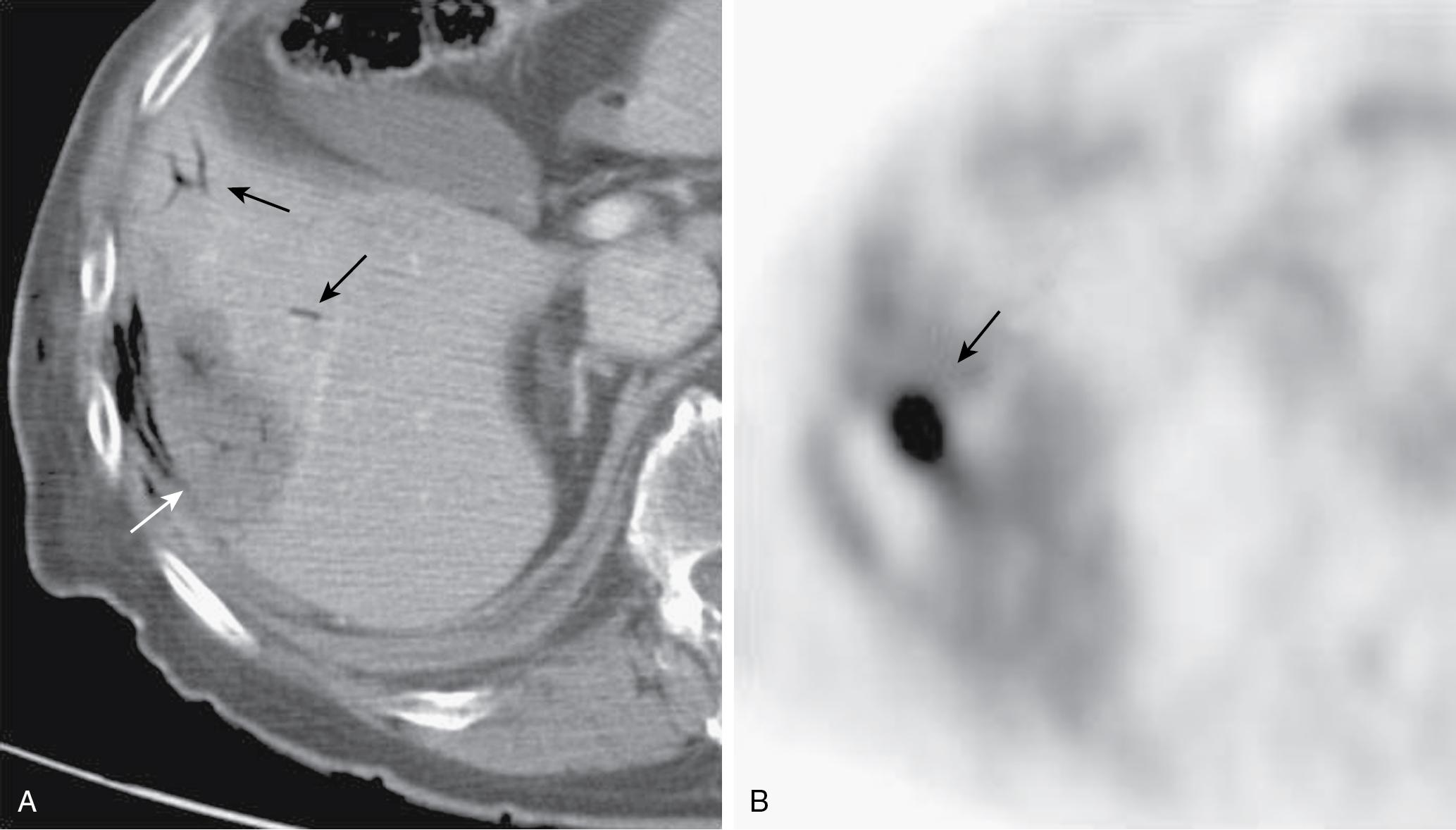
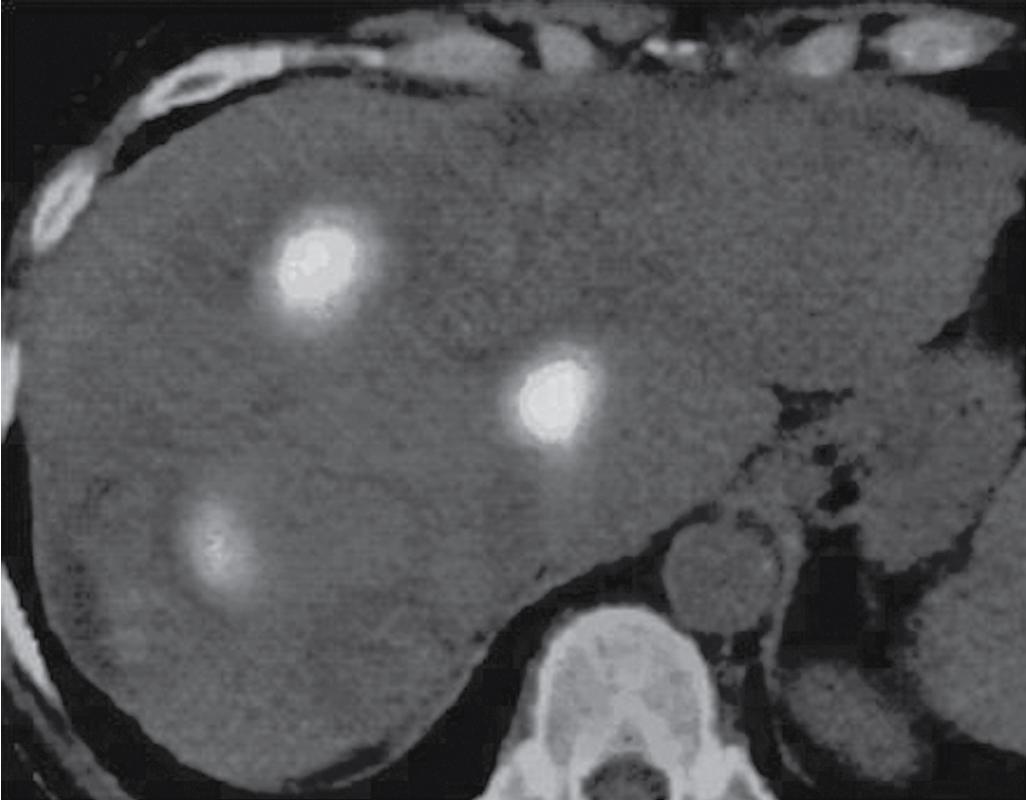
Surveillance of the tumor should use both laboratory testing and imaging. Tumor markers such as carcinoembryonic antigen (CEA) for colorectal cancer metastases and α-fetoprotein (AFP) for HCC should be drawn and compared with preablation levels. Contrast-enhanced ultrasound, MRI, CT, or PET may be chosen and should be consistently used during follow-up. , As there is not a standardized postablation surveillance protocol, the authors recommend postablation clinical follow-up at 1 month with imaging, followed by imaging with the same modality at 3 months, and then quarterly. Periablation enhancement due to inflammation can appear as residual disease, thus necessitating at least a 1-month delay before initial postablation imaging. , FDG-PET scanning is particularly sensitive in assessing the adequacy of ablation, identifying recurrence at the margin of ablation ( Fig. 96B.5 ), or distinguishing viable tumor from areas of previous surgical or ablative therapy ( Fig. 96B.6 ) (see Chapter 18 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here