Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Radiation therapy can lead to various forms of cardiovascular disease, including cardiomyopathy, heart failure, coronary artery disease, valvular heart disease, pericardial disease, and autonomic dysfunction.
Dose sparing is the single most important preventive strategy, accomplished by shifting from a large field (e.g., mantle radiation) to an involved field, from photons to protons, and from none to standard use of ancillary techniques such as breath holding and prone positioning.
Whereas advancements in the delivery of radiation therapy are expected to decrease the long-term risk of radiation-induced heart disease, no “safe” radiation dose threshold has been defined and the risk may be rather linear even in the low-dose range spectrum.
Besides dose, risk factors for radiation-induced heart disease to consider include age at time of radiation exposure (<5 years and >65 years), additional cancer therapies (especially anthracyclines), and the presence of cardiac comorbid conditions (esp., ischemic heart disease and myocardial infarction).
All risk factors should be considered to direct to the appropriate radiation techniques and patients should be appropriately counseled regarding risks and benefits mitigation strategies.
Among cardiac surveillance parameters, strain imaging might be the most promising, indicating subclinical cardiac dysfunction during and early after radiation therapy; however, the long-term significance of those changes, including implications for treatment and long-term cardiotoxicity, are unknown.
Radiation therapy (RT) is commonly used to cure, halt, or palliate the manifestations and/or symptoms of many types of cancers (e.g., Hodgkin lymphoma [HL], breast, lung, and esophageal cancer), often in combination with surgical resection and/or chemotherapy. Although RT can provide significant benefit for the treatment of cancer, it is important to recognize that RT carries significant risks to healthy tissue that may inadvertently be exposed. RT causes tissue injury primarily through the generation of oxidative stress; inflammation is seen acutely and fibrosis over time.
Radiation-induced heart disease (RIHD) is typically noted in patients who receive high doses of radiation for thoracic malignancies where the cardiac silhouette overlaps with the radiation field. RIHD can manifest in a variety of disease states, including cardiomyopathy, coronary artery disease, valvular dysfunction, and pericardial disease (see Central Illustration). The risk of RIHD is influenced by multiple factors, including the radiation dose and technique, concomitant administration of cardiotoxic chemotherapy such as with anthracycline agents, age at the time of exposure, time interval since exposure, and patient-specific cardiovascular risk factors ( Table 4.1 ). It is critical for providers to consider the risk, appropriately counsel patients, and to participate in discussions with care team providers regarding the best modes of therapies and risk mitigation strategies before radiation therapy is applied. The specific disease elements of RIHD, including screening and management, will be discussed in Chapter 26 .
| RADIATION-INDUCED HEART DISEASE | RISK FACTORS |
|---|---|
| Pericarditis | Radiation dose |
| Ischemic heart disease | History of coronary artery disease, cardiovacular risk factors, younger age at time of exposure |
| Cardiomyopathy/congestive heart failure | Anthracycline use, cardiovacular risk factors |
| Valve disease | Radiation dose, anthracycline use |
Modern RT for the treatment of HL, breast, lung, and esophageal cancer is performed using medical linear accelerators to produce megavoltage x-ray beams, with the beam being tailored to the tumor using collimators and blocks. Radiation dose is commonly described in terms of gray (Gy), the International System (SI) unit for absorbed radiation dose ( Table 4.2 ). Therapeutic doses of radiation for common malignancies range from 30 to 60 Gy delivered to the tumor. They are fractionated into multiple doses separated temporally ( Table 4.3 ). Dose-sparing is the single most important preventive strategy; a list of techniques used to reduce radiation exposure to the heart is provided in Table 4.4 .
| UNIT | TYPE OF UNIT | CONVERSION FACTOR |
|---|---|---|
| Rad a | Absorbed radiation dose | 1 rad = 0.01 Gy |
| Gray (Gy) a | Absorbed radiation dose; SI unit | 1J/kg = 1 Gy = 100 rad |
| Rem b | Dose equivalent | 1 rem = 0.01 Sv; 1 rem = 1 rad c |
| Sievert (Sv) b | Dose equivalent; SI unit | 1 Sv = 100 rem; 1 Sv = 1 Gy c |
a Rad and grays are units of energy per mass.
b Rem and sieverts are units of energy per mass adjusted by a dimensionless factor to account for a potential for biological damage.
c Rem and rad are equivalent and sieverts and grays are equivalent for radiograph and gamma radiation.
| MALIGNANCY | DOSE (Gy) |
|---|---|
| Hodgkin lymphoma | 30–36 |
| Breast cancer | 45–50 |
| Gastric carcinoma | 45–50 |
| Esophageal carcinoma | 45–50 |
| Lung cancer | 50–60 |
| Thymoma | 60 |
| TECHNIQUE | CARDIAC-SPARING MECHANISM |
|---|---|
| Breath hold | With inspiration, distance from chest wall to the heart increases |
| Prone position |
|
| Intensity modulated RT | Computerized leaves and dose planning algorithms allow for shaping of radiation field to limit cardiac dose |
| Proton beam irradiation | Utilizes difference in properties of protons compared with photons to allow for reduced dose fall off |
| Accelerated partial breast irradiation | Smaller target volume allows for possible decreased dose to the heart |
| Intraoperative RT | Smaller target volume and, in some cases. lower energy reduced dose to the heart |
Historically, large areas (e.g., mantle field radiation) and high doses of radiation (40 to 45 Gy) were used for the treatment of HL. Of note, doses of 30 Gy or higher have been associated with the greatest proportion of morbidity and mortality caused by RIHD. With the aforementioned dose-sparing techniques, such as radiation blocks (shielding), smaller dose fractions, and involved-node radiation therapy (in which only the involved nodes are irradiated), , the relative cardiovascular mortality risk could be reduced from 5.3 to 1.4. Acute manifestations, such as pericarditis, are nearly eliminated nowadays.
The cardioprotective benefit of dose fractionation is supported by experimental studies. , , For patients, the ideal fractionation regimen to reduce RIHD is not known, but hypofractionated whole breast irradiation (42.56 Gy/16 fractions) resulted in a lower rate of acute toxicity compared with conventional radiation (50 Gy/25 fractions). Otherwise there are no indications of inferior outcomes at 10 years when hypofractionated RT is compared with conventional RT. Evidence of obstructive coronary artery disease and abnormalities on myocardial perfusion scans correlate with the left ventricular volume included in the radiation therapy field. Newer radiation techniques with smaller radiation fields help to minimize the radiation volume. Intensity-modulated radiation therapy (IMRT), for example, can improve dose distribution with the ultimate goal of delivering homogeneous radiation to target tissue and minimizing doses absorbed by critical structures ( Fig. 4.1 A) . IMRT may be particularly beneficial in patients undergoing repeat RT for relapsed disease or for patients with very large tumor burden.
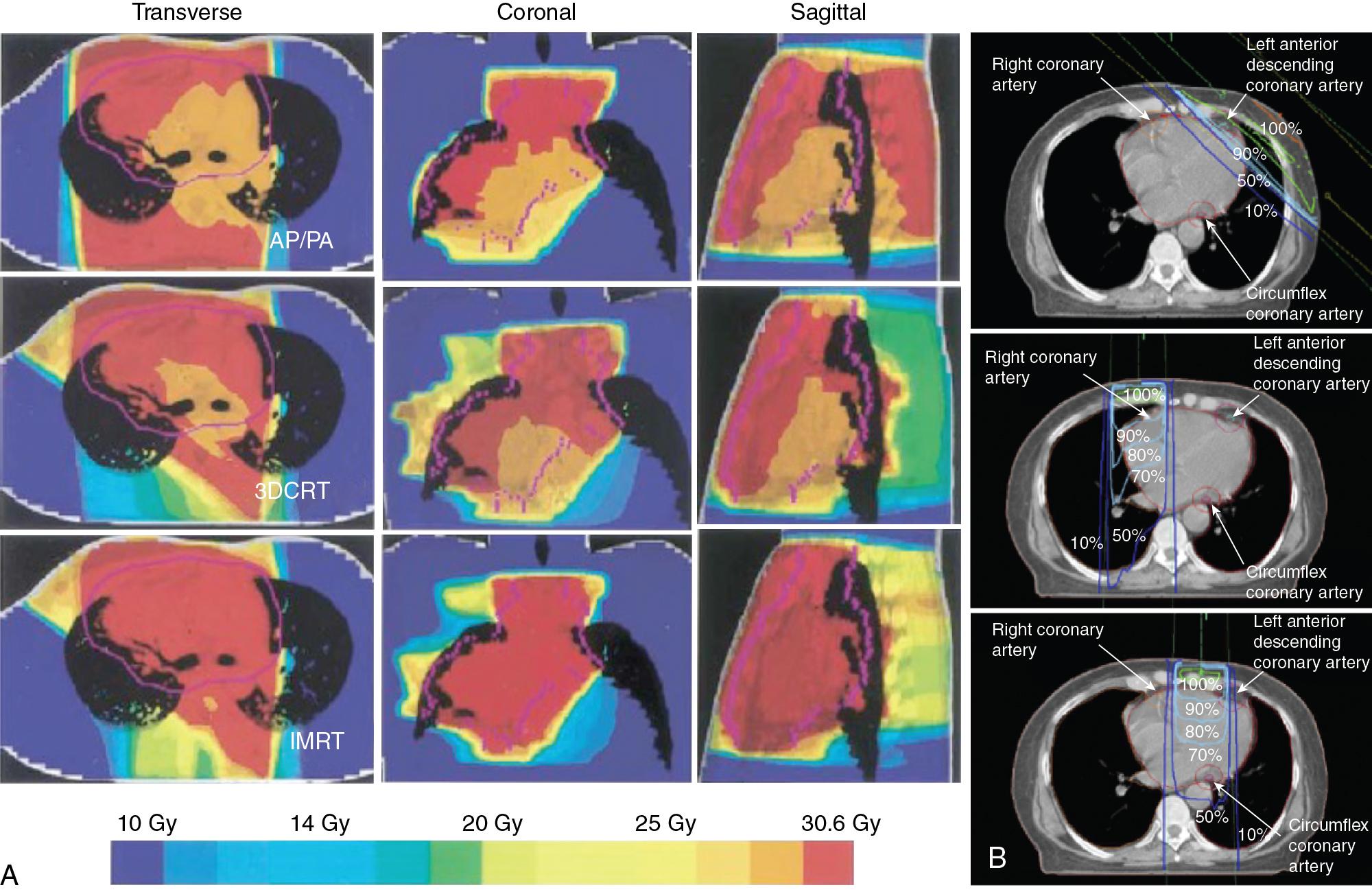
In addition to fractionation and minimizing the delivered dose, RT planning and custom radiation blocks can reduce the dose absorbed by the heart. In the case of breast cancer, RIHD is primarily a concern with RT of the left breast, which results in at least twice the radiation dose to the heart compared with that to the right breast, and a higher risk for accelerated atherosclerosis. , No safe threshold of cardiac radiation dose exists: for every gray of absorbed dose there is an approximate 7% increased risk of coronary artery disease (CAD), with a higher risk observed in patients with conventional CAD risk factors. The lowest dose that has been found to be associated with CAD is 2.8 Gy. This being said, no “safe” radiation dose threshold has been defined and the risk may be rather linear even in the low-dose range spectrum ( Fig. 4.2 ).

Strategies to reduce cardiac dose during left breast RT include computed tomography planning to ensure the heart is not within the radiation field (see Fig. 4.1 B), tangential (as opposed to anterior) radiation beams, and cardiac radiation protection blocks. Historically the myocardium involving the left anterior descending coronary artery would receive higher doses, but with contemporary RT CAD is no longer lateralized, depending on which breast is treated. , Furthermore, the recent Danish Breast Cancer Cooperative Group trials, which randomized patients to RT and surgery or surgery alone, found no increase in atherosclerotic cardiovascular disease with RT. , These more recent studies suggest that modern cardiac dose reduction strategies are proving effective at minimizing RIHD. RT of the internal mammary chain of lymph nodes is also utilized. Internal mammary node RT, which is often delivered using anterior fields, increases the absorbed dose of the heart and techniques between 1979 and 1986 continued to be associated with an elevated risk of heart failure. With modern techniques the overall cardiac toxicity of internal mammary RT appears to be low at least on short-term follow up. Internal mammary RT has not been found to result in increased RIHD-related mortality at 10-year follow up and it reduces the risk of breast cancer recurrence. ,
Children are more vulnerable to serious radiation-related complications compared with adults, both owing to growing and developing organs and to a longer life expectancy with more time to develop complications. , Adult childhood cancer survivors from the Childhood Cardiac Registry in the Netherlands had a 27% prevalence of cardiac dysfunction based on screening with echocardiography. Multivariate regression analysis showed that younger age at diagnosis (age 0 to 5 had an odds ratio [OR] of 2.94 compared with age >15 years), time since diagnosis (>25 years following diagnosis had an OR of 0.11 compared with 5 to 10 years following treatment), anthracycline dose (cumulative doses of 151 to 300 mg/m 2 had an OR of 3.98, whereas cumulative doses of >450 mg/m 2 had an OR of 10.58 when compared with 1 to 150 mg/m 2 ), and thoracic radiotherapy were all predictive of left ventricular dysfunction. It is worth noting that two-thirds of the patients had also received chemotherapy with anthracyclines, which are known to cause cardiomyopathy. Children and adolescents with HL treated with radiation and/or chemotherapy at Stanford Hospital between 1961 and 1991 had high risks of death from heart disease (relative risk [RR], 29.6), death from acute myocardial infarction (MI; RR, 41.5), and death from other cardiac disease (RR, 21.2). Patients who died had received between 42 and 45 Gy of radiation to the mediastinum between the ages of 9 and 20 years. A second analysis on a broader spectrum of 2232 patients with HL treated with radiation therapy (72% mantle field) at Stanford Hospital between 1960 and 1990 confirmed a 45 times higher risk of death owing to acute MI with radiation exposure before age 19.
As alluded to above, the risk of RIHD and cardiac mortality increases with a longer duration after radiation therapy. In the Stanford study noted above, the risk of cardiac death increased substantially with increasing duration of follow up: the relative risk of death caused by an acute MI was 2 for patients within 5 years of treatment compared with a relative risk of 5.6 at 20 years following radiation. A retrospective cohort study of the medical records of 2524 Dutch patients with HL treated between 1965 and 1995 evaluated more types of cardiac disease, which showed a significant increase in the risk of ischemic heart disease, as well as cardiomyopathy/congestive heart failure (HF) and valvular heart disease even 35 years or more after treatment. The highest risk of cardiac disease was noted in patients treated before age 25 and in those who were 20 to 47 years posttreatment (when compared with those patients treated 5 to 10 years ago). Similar results have been shown for patients with breast cancer where the excess risk of cardiac death may not be apparent until up to 20 years following treatment in patients with left-sided disease compared with right-sided disease. In a large, long-term follow-up study of 7425 patients with breast cancer, longer follow-up time was associated with increasing risk of cardiovascular death: HR 1.0 at ≤10 years, HR 1.5 at 10 to 20 years, and HR 2.9 >20 years. A review of 19 published reports on patients with breast cancer is likewise in agreement with the conclusion that extended follow-up duration is associated with excess risk of cardiac mortality.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here