Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the previously untreated neck, lymph node infiltration follows a predictable pattern.
Except for nasopharyngeal carcinoma, selective irradiation can be applied for patients with an N0 to N1 neck.
Three-dimensional (3D) delineation of the neck levels should be performed on thin-slice contrast-enhanced computed tomography sections following the consensus guidelines.
A high (>90%) control rate of the node-negative neck is achieved with a prophylactic (a total dose equivalent to 50 Gy with 2 Gy per fraction over 5 weeks) dose of ionizing radiation; for the node-positive neck, a higher dose of ionizing radiation (a total dose equivalent to 70 Gy with 2 Gy per fraction over 7 weeks) is required.
After node-positive neck treatment with radiotherapy (RT), neck dissection should be restricted to those patients who did not achieve a complete response.
Postoperative irradiation is required for a pathologic node-positive neck (>pN1).
Postoperative concomitant chemoradiotherapy (CRT) is required for patients with extracapsular rupture.
Proton beam irradiation can be designed to yield a uniform dose across the target and to deliver virtually zero dose deep and a lower dose proximal to the target.
CyberKnife is a frameless robotic radiosurgical device that has been developed mainly to treat extracranial lesions.
Intensity-modulated radiation therapy (IMRT) has the ability to treat an irregularly shaped target regardless of size, while minimizing the dose delivered to the surrounding normal tissues.
Treatment of esthesioneuroblastoma typically consists of combination therapy, either surgery followed by RT, possibly with chemotherapy, or CRT followed by surgery for salvage.
Sinonasal undifferentiated carcinoma portends a poor prognosis owing to its high rates of locoregional failures and distant metastases.
The preferred irradiation technique for chordomas is proton therapy.
High rates of locoregional control can be achieved using IMRT for nasopharyngeal carcinoma.
Distant metastases are the predominant cause of treatment failure in patients with nasopharyngeal carcinoma.
The preferred treatment approach for sinonasal cancer is complete surgical resection followed by postoperative RT.
Assessment and treatment of regional lymph nodes (LNs) in the neck are of utmost importance in the management of patients with head and neck squamous cell carcinoma (HNSCC). The philosophy of treatment of the neck has evolved over past decades. Radiation oncologists and head and neck surgeons progressively realized that extensive treatments were associated with more morbidity, but not always with a better oncologic outcome, than less extensive procedures. Today, a comprehensive approach of the treatment of the neck needs to be multidisciplinary, and it must take into account the quality of life of the patients without jeopardizing cure and survival. A better understanding of the patterns of LN metastasis has promoted the use of not only selective dissection but also selective irradiation in subgroups of patients.
This chapter discusses only the management of the neck for oral cavity, oropharyngeal, hypopharyngeal, and laryngeal squamous cell carcinomas (SCCs), and malignant skull base tumors including nasopharyngeal carcinoma. The specific management of the neck for thyroid, nasal cavity, paranasal sinus, and skin cancers is covered in the respective chapters that deal with these anatomic sites.
The head and neck region has a rich network of lymphatic vessels that drain from the base of the skull through the jugular, spinal accessory, and transverse cervical nodes to the venous jugulosubclavian confluence or the thoracic duct on the left side and the lymphatic duct on the right. The whole lymphatic system of the neck is contained in the celluloadipose tissue delineated by aponeurosis that envelopes the muscles, vessels, and nerves. The lymphatic drainage is mainly ipsilateral, but structures like the soft palate, tonsils, base of the tongue, posterior pharyngeal wall, and especially the nasopharynx have bilateral drainage. On the other hand, sites such as the true vocal cords, paranasal sinuses, and middle ear have few or no lymphatic vessels at all.
The nomenclature of head and neck LNs has been complicated by various confusing synonyms that are still in use in major textbooks and articles. More recently, several expert bodies have proposed the adoption of systematic classifications aimed at standardizing the terminology. Following the description by Rouvière, the TNM Atlas proposed a terminology that divides the head and neck LNs into 12 groups. In parallel to this classification, the Committee for Head and Neck Surgery and Oncology of the American Academy for Otolaryngology–Head and Neck Surgery has been working on a classification, the so-called Robbins classification, which divides the neck into six levels that include eight node groups ( Fig. 117.1 ). This classification is based on the description of a level system that has been used for some time by the Head and Neck Service at the Memorial Sloan Kettering Cancer Center (MSKCC). Because one of the objectives of the Robbins classification was to develop a standardized system of terminology for neck dissection procedures, only the LN groups routinely removed during neck dissection were considered. The terminology proposed by Robbins was recommended by the Union for International Cancer Control (UICC). A comparison between the TNM classification and the Robbins terminology is shown in Table 117.1 . The major advantage of the Robbins classification over the TNM terminology is the definition of the boundaries of the node levels. The delineation of these boundaries is based on anatomic structures—such as major blood vessels, muscles, nerves, bones, and cartilage—that are easily identifiable by the surgeon during neck dissection procedures. It is beyond the scope of this chapter to go into a detailed description of the node levels, and the reader is referred to a review article outlining the anatomic basis of the neck node classification.
| TNM Atlas | Robbins Classification | ||
|---|---|---|---|
| Group | Terminology | Level | Terminology |
| 1 | Submental nodes | IA | Submental group |
| 2 | Submandibular nodes | IB | Submandibular group |
| 3 | Cranial jugular nodes | II | Upper jugular group |
| 4 | Medial jugular nodes | III | Middle jugular group |
| 5 | Caudal jugular nodes | IV | Lower jugular group |
| 6 | Dorsal cervical nodes along the spinal accessory nerve | VA | Posterior triangle group |
| 7 | Supraclavicular nodes | VB | Posterior triangle group |
| 8 | Prelaryngeal and paratracheal nodes | VI | Anterior compartment group |
| 9 | Retropharyngeal nodes | ||
| 10 | Parotid nodes | ||
| 11 | Buccal nodes | ||
| 12 | Retroauricular and occipital nodes | ||
The eighth edition (2017) of the UICC's/AJCC's TNM classification of malignant tumor staging for neck node metastasis is presented in Table 117.2 . For oropharyngeal, the new classification distinguishes between p16-negative (HPV negative) and p16-positive (HPV positive) tumors. This classification does not apply to nasopharyngeal carcinoma (NPC), thyroid, or skin cancers. The classification for nodal staging does apply irrespective of the modality used for the neck assessment (i.e., clinical examination or imaging). However, the routine use of computed tomography (CT) or magnetic resonance imaging (MRI) and, in expert hands, ultrasonography is recommended, especially to assess nodes not clinically identifiable—such as retropharyngeal, intraparotid, or superior mediastinal nodes—or in patients for whom clinical palpation of the neck is less sensitive; for example, those with thick or small necks. Lastly, it should be emphasized that the Nx classification only applies when the neck was not assessed or could not be assessed.
| Stage | Definition for Oral Cavity, Hypopharyngeal, Laryngeal, and p16-negative Oropharyngeal Tumors |
|---|---|
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis in a single ipsilateral node, ≤3 cm in greatest dimension |
| N2a | Metastasis in a single ipsilateral node, >3 cm but ≤6 cm in greatest dimension |
| N2b | Metastasis in multiple ipsilateral nodes, ≤6 cm in greatest dimension |
| N2c | Metastasis in bilateral or contralateral nodes, ≤6 cm in greatest dimension |
| N3a | Metastasis in a lymph node >6 cm in greatest dimension without extracapsular spread |
| N3b | Metastasis in single or multiple node(s) with extracapsular spread |
| Stage | Definition for p16-Positive Oropharyngeal Tumors |
| Nx | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis |
| N1 | Unilateral metastasis ≤6 cm in greatest dimension |
| N2 | Metastasis in contralateral nodes ≤6 cm in greatest dimension |
| N3 | Metastasis in a lymph node >6 cm in greatest dimension |
The metastatic spread of head and neck tumors into the cervical LNs is rather consistent and follows predictable pathways, at least in the neck that has not been violated by previous surgery or RT. In Figs. 117.2 to 117.6 , the frequency of metastatic LNs is expressed as a percentage of node-positive patients.
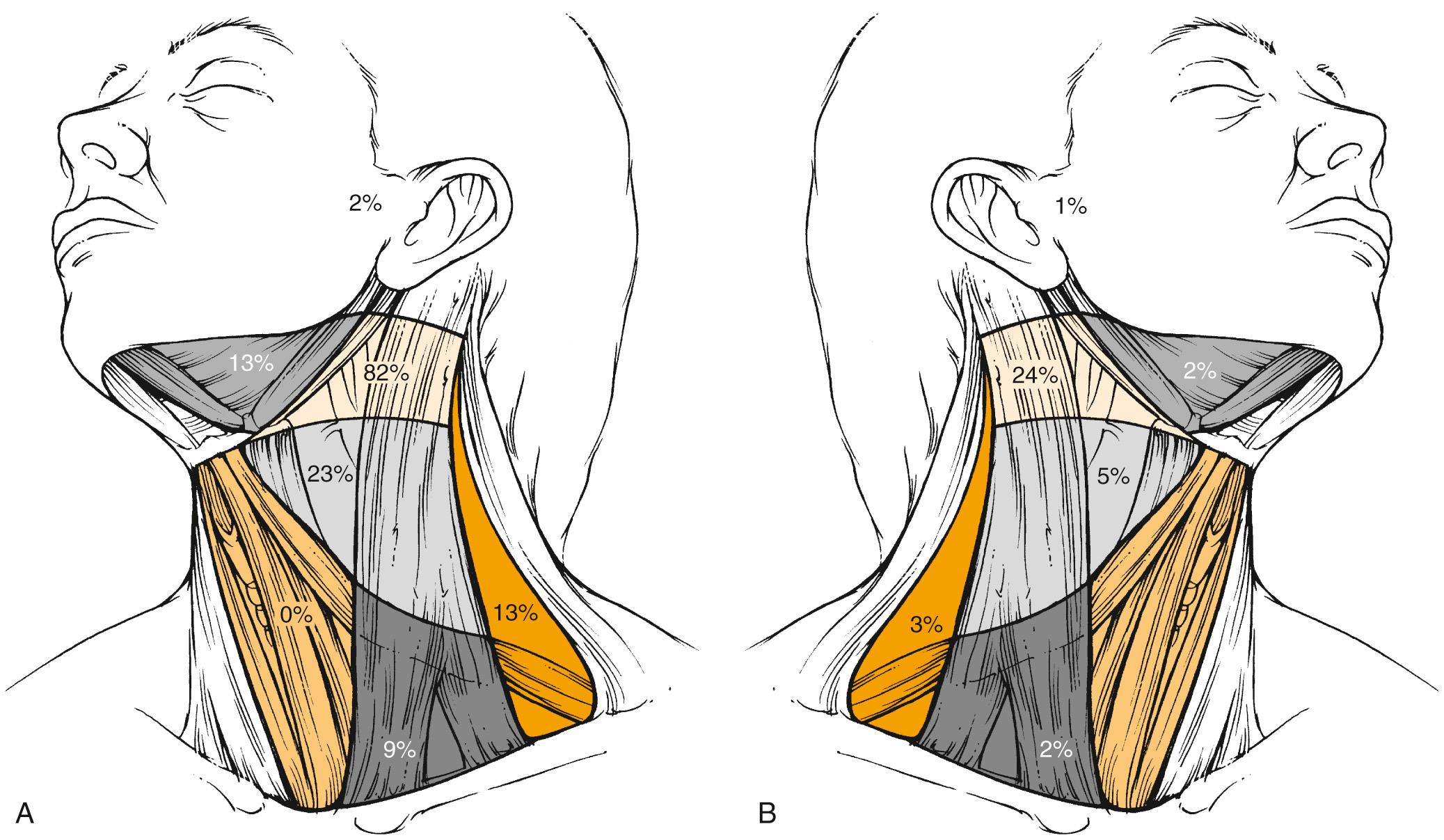
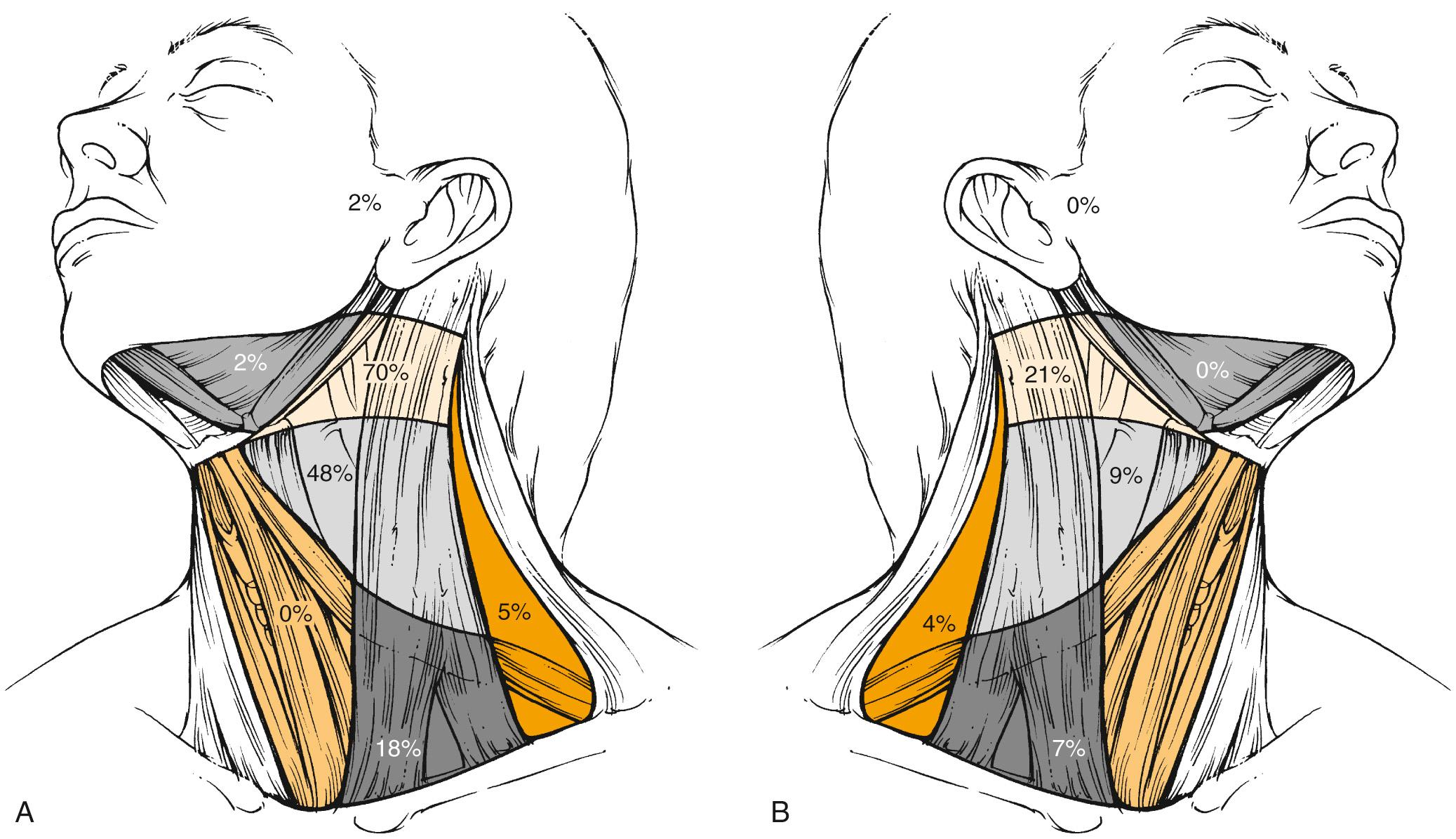

The frequency of neck node metastases and the distribution of clinically involved nodes depend to a major extent on the primary tumor site. Typically, hypopharyngeal tumors have the highest propensity for nodal involvement, which occurs in 70% of cases. Cranial and anterior tumors, such as oral cavity tumors, mainly drain into levels I, II, and III, whereas more caudally located tumors, such as laryngeal tumors, mainly drain into levels II and III, and to a lesser extent into Levels IV and V. Contralateral nodes are rarely involved except for midline tumors or tumors in those sites where bilateral lymphatic drainage has been reported, such as the soft palate, base of the tongue, and pharyngeal wall. Even in these tumors, the incidence of contralateral involvement is much lower; for example, in base-of-tongue tumors with clinically positive nodes, it reaches 31% in contralateral level II compared with 73% in ipsilateral level II (data not shown). Interestingly, node distribution follows the same pattern in the contralateral neck as in the ipsilateral neck. Except for nasopharyngeal tumors, involvement of ipsilateral level V is a rather rare event and occurs in less than 1% of all oral cavity tumors, in less than 10% of all oropharyngeal and laryngeal tumors, and in about 15% of all hypopharyngeal tumors. It almost never occurs in contralateral level V. Nasopharyngeal tumors behave differently than other head and neck tumors. These highly lymphophilic tumors carry almost the same risk of nodal involvement in the ipsilateral and contralateral neck, with the preferential involvement of level V in almost one-third of patients. On the other hand, the incidence of level I infiltration is much lower.
Metastatic LN involvement in the neck depends on the size of the primary tumor, and it increases with the T stage. In the series reported by Bataini and colleagues, 44% of patients with a T1 tumor had clinical LN involvement; this increased to 70% for patients with T4 lesions. However, there are no data to suggest that the relative distribution of involved neck levels varies with the T stage.
Retropharyngeal LNs represent a special entity inasmuch as they are usually not clinically detectable. The incidence of retropharyngeal LN involvement can only be precisely estimated from series in which CT or MRI of the retropharynx has been systematically performed as part of the diagnostic procedure. Retropharyngeal node involvement occurs in primary tumors that arise from or invade the mucosa of the occipital and cervical somites (e.g., of the nasopharynx, pharyngeal wall, and soft palate). Interestingly, the incidence of retropharyngeal LNs is higher in patients in whom involvement of other neck node levels has also been documented. However, in clinically N0 patients with nasopharyngeal tumors, and to a lesser extent in those with posterior pharyngeal wall tumors, the incidence of retropharyngeal nodes is still significant, between 16% and 40%. Also, as already described for the other LN levels, involvement depends on the T stage and is typically lower for T1 tumors. However, accurate figures are not available.
The distribution of pathologic LN metastasis in patients with primaries of the oral cavity, oropharynx, hypopharynx, and larynx can be derived from retrospective series in which a systematic radical neck node dissection was proposed as part of the initial treatment procedures. In essence, retrospective series are biased regarding patient and treatment selection, but these series from MSKCC are the largest and most consistent data ever published on the subject; the results of these retrospective studies are shown in Table 117.3 . The data are presented in terms of the number of neck dissections with positive LNs over the total neck dissection procedures and are expressed as percentages. The vast majority of patients—over 99% of N0 neck patients and 95% of N+ neck patients—had only unilateral treatment, and no distinction between the ipsilateral and contralateral neck was made.
| Tumor Site | Elective RND | Therapeutic (Immediate or Subsequent) RND | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | I | II | III | IV | V | No. | I | II | III | IV | V | |
| Oral cavity | 192 | 20% | 17% | 9% | 3% | 1% | 323 | 46% | 44% | 32% | 16% | 3% |
| Oropharynx | 48 | 2% | 25% | 19% | 8% | 2% | 165 | 15% | 71% | 42% | 27% | 9% |
| Hypopharynx | 24 | 0% | 12% | 12% | 0% | 0% | 104 | 10% | 75% | 72% | 45% | 11% |
| Larynx | 79 | 5% | 19% | 20% | 9% | 3% | 183 | 7% | 57% | 59% | 30% | 4% |
Overall, metastatic disease was detected in 33% of the elective neck dissections and in 82% of the therapeutic neck dissections. As already observed with the pattern of clinical metastatic LNs, the distribution of pathologically confirmed metastatic LNs depended on the primary tumor site. Typically, in clinically N0 patients, metastatic LNs were observed in levels I to III for oral cavity tumors and in levels II to IV for oropharyngeal, hypopharyngeal, and laryngeal tumors. This pattern of node distribution is similar to that determined from the clinical palpation of the neck. It should be noted that the T-stage distribution was different in the various groups. Of patients with laryngeal tumors, 53% (42/79) had T3 to T4 tumors, mainly of the supraglottis, compared with 27% (52/192), 25% (6/24), and 17% (8/47) in patients with oral cavity, hypopharyngeal, and oropharyngeal tumors, respectively. Such a difference in T stage presumably explains the high incidence of microscopic node metastases in the larynx group.
When considering the patients who underwent therapeutic neck dissection, the pattern of metastatic node distribution was similar to that observed in clinically N0 patients, with the difference being that significant pathologic infiltration of an additional nodal level was typically observed (i.e., level IV for oral cavity tumors and levels I and V for oropharyngeal, hypopharyngeal, and, to a lesser extent, laryngeal tumors). Overall, this observation illustrates the gradual infiltration of various node levels in the neck.
The incidence of pathologic nodal infiltration depends on the tumor stage. In a study of 515 patients with node-negative oropharyngeal SCC, primarily tonsillar fossa and base-of-tongue tumors, who benefited from some form of neck node dissection (mainly modified radical neck node dissection), pathologic nodes were observed in 6.8% (9/132), 16.4% (36/220), 21.8% (22/101), and 12.9% (8/62) for T1, T2, T3, and T4 tumors, respectively. The rather low incidence of pathologic neck infiltration for the T4 tumors in that series is probably the result of a selection bias; patients with locally advanced tumors are mainly referred for concomitant chemoradiation (CRT).
Few data are available on the pattern of pathologic node distribution in the contralateral neck. Bilateral neck dissection was only performed when the surgeon perceived a high risk of contralateral node involvement; for example, tumors of the oral cavity or the oropharynx that reach or extend beyond the midline and hypopharyngeal and supraglottic tumors. Obviously, in such cases, bilateral radical neck dissection was never performed, so an accurate estimate of the pattern of node involvement in levels I to V of the contralateral neck is not possible. Furthermore, in almost every study, data on both sides of the neck were pooled for presentation.
Kowalski and colleagues presented data on 90 patients who underwent bilateral supraomohyoid neck dissection and in whom the pattern of node distribution in each side of the neck was reported separately. The majority of these patients had SCC of the lip or oral cavity. In the ipsilateral neck, pathologic infiltration in levels I, II, and III reached 20%, 15%, and 15%, respectively. In the contralateral neck, corresponding values reached 13%, 11%, and 0%, respectively. These figures are in agreement with data on clinical node distribution, which shows that both sides of the neck exhibit a similar pattern of node distribution, but with a lower incidence in the contralateral neck. In the study of Olzowy and colleagues, in the 352 node-negative patients with oropharyngeal SCC who underwent bilateral neck node dissection, the overall incidence of bilateral neck node infiltration reached 20.8% and was typically lower for T1 tumors (12%) compared with T2 to T4 tumors (20% to 25%). Bilateral node infiltration was predominantly observed in base-of-tongue and soft palate SCC. In that study, no data were reported on the nodal distribution by level in the contralateral neck. Foote and colleagues reported the rate of contralateral neck failure in a limited series of 46 clinically N0 patients with base of the tongue tumors treated by some form of glossectomy and ipsilateral neck dissection. None of these patients received postoperative RT. Ten patients (22%) had contralateral neck recurrence, and the most common sites were in levels II, III, or IV. It appears that in two of these patients, recurrence was also observed at the primary site. The development of delayed contralateral neck metastases was not related to the clinical or pathologic extent of the base of the tongue tumor. O'Sullivan and associates reported a retrospective series of 228 patients with tonsillar carcinoma who were treated on the primary tumor and ipsilateral neck only with RT. The vast majority of these patients had T1 to T2 and N0 to N1 disease. Contralateral recurrence in the neck was only observed in eight patients (3.5%), including five patients with local recurrence. No contralateral neck recurrence was observed in the 133 N0 patients. Although not significant because of the small number of events, involvement of midline structures, such as soft palate and base of tongue, appeared to be a prognostic factor for contralateral neck recurrence. A recent reevaluation of such finding was performed on 102 subsequent patients treated unilaterally by RT from 1999 to 2014, and confirmed the value on unilateral treatment in those selected patients. Similar results were reported in a series of 101 node-negative tonsil carcinoma patients (mainly T1 to T3) treated unilaterally. Only two neck recurrences were observed in the contralateral neck.
The data presented in the previous sections indicate that metastatic LN involvement of primary SCC of the oral cavity, pharynx, and larynx typically follows a predictable pattern. Data on both clinical and pathologic neck node distribution and on neck recurrence after selective dissection procedures support the concept that not all the neck node levels should be treated as part of the initial management strategy of head and neck primaries of squamous cell origin. However, the clinician should bear in mind that the data on which such a concept is based have come from retrospective series, and thus they may include possible bias (e.g., patient selection, series from the pre-imaging area), which could limit their validity.
Tables 117.4 to 117.7 present recommendations for the selection of the target volumes in the neck for oral cavity and pharyngo-laryngeal SCCs . These guidelines can be applied irrespective of the treatment modality (surgery or RT) . The discussion of the choice between these two modalities is beyond the objective of this chapter but should be considered relative to the neck stage, treatment options for the primary tumor, performance status of the patient, toxicity and functional outcome of the two modalities, and the institutional policy agreed on by a multidisciplinary head and neck tumor board. The denomination of the levels is an extension of the Robbins classification to also include levels that are not routinely removed during a neck dissection procedure.
| Tumor Location | Staging (8th AJCC/TNM Classification) | |||
|---|---|---|---|---|
| N0 a | N1 a | N2a–N2b–N3 | N2c | |
| Mobile tongue | I–III b | I–III b , c | I–V d , e | f |
| Floor of mouth | I–III b | I–III c | I–V d , e | f |
| Lower gum | I–III b | I–III c | I–V d , e | f |
| Upper gum | I–III | I–III c | I–V d , e | f |
| Cheek mucosa | I–III, IX b | I–III, IX b | I–V d , e | f |
| Inferior lip | I–III b | I–III b | I–V d , e | f |
| Hard palate | I–III | I–III | I–V d , e | f |
a Ipsilateral treatment for tumor of the gum, cheek, floor of mouth and mobile tongue not approaching the midline by more than 5 mm.
b Include level IVa for tumor of the tip of the mobile tongue.
c Include level IVa and V when positive node is located in level II or III.
d Include level IVb when positive nodes are located in level IVa.
e Include level VIIb when positive nodes are located in the upper level II.
f As a function of the staging on each side of the neck separately.
| Tumor Location | Staging (8th AJCC/TNM Classification) | |||
|---|---|---|---|---|
| N0 a | N1 a | N2a–N2b–N3 a | N2c | |
| Tonsil fossa | II–IVa | II–IVa b , c | Ib–V, VIIa b , c | d |
| Soft palate | II–IVa | II–IVa b , c | Ib–V, VIIa b , c | d |
| Base of tongue | II–IVa | II–IVa b , c | Ib–V, VIIa b , c | d |
| Posterior pharyngeal wall | II–IVa, VIIa | II–IVa, VIIa b , c | Ib–V, VIIa b , c | d |
a Ipsilateral treatment for tonsil fossa tumor not infiltrating the soft palate and/or the base of tongue.
b Include level IVb when positive node is located in level IVa.
c Include level VIIb when positive nodes are located in the upper level II.
d As a function of the staging on each side of the neck separately.
| Tumor Location | Staging (8th AJCC/TNM Classification) | |||
|---|---|---|---|---|
| N0 a | N1 a | N2a–N2b–N3 a | N2c | |
| Piriform sinus | II–IVa b | Ib–V, VIIa b , c , d | Ib–V, VIIa b , c , d | e |
| Retro-cricoïde area | II–IVa b | Ib–V, VIIa b , c , d | Ib–V, VIIa b , c , d | e |
| Posterior pharyngeal wall | II–IVa, VIIa b | Ib–V, VIIa b , c , d | Ib–V, VIIa b , c , d | e |
a Ipsilateral treatment for tumor of the lateral wall of the piriform sinus.
b Include level VI when tumor infiltrates the apex of piriform sinus and/or the junction with the cervical esophagus.
c Include level IVb when positive node is located in level IVa.
d Include level VIIb when positive nodes are located in the upper level II.
e As a function of the staging on each side of the neck separately.
| Tumor Location | Staging (8th AJCC/TNM Classification) | |||
|---|---|---|---|---|
| N0 a | N1 a | N2a–N2b–N3 a | N2c | |
| Supraglottic larynx | II–IVa b | II–IVa b , c , d | II–V, b , c , d | e |
| Glottic larynx f | II–IVa b ,g | II–IVa b , c , d | II–V, b , c , d | e |
| Subglottic larynx | II–IVa, VI | II–IVa, VI c , d | II–V, VI c , d | e |
b Include level VI in case of trans-thyroid cartilage infiltration and/or subglottic extension.
c Include level IVb when positive node is located in level IVa.
d Include level VIIb when positive nodes are located in the upper level II.
e Ss a function of the staging on each side of the neck separately
f For T2 glottic SCC only infiltration the floor of the ventricule, no neck treatment can be considered.
For clinically N0 patients with HNSCC of the oral cavity, oropharynx, hypopharynx, and larynx, selective treatment of the neck is appropriate. Typically, levels I to III should be treated for oral cavity tumors, and levels II to IVa are treated for oropharyngeal, hypopharyngeal, and laryngeal tumors. Robbins has suggested that elective treatment of level IIb is probably not necessary for N0 patients with a primary tumor of the oral cavity, larynx, or hypopharynx. On the other hand, two studies have suggested that level IVa be included in the treatment of the mobile tongue because of the high incidence (>10%) of skip metastases. However, this finding has not always been observed. Retropharyngeal nodes should be treated in tumors of the posterior pharyngeal wall. For subglottic tumors, tumors with subglottic or transglottic extension, or hypopharyngeal tumors with esophageal extension, level VI nodes also should be included in the treatment volume.
As proposed by Byers, similar guidelines also could be recommended for N1 patients without radiologic evidence of extracapsular infiltration. However, when an involved LN is located at the boundary with a level that has not been selected in the target volume, it has been recently recommended to extend the selection to include the adjacent level. Typically, this will only apply for oropharyngeal tumors with a single LN in level II at the boundary with level Ib or for an oral cavity tumor with an N1 node in level III at the boundary with level IV.
For patients with multiple involved nodes (N2b), the available data suggest that adequate treatment should include levels I through V. However, Level I could be omitted for laryngeal tumors, and level V could be omitted for oral cavity tumors with neck involvement limited to levels I through III. Elective treatment of the retropharyngeal nodes should be recommended for oropharyngeal and hypopharyngeal tumors. As for N0 patients, level VI nodes also should be treated for subglottic tumors, those with subglottic or transglottic extension, or hypopharyngeal tumors with esophageal extension. Recently, for patients with involved nodes in the upper neck (i.e., upper level II), it has been proposed to extend the upper limit of the target volume to include the retrostyloid space (level VIIa). Similarly, the supraclavicular fossae also should be included in the target volume in case of lower neck involvement (i.e., level IVb or Vb nodes).
No data on the distribution of pathologic metastatic neck nodes in patients who come to medical attention with a single ipsilateral large node (N2a or N3) or with bilateral or contralateral nodes (N2c) are available. For patients with a single large node, in the absence of data, it appears prudent not to recommend selective treatment. Also, for N3 patients, the type of treatment of the neck is likely to be dictated by the local extension of the node into the adjacent structures (e.g., paraspinal muscles, parotid gland, blood vessels). For N2c patients, one proposal is to consider each side of the neck separately (e.g., selective treatment in both sides for a small single node in each side, selective treatment for a small single node in one side, and more extensive treatment in the other side in the case of multiple nodes). The rationale behind this proposal is that although patients with bilateral neck nodes harbor a worse global prognosis, regional control will only be affected by the extent of disease on each side of the neck.
Elective treatment of the contralateral N0 neck is still a gray area, and treatment is likely to be based on clinical judgment rather than on strong scientific evidence. Typically, patients with midline tumors or tumors that originate from or extend to a site that has bilateral lymphatic drainage—base of tongue, vallecula, posterior pharyngeal wall—are thought to benefit from bilateral neck treatment, whereas well-lateralized tumors, such as those at the lateral border of the tongue or in the retromolar trigone or tonsillar fossae, can be spared contralateral treatment. Also, it has been reported in tumors of the oral cavity, pharynx, and larynx that the risk of contralateral neck metastases increases with involvement of the ipsilateral neck. Putting all these data together, the clinician could recommend restricting the treatment to the ipsilateral neck for N0 and N1 tumors of the lower gum (not approaching the midline), lateral border of the mobile tongue, upper gum, cheek, retromolar trigone, tonsillar fossa (without extension to the base of the tongue, soft palate, or posterior pillar), and lateral wall of the piriform sinus. For those tumors that manifest with larger or multiple ipsilateral nodes (N2a, N2b), it is not known whether a unilateral neck treatment is adequate, and the decision is left to the treating physician. In the other situations, where elective contralateral neck treatment is recommended, the selection of the node levels to be treated should follow similar rules to those for the ipsilateral neck.
In patients with p16-positive oropharyngeal SCC, there are no data to suggest that the selection of the neck node levels should be different than for the p16-negative tumors. Owing to the new TNM classification, it is recommended to consider the number, the location, and the laterality of the positive nodes to select the levels to be treated.
Because of the lymphophilicity of NPC tumors, bilateral treatment of levels II through V and treatment of retropharyngeal nodes is recommended even for patients with a node-negative neck ( Table 117.8 ). For patients with bulky involvement of level II, additional treatment of level Ib might be considered.
| Tumor Location | Staging (8th AJCC/TNM Classification) | ||
|---|---|---|---|
| N0 a | N1–N2 a | N3 a | |
| All sites | II–V, VIIa, VIIb | II–V, VIIa, VIIb b , c , d | Ib–IVb, Va,b,c, VIIa, VIIb |
b Include level IVb when positive node is located in level III or IVa.
c Include level Vc when positive nodes are located in level Va,b.
d Inclusion of level Ib can be considered in case of bulky infiltration of level II.
In principle, a similar approach should apply for the definition of the node levels to be irradiated postoperatively. However, if the selection criteria for postoperative RT can be agreed upon (i.e., extracapsular spread, patients with a metastatic node >3 cm in diameter or with more than one metastatic node), irradiation of levels I through V is typically performed. As for primary RT, the retrostyloid space (level VIIb) and the supraclavicular fossae (level IVb) should be included in the target volume, depending on the location of the metastatic nodes. For laryngeal tumors, level I could be omitted. For oral cavity tumors, postoperative irradiation of level V could be omitted in the case of metastatic nodes located in level I and/or II only. Retropharyngeal and level VI nodes should be treated as mentioned earlier.
The clinician should question the necessity of systematic bilateral postoperative irradiation in situations where a proper bilateral neck node dissection has been performed and where one side of the neck was free of disease on pathologic examination. In a retrospective study from St-Luc University Hospital in Brussels (Belgium) involving 105 patients with HNSCC (50% oral cavity, 85% pathologic stages III and IV) treated primarily with up-front surgery, including unilateral or bilateral neck dissection according to tumor location, and for whom postoperative irradiation was only delivered on the positive neck after pathologic examination, the locoregional disease control rate reached 78% at 5 years (V. Grégoire, unpublished data). Among the 24 patients who had locoregional recurrence, only seven patients did so in an area treated by surgery but that did not receive postoperative irradiation; it is unknown whether postoperative irradiation on that area would have prevented a locoregional recurrence. This study suggests that a new paradigm should be properly tested and validated regarding the extent of postoperative irradiation.
Since the late 1990s, several authors have proposed recommendations for the delineation of the neck node levels. A critical review of the various proposals was undertaken in 2003 in collaboration with representatives of the major European and North American clinical cooperative groups to generate an international set of guidelines for the delineation of the neck node levels in the node-negative neck. Subsequently, few amendments were proposed to take into account the node-positive neck, the delineation of extranodal structures in case of muscular infiltration, the inclusion of the retrostyloid space in case of infiltration of level II, and the inclusion of the supraclavicular fossae in case of infiltration of level IV and/or Vb.
Although it appears that these recommendations are well accepted and used among the radiation oncology community, they are also associated with some shortcomings. First, not all the neck node areas described in the TNM atlas were included, and in particular, the nodal areas in the lower and posterior neck typically involved in NPC were not properly discussed; also the LN regions draining the face, the scalp, and those nodal regions close to the base of skull received inadequate attention. Second, it appears that the description of the anatomic boundaries of some of these levels (e.g., in the lower neck) was not sufficiently accurate and thus required some interpretation from the users. Third, in the guidelines for the node-positive neck, the proposed extension around positive nodes into normal structures to generate the clinical target volume (CTV) was both arbitrary and potentially imprecise. In this context, a new set of international guidelines applicable to the node-negative, the node-positive, and the postoperative neck was proposed. It is beyond the scope of this chapter to present an in-depth discussion of the boundaries of the various levels. Interested readers are invited to refer to the original publication. Fig. 117.7 presents several CT cuts of the neck with selected levels.

With the use of intensity-modulated radiation therapy (IMRT), there is no longer a standard recipe for how to set up the field sizes and borders according to bony landmarks. Instead, the irradiation technique should be selected and adapted so that the entire planning target volume receives the prescribed dose within the adopted dose-volume constraints and in full respect of the International Commission on Radiation Units and Measurements recommendations.
The dose prescription depends on various factors—such as elective versus therapeutic irradiation, the use of combined modality treatment, planned neck node dissection, and postoperative irradiation—that are beyond the scope of this section for comprehensive review. Typically, for primary RT, an elective dose equivalent to a dose of 50 Gy in about 2 Gy per fraction over 5 weeks and a therapeutic dose equivalent to a dose of 70 Gy in about 2 Gy per fraction over 7 weeks will be prescribed. In 2018, irradiations are typically performed using a simultaneous integrated boost (SIB) approach with therapeutic dose of 70 Gy (35 × 2 Gy per fraction over 7 weeks) and an elective dose of 54.25 Gy (35 × 1.55 Gy per fraction over 7 weeks).
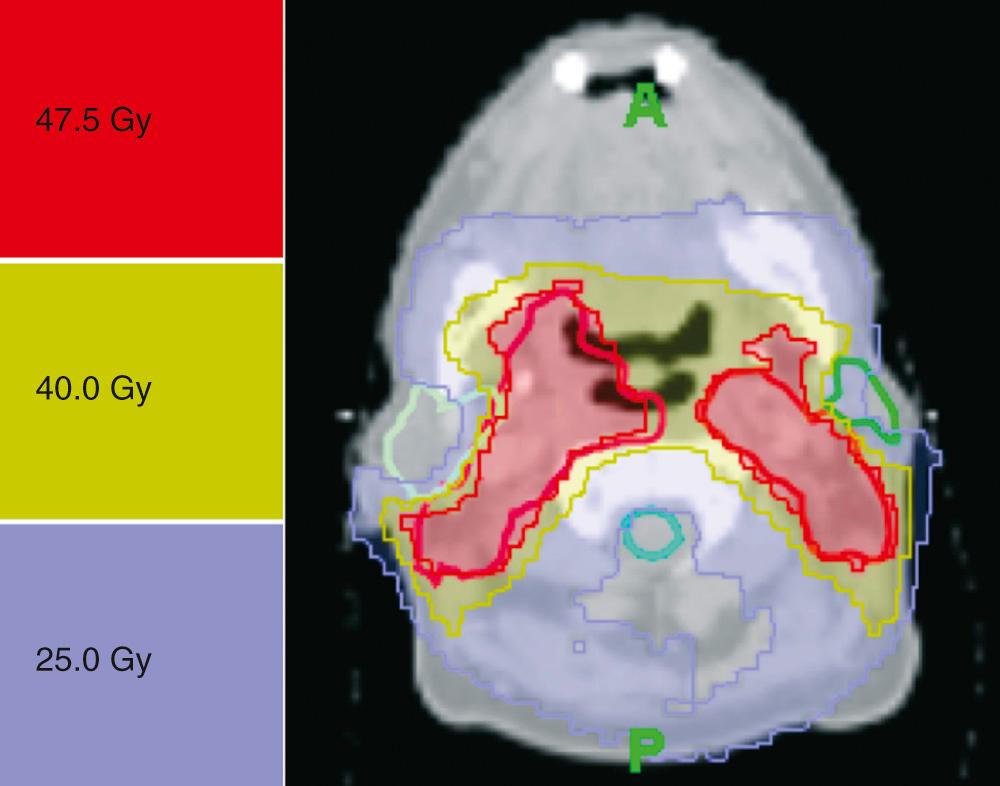
For postoperative irradiation, depending on the risk factors, doses will range from 60 to 66 Gy, in 2-Gy fractions over 6 to 6.5 weeks. A typical example of neck irradiation is presented in Fig. 117.8 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here