Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The annual, population-wide exposure to ionizing radiation from medical sources increased a staggering 750% from 1980 to 2009, and medical imaging now comprises nearly one-half of Americans' radiation exposure. The World Health Organization classifies x-rays officially as carcinogens, and indeed a report by the National Academy of Science indicates that a 100 mGy dose is associated with a lifetime attributable risk for development of a solid cancer or leukemia of 0.1%. Efforts were undertaken to reduce unnecessary exposure, and in 2011 the Joint Commission on Accreditation of Healthcare Organizations published a report that suggests risks can be reduced by the following: (1) raising awareness among staff and patients of the increased risk associated with cumulative doses; (2) providing the right test and the right dose (as low as reasonably achievable [ALARA]) through efficient processes and safe technology; and (3) promoting a culture of safety to make sure that doses are as low as possible while achieving the purpose of the study.
Such risks associated with exposure to ionizing radiation during medical care are sources of anxiety and concern in the pregnant patient, based on questions submitted to Health Physics Society's “Ask the Expert” website. Furthermore, clinicians add a great deal of confusion and fear by providing exposed women with potential erroneous or inadequate information. Clinicians, nurses, and even radiologists may be ignorant of the effects of ionizing and nonionizing radiation. This misinformation contributes not only to undue and unnecessary anxiety and confusion but may lead to inappropriate abortions and litigation. For example, 23% of pregnancies in Greece were terminated after the Chernobyl incident because of unsubstantiated fears. A better understanding of the risks and effects enables providers to make informed decisions and counsel patients that appropriate radiology examinations provide more benefit than harm.
According to the American College of Obstetricians and Gynecologists, “With few exceptions, radiation exposure through radiography, computed tomography scan, or nuclear medicine imaging techniques is at a dose much lower than the exposure associated with fetal harm. If these techniques are necessary in addition to ultrasonography or magnetic resonance imaging or are more readily available for the diagnosis in question, they should not be withheld from a pregnant patient.” The risks of medically-indicated diagnostic studies generally outweigh the future risks of irradiation, and nowhere is this more evident than in the emergency setting, where the risk of not obtaining an indicated study can be high.
When evaluating the pregnant patient who has been or may be exposed to ionizing radiation and attempting to estimate risk, consideration must be given to a number of factors. These include not only factors related to gestational age, dose and type of radiation, and the studies performed or to be performed, but also to the mother herself, as studies raise concern about exposure of maternal breast tissue to ionizing radiation and the future risk of breast cancer. This chapter provides a review of ionizing and nonionizing radiation, its uses in diagnostic radiological examinations, the risks of its use, and strategies for minimizing risk to assist the clinician in counseling the patient to arrive at the most appropriate shared decision.
Simply stated, radiation is the emission or transmission of energy, which can take the form of electromagnetic radiation, such as visible light and x- and gamma-rays (the difference lying in the origin of the photon: electron orbital and nucleus, respectively); particle radiation, such as alpha particles (helium nuclei), beta particles (electrons, positrons), and neutrons; and acoustic radiation. As radiation propagates through space, it can impart or give-up some or all of its energy to surrounding material or tissues, and detection of this energy or its absence forms the basis of diagnostic medical imaging. Radiation is further classified by its effects on the absorbing material. Nonionizing radiation lacks sufficient energy to liberate electrons, and its deposited energy subsequently manifests as heat, whereas ionizing radiation is of sufficient energy to liberate electrons, thereby breaking chemical bonds, forming highly reactive free radicals, and damaging DNA and cellular machinery.
The detrimental effects of radiation on health are grouped broadly into tissue reactions (also called deterministic effects, nonstochastic effects) and stochastic effects ( Table 71.1 ). Tissue reactions are the result of cell killing and manifest as lethality, central nervous system (CNS) abnormalities, cataracts, growth retardation, malformations, and behavioral disorders. Tissue effects are dependent on the energy imparted to the tissue and demonstrate a threshold below which they are not observed but above which they are reliably observed, with their severity rising with dose. Stochastic effects include cancer and potential hereditary effects. Unlike tissue reactions, they have no identifiable threshold. Their probability of occurrence rises with dose, and their severity is independent of the dose.
| PHENOMENON | PATHOLOGY | DISEASES | RISK | DEFINITION |
|---|---|---|---|---|
| Stochastic | Damage to a single cell may result in disease | Cancer, germ cell mutation | Some risk exists at all dosages; at low doses the risk may be less than the spontaneous risk | The incidence of the disease increases with dose, but the severity and nature of the disease remain the same |
| Tissue Reactions | Multicellular injury | Intrauterine death, organ malformations, mental impairment, growth retardation | No increased risk below the threshold dose | Both the severity and the incidence of the disease increase with the dose |
The quantity of energy that is imparted by radiation to the material or tissue is a physical quantity, can objectively be measured, and is called the absorbed dose (D) . It is defined as the mean energy imparted to matter per unit mass, and the mean absorbed dose (D T ) in an organ or tissue is the average absorbed dose over that organ or tissue. The Systeme International (SI) unit for both absorbed dose and mean absorbed dose is the joule per kilogram (J/kg) with the special name grey (Gy). In the centimeter-gram-second (CGS) variant of the metric system, the unit is given the special name rad : 1 rad = 100 erg/g = 0.01 J/kg = 0.01 Gy = 10 mGy. This unit is deprecated but remains in use in some countries (notably the United States).
The many forms of radiation differ in their abilities to impart energy to the absorbing material and cause ionizing events. Neutrons, alpha particles, and other heavy charged particles (e.g., carbon ions) impart their energy more readily, thereby producing more densely spaced ionizing events, whereas the converse is true for electromagnetic radiation and light charged particles (e.g., beta particles). Another quantity, the equivalent dose (H T ) , is therefore necessary and derived from the mean absorbed dose by scaling with a radiation weighting factor (w R ), H T = w R D T , and the effective dose for an organism is the tissue-weighted sum of the equivalent doses for all tissues, radiation sources, and energies. The radiation weighting factor, previously called the quality factor, is dependent only on the type of radiation (and for neutrons, its energy) and is dimensionless. Per the International Committee on Radiological Protection, w R = 1 for all photons and electrons; w R = 2 for protons and charged pions; w R = 20 for alpha particles, heavy ions, and fission fragments; and w R is a continuous function of energy for neutrons. Equivalent dose and effect dose therefore have SI units of joule per kilogram (J/kg) with the special name sievert (Sv). In CGS, they have units of roentgen equivalent in man (rem), and 1 rem = 100 erg/g = 0.01 J/kg = 0.01 Sv = 10 mSv. As diagnostic radiology examinations involve essentially only photons, the equivalent and effective doses have essentially the same magnitude as the absorbed dose. As an example, a mean absorbed dose of 100 mGy results in an effective dose of 100 mSv if the source is electromagnetic radiation or 2 Sv if the source is alpha radiation.
Radioactive decay or radioactivity is the process by which the nucleus of an unstable atom emits energy in the form of radiation. At the quantum level, the process is stochastic, but at the macroscopic level, the decay of a sufficiently large collection of unstable atoms can be calculated and is represented by the half-life, or the time it takes for the collection of unstable atoms to reduce by half. The SI unit of radioactivity is the becquerel (Bq) and is defined as one nuclear decay event per second. Its units are therefore inverse seconds (1 Bq = 1 s −1 ), as a nuclear decay event is dimensionless. A frequently encountered, non-SI unit of radioactivity is the curie (Ci). Its definition is based on the amount of radioactivity in one gram of radium and also has units of events per second; 1 Ci = 3.7 × 10 10 Bq = 37 GBq. Doses of radioactive substances used in medicine are typically on the MBq and mCi scale (e.g., 1 mCi = 37 MBq). These units encode neither the type nor energy of the radiation emitted by the nuclear decay process.
Exposure to radiation is among many recognized agents and environmental exposures which can produce deleterious effects, resulting in mental retardation, neurobehavorial effects, convulsive disorders, congenital malformations, fetal growth retardation, embryonic death, and cancer. The significant background prevalences of these events are 0.5% to 1% for mental retardation, 3% for major congenital malformation, 3% for growth retardation, 15% for miscarriage (with a reported variation as wide as 30% to 50%), 11% for genetic disease, and 7% for prematurity, and in the United States there is a 21% background lifetime risk of fatal cancer in the offspring.
As previously discussed, the effects of radiation are broadly divided into two categories (see Table 71.1 ): stochastic effects (which include cancer and hereditary disease) have no dose threshold, and the frequencies of which rise with increasing dose; and tissue reactions (which include the remaining effects) have identifiable thresholds above which they are reliably observed, the severities of which do not depend on dose, and whose manifestations depend on the stage of fetal development in which the exposure occurs. Dependence on the duration of time over which the exposure occurs is also noted, as a protracted exposure is less likely to produce an effect than a single exposure of high intensity (e.g., the dose from continuous exposure during flight). Fractionation of the dose (e.g., multiple radiographs over a period of hours or days), like protraction, also decreases the severity of and increases the threshold for tissue reactions. The dependence of the effects of radiation on the stage of development is demonstrated in Fig. 71.1 .
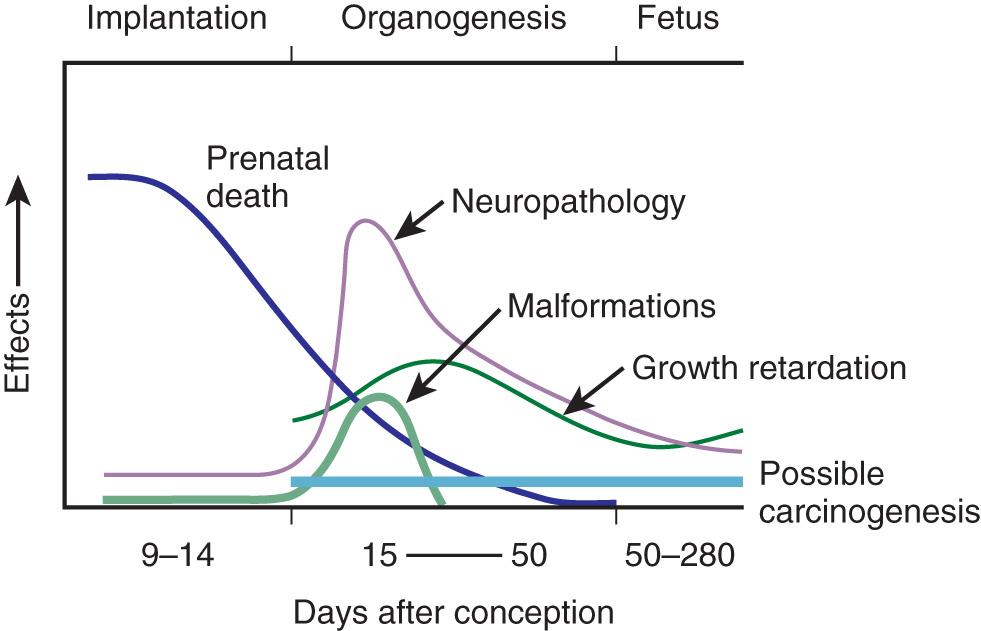
Development of the fetus is expressed as postconception age and divided into three major stages: (1) the preimplantation and implantation phase (conception to implantation), (2) organogenesis (3RD to 8th week), and (3) fetal development (9th week until birth), which includes the majority of CNS development (8th to 25th week). The risks of radiation are most significant during organogenesis and the early fetal period, somewhat less in the second trimester, and least in the third trimester. This relationship is demonstrated in Fig. 71.1 , and the main effects and their thresholds are summarized in Table 71.2 .
| HUMAN GESTATIONAL AGE (WEEKS) [POSTCONCEPTION AGE] | MINIMUM LETHAL DOSE TO EMBRYO OR FETUS (Gy) | APPROXIMATE LD50 | MINIMUM DOSE FOR PERMANENT GROWTH RETARDATION IN THE ADULT (Gy) | MINIMUM DOSE FOR GROSS ANATOMIC MALFORMATIONS (Gy) | INCREASED INCIDENCE OF MENTAL RETARDATION (Gy) |
|---|---|---|---|---|---|
| 3rd to 4th [1st to 2nd pc] |
0.15 to 0.2 | < 1 | No increased incidence of GR in survivors | No increased incidence of congenital malformation in survivors | |
| 5th to 7th [3rd to 5th pc] |
0.25 to 0.5 | 1.4–2 | 0.2–0.5 | > 0.2 but most malformations require > 0.5 | |
| 8th to 15th [6th to 13th pc] | > 1 | > 2 | 0.25–0.5 | ||
| 16th to term [14th pc to term] |
> 1.5 | Same as for the mother | > 0.5 | ||
| 10th to 27th [8th to 25th pc] |
SMR at doses > 0.5; (lower 95% CI value of ~ 0.3) |
The preimplantation and implantation phase has been designated an “all or none” period, as embryonic cells are few in number and totipotential, and can replace damaged neighboring cells. It is in this stage of development that the embryo is least likely to be malformed, and the predominant effect of radiation exposure is failure to implant, resulting in abortion. The threshold for mortality in this stage of development is quite high, estimated at 100 to 200 mGy and rises with fetal age such that at term the threshold for mortality is 1 Gy to 1.5 Gy (see Table 71.2 ). Embryos that survive exposure in this period of development demonstrate no increased risk for malformations or growth retardation.
Exposure to high doses of radiation during the period of organogenesis is likely to affect the organ system or systems under development at the time of the exposure and results from reduced ability to replace those cells damaged or killed by the exposure. Although any organ system can be affected, the predominantly observed effects are microcephaly and growth retardation, and have a dose threshold of 50 to 200 mGy, which is significantly higher than doses obtained from diagnostic radiology examinations. Temporary growth retardation can be observed with doses of 100 to 250 mGy, but these infants recover fully and attain normal adult stature and weight. The dose threshold for permanent growth retardation depends on postconception age and is 200 to 500 mGy in the 3rd to 5th weeks, 250 to 500 mGy in the 6th to 13th weeks, and greater than 500 mGy from the 14th week onward (see Table 71.2 ). Exposure to less than 50 mGy during the period of organogenesis results in no change in the risk for organ malformations or growth retardation.
At the end of the period of organogenesis, the fetus has diminished vulnerability to multiple organ system teratogenesis, but beginning in the 8th week and continuing through the 25th week is development of the CNS, during which it can be seriously affected by high doses of ionizing radiation, resulting in mental impairment, mental retardation, neurobehavioral effects, and convulsive disorder. CNS sensitivity to ionizing radiation is not uniform throughout its development, with highest sensitivity from the 8th through 15th weeks, during which there is a rapid increase in the number of neurons that migrate and differentiate, losing their ability to divide. This process is largely concluded by the 15th week, at which point and lasting until the 25th week, further differentiation and architectural definition occur, and the CNS becomes slightly less susceptible to damage.
It is important to note that all clinical observations of significant IQ reduction and severe mental retardation (SMR) are associated with high doses of radiation in excess of 500 mGy, a level not easily approached with diagnostic radiology examinations. The highest risk for SMR exists in the 8th through 15th weeks and with a high dose threshold of 1 Gy, and the severity of the retardation does appear to have some dependence on the dose. For survivors of the atomic bombings of Japan who were between 8 and 15 weeks after conception at the time of their exposure, a drop of 0.3 IQ points per 10 mGy of exposure, with a threshold of 500 mGy, was observed. There is no documented risk for mental retardation in humans at gestational ages less than 8 weeks or greater than 25 weeks with exposure less than 500 mGy. Furthermore, the risk of exposure to 100 mGy or less is far smaller than the 3% background risk of a child being born with an IQ less than 70, and exposure to less than 100 mGy cannot be clinically identified with reduction in IQ.
Convulsive disorders are a broad category encompassing various diagnoses, etiologies, and manifestations. The risk for convulsive disorder following in utero radiation exposure was evaluated in several studies on Japanese survivors of the atomic bombing. Among these survivors, no convulsive disorders were identified in those receiving a dose less than 100 mGy. Those at highest risk for seizure disorder were those exposed in the 8th through 15th weeks postconception and who were also mentally retarded. The association of convulsive disorders with radiation exposure between the 8th and 15th weeks postconception without mental retardation was borderline significant. There is no increase in convulsive disorders from exposure before the 8th week or after the 25th week.
Growth retardation can occur with exposure to ionizing radiation in the fetal period, though exposure is more likely to result in microcephaly. Studies on the atomic bombing survivors who were exposed in utero show divergent thresholds for microcephaly. For survivors of Nagasaki, the threshold is estimated at 1.5 Gy, whereas for the survivors of Hiroshima, a threshold of 100 to 190 mGy is reported. This latter finding is consistent with a threshold of 100 to 200 mGy for microcephaly that was observed in mammalian animal models. Irradiation of the human fetus at doses less than 100 mGy has not been observed to cause congenital malformations or growth retardation (see Table 71.2 ).
The remaining effects (cancer and hereditary effects) are stochastic, notably in that risk exists at all levels of exposure (see Table 71.1 ). Childhood malignancies account for less than 1% of total cancer diagnoses in the United States, and the cumulative risk to 15 years of age is 1 to 2.5 per 1000 in most western countries. Additionally, lymphoma, leukemia, and brain tumors account for 70% of childhood cancers, which stands in contrast with adult malignancies, the majority of which are epithelial in origin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here