Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Aortic stenosis (AS) is the most common cardiac valve lesion in developed countries, including North America and Europe, with an incidence of 2% to 9% in patients older than 65 years of age. Moreover, the incidence is increasing as the population ages. Aortic sclerosis, the precursor of AS, is present in nearly one-third of patients older than age 65 years.
AS is suspected clinically when a harsh systolic ejection murmur is heard, a delayed carotid upstroke is palpated, or typical symptoms (angina pectoris, exertional dyspnea, or exertional syncope) occur. However, the clinical diagnosis of AS can be challenging. Clinical signs and symptoms are limited for distinguishing critical AS from noncritical AS, and these signs have reduced sensitivity and specificity in older adults. , Cardiac catheterization, once considered the gold standard for quantitation of AS, is invasive, and the frequency of complications increases with age. Omran and coworkers demonstrated evidence of acute, focal embolic events on magnetic resonance imaging (MRI) in 22% of 152 patients who underwent retrograde catheterization.
In contrast, echocardiography provides noninvasive assessment of both valve morphology and hemodynamics. Because of its versatility, noninvasiveness, reproducibility, and accuracy, current guidelines endorse echocardiography as the diagnostic method of choice for the assessment and management of AS. , Cardiac catheterization is no longer recommended and is only performed in a limited subset of patients in whom echocardiography is nondiagnostic or discrepant with clinical parameters. , In most situations, transthoracic echocardiography (TTE) is sufficient, and it is the current standard procedure for assessing both severity and serial evaluations of AS. Moreover, the prediction of clinical outcomes of patients with AS has been studied mainly using TTE.
Precise assessment of AS severity is necessary for clinical decision making. The primary hemodynamic parameters recommended for the quantitation of AS severity are peak jet velocity, transaortic gradients, and aortic valve area (AVA) calculated by the continuity equation. Box 79.1 lists the echocardiographic and Doppler parameters that should be evaluated in patients with valvular AS. These are subsequently discussed in the following.
Two-dimensional (2D) measurement of the left ventricular outlet tract (LVOT) diameter and aortic annulus
LVOT velocity (V1)—by pulsed-wave Doppler
Velocity across the aortic valve (V2 or Vmax) by continuous wave Doppler (from apex, right parasternal view, suprasternal notch, subxiphoid view)
Calculation of peak instantaneous gradient and mean gradient
Calculation of aortic valve area by the continuity equation
Dimensionless index
M-mode or 2D measurements of left ventricular size
Calculation of LV mass
Assessment of aortic insufficiency
Assessment of other cardiac defects
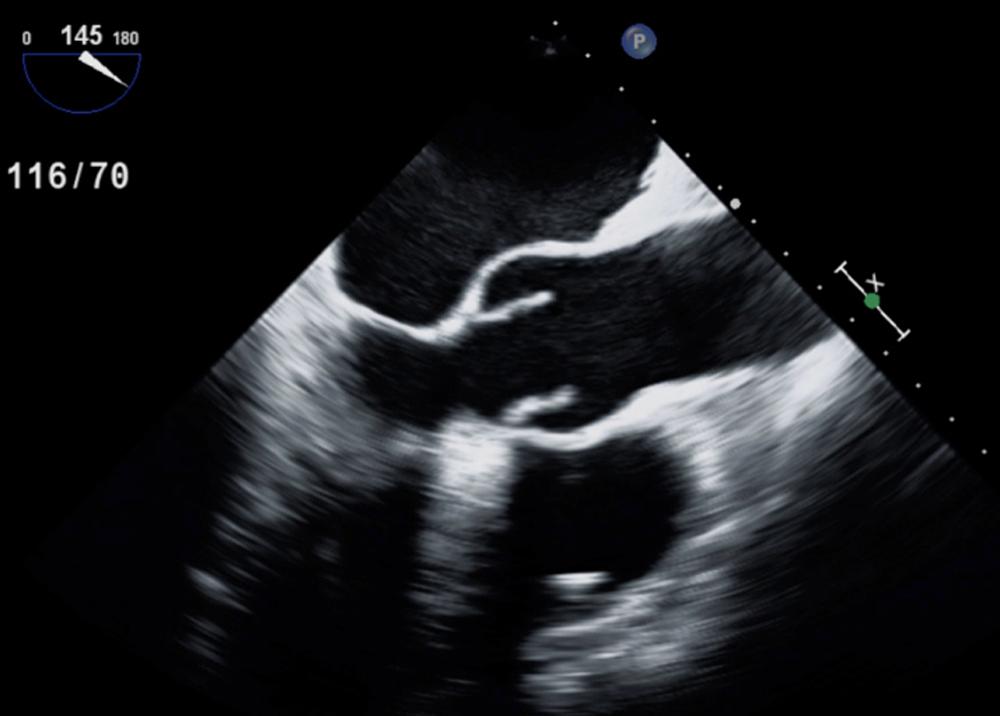
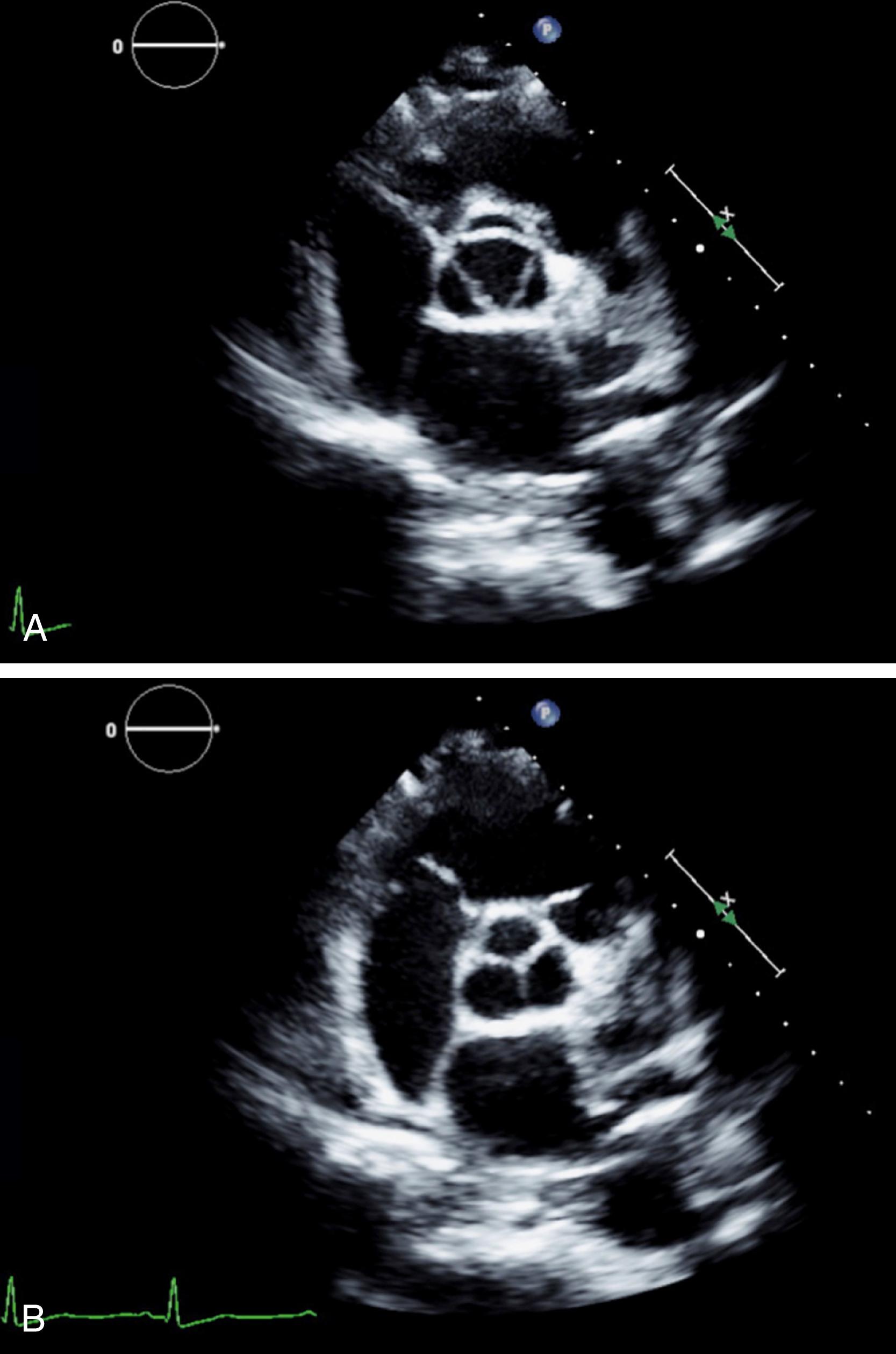
The normal aortic valve is composed of three leaflets or cusps (the left, right, and noncoronary cusps [NCCs]) of equal or nearly equal size. Two-dimensional (2D) TTE of the normal aortic valve in the parasternal long-axis (PLAX) view shows two leaflets: (1) the right coronary cusp, which is the most anterior cusp, and (2) either the noncoronary cusp [NCC] (most commonly) or the left coronary cusp. Normal aortic valve cusps appear thin and delicate. In the PLAX view, the cusps open rapidly in systole and appear as parallel lines close to the aortic walls ( Fig. 79.1 ). In diastole, the leaflets come together and appear as a linear density in the center of the aortic root, parallel to the aortic walls. The aortic leaflets are seldom seen during the opening and closing because their motion is very rapid relative to the frame rate of the 2D ultrasound system. In the short-axis (SAX) view, the three thin leaflets open in systole to form a triangular or circular orifice ( Fig. 79.2 ). During diastole, the closure lines of the three leaflets form a Y shape (an inverted Mercedes Benz sign). Sometimes there is a slight thickening of the midportion of each closure line formed by nodules known as the nodules of Arantius . In the SAX view, the NCC is located posteromedially. The atrial septum always points to the NCC. The left coronary cusp is located posterolaterally.
M-mode echocardiography of the aortic valve is formed by directing the M-mode echo beam through the aortic leaflets. This can be done from both the PLAX and SAX views. At the onset of systole, the leaflets open rapidly and become parallel to, and nearly oppose, the walls of the aortic root ( Fig. 79.3 ). They remain open throughout systole and rapidly close again at end-systole, forming a box or parallelogram. Normally, these leaflets show fine, regular vibrations during systole. These fine vibrations actually indicate that the leaflets are thin and are able to luff, like a sail, because of the rapid flow through them on one side (their ventricular surface) and eddy currents swirling behind the leaflets on the aortic side, resulting in opposing forces that cause these vibrations. During diastole, the coapted leaflets form a single (or sometimes multiple parallel) central closure line(s) midway between the aortic walls (see Fig. 79.3 ). The left ventricular (LV) ejection time can be measured from the point of the cusp opening to the point of the cusp closing.
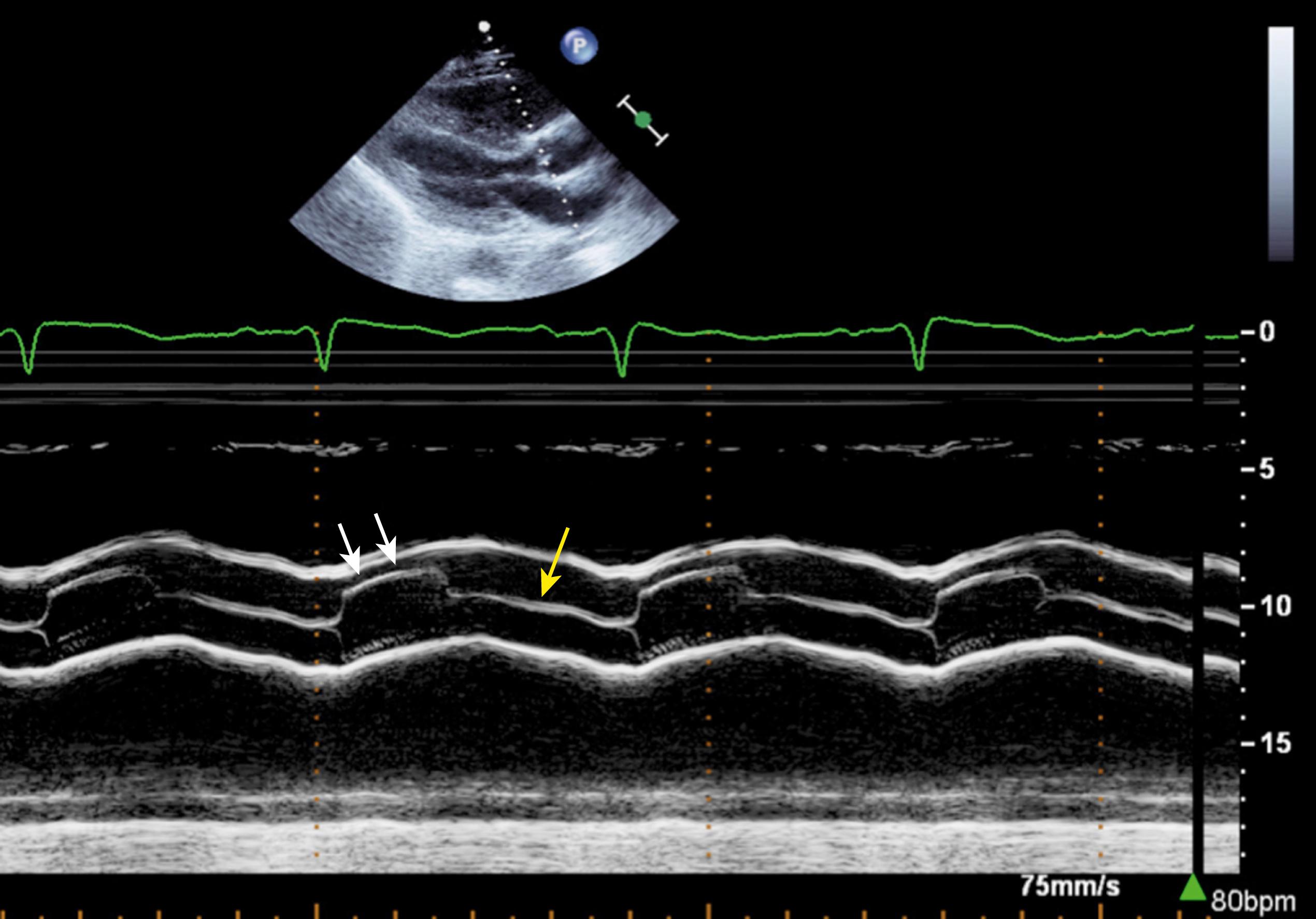
A rough estimate of the severity of AS can be obtained by noting the maximal degree of separation of the leaflets at the onset of systole. In patients with valvular AS, the thickened leaflets (caused by fibrosis, calcium, or both) appear as dense echoes in both systole and diastole. In systole, the thickened rigid leaflets fail to open widely. The distance between the anterior cusp (right coronary cusp) and the posterior cusp (usually the NCC; sometimes, the left coronary cusp) is reduced or not even visible, which suggests moderate or severe AS ( Fig. 79.4 ). In the absence of a bicuspid valve, a maximal opening of the leaflets of at least 1.5 cm virtually excludes significant valvular AS. , When any of the three leaflets opens normally or maximally, regardless of the degree of limitation of the other two, the degree of AS is not more than mild.
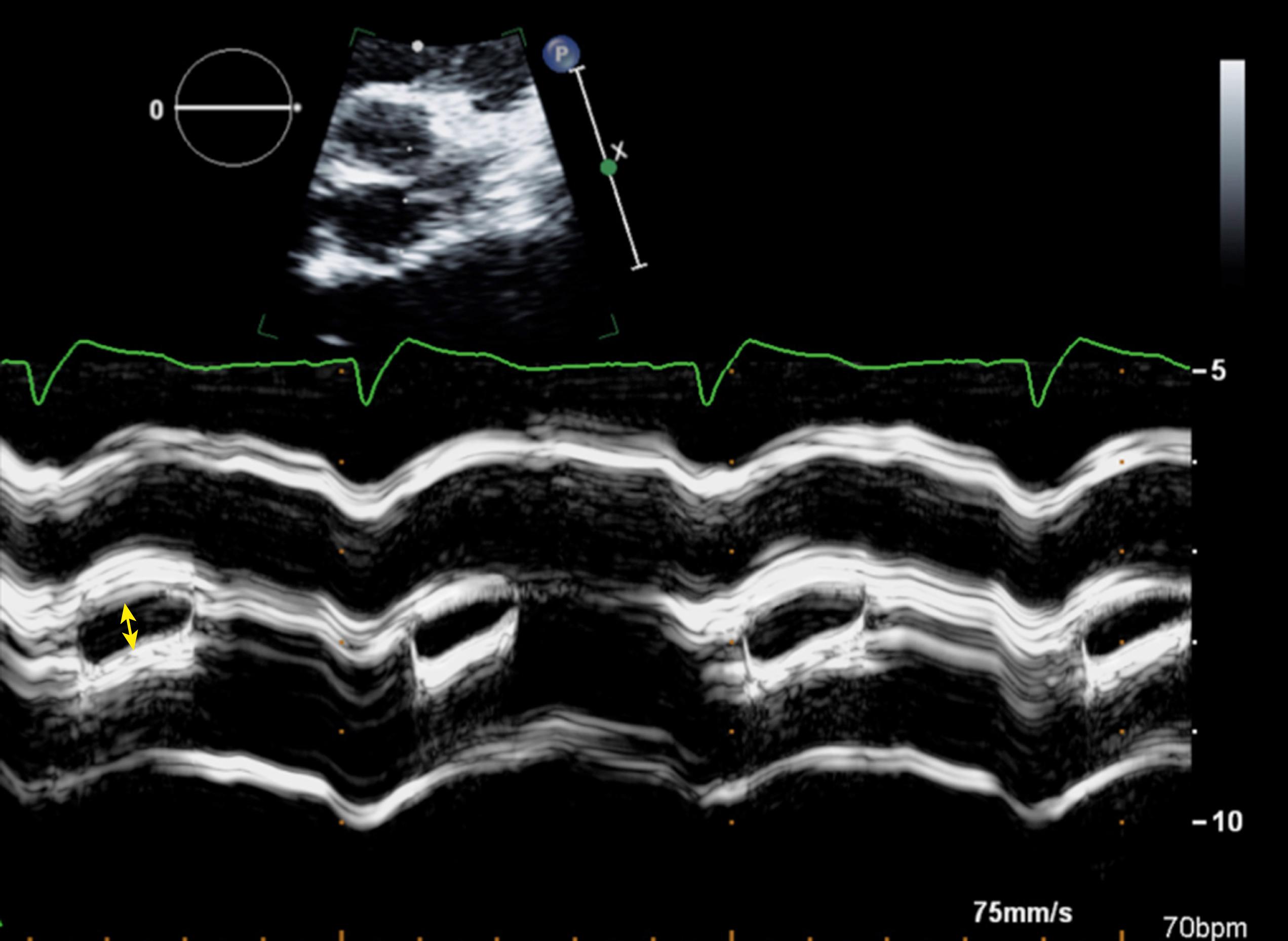
With the development of acquired AS, the cusps become thickened, and their motion is restricted. The degree of thickening and restriction progresses as the severity of AS increases. In severe AS, the leaflets become markedly thickened and calcified, and there is nearly a total lack of mobility. Identification of individual cusps is often difficult or impossible. Moreover, attempts to planimeter the aortic valve orifice by TTE have been largely unsuccessful. Nevertheless, a qualitative estimation of AS severity should be attempted and correlated with quantitative methods. If leaflet separation is at least 15 mm or if at least one cusp moves normally, critical AS is unlikely. However, as will be discussed later, planimetry is possible in the majority of patients using transesophageal echocardiography (TEE).
The previously mentioned, 2D and M-mode features are useful for detecting AS, but they are unreliable for quantitating AS. The severity of AS is determined by a combination of 2D and Doppler echocardiography. As the aortic valve becomes stenotic and obstruction to blood flow occurs, a pressure gradient develops across the valve. This obstruction is associated with an increase in transaortic jet velocity. The primary routine parameters used to quantitate AS include the peak aortic jet velocity, the mean pressure gradient, and the AVA.
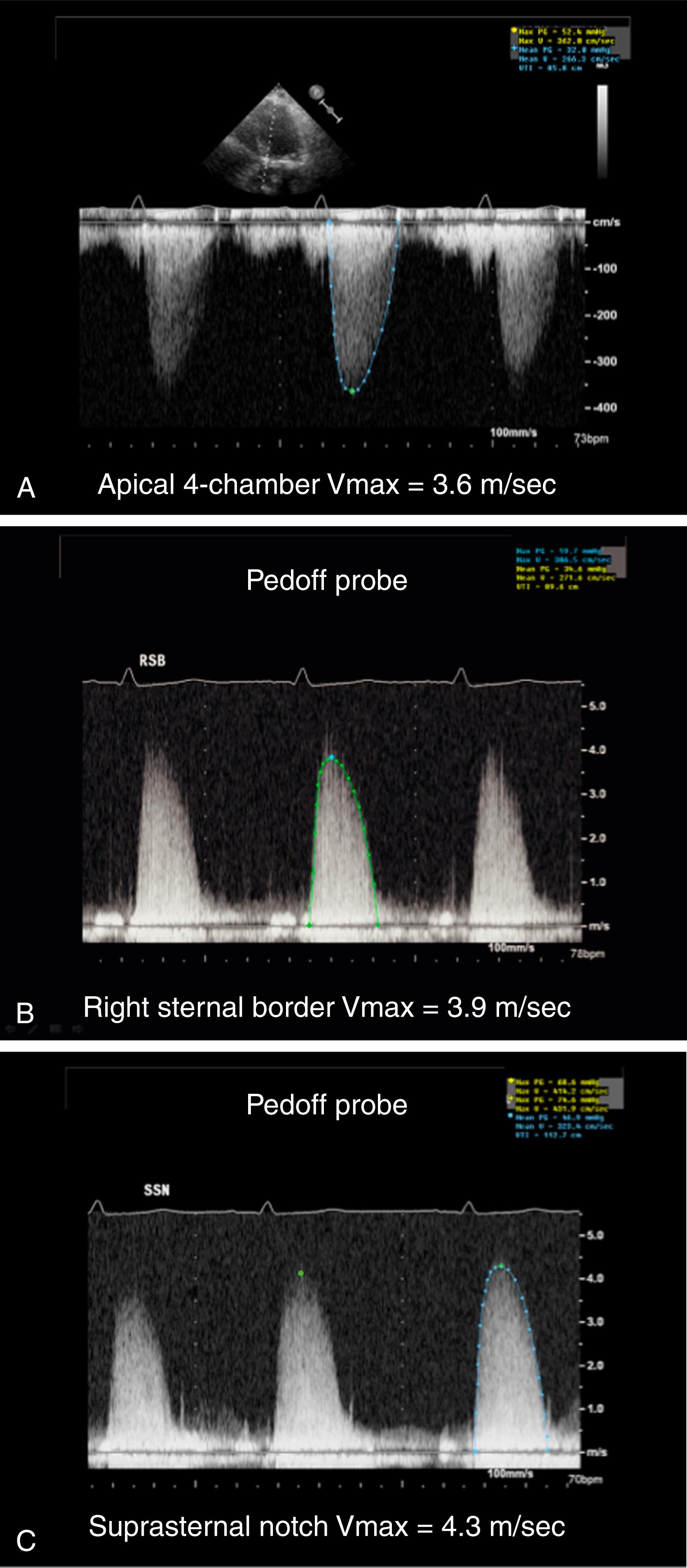
Transaortic jet velocities are directly obtained using a continuous-wave (CW) Doppler probe. To obtain the highest velocity, the angle of interrogation should be as parallel to flow as possible. Therefore, multiple transducer windows should be used to obtain the Doppler signal that is aligned most parallel to the direction of the stenotic jet. These windows include the apical three- and five-chamber views, the right sternal border, the suprasternal notch (SSN), and subxiphoid views. A careful, thorough, meticulous manipulation of the transducer is necessary to achieve optimal alignment and to determine the highest velocity possible ( Fig. 79.5 ). The highest velocity obtained from any window is used in the calculation of the gradient and the AVA. Lower values from the other windows are ignored. Using a nonimaging CW Doppler probe (so-called Pedoff probe or pencil probe ) is recommended because it is smaller, easier to manipulate between the ribs and in the SSN, and has a higher signal-to-noise ratio.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here