Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The incidence of thromboembolic disease is difficult to estimate because of the nonspecific nature of presenting symptoms and a lack of awareness of the disorder.
Chronic thromboembolic pulmonary hypertension (CTEPH) results from incomplete resolution of a pulmonary embolus (PE) or from recurrent PE.
The cause of CTEPH after acute PE is not fully understood. Proposed mechanisms include abnormalities in fibrinolytic enzymes or resistance of the thrombus to fibrinolysis.
Pulmonary thromboendarterectomy (PTE) is the most effective treatment for patients with CTEPH.
Patients typically present with progressive exertional dyspnea and exercise intolerance because of increased pulmonary vascular resistance (PVR), decreased cardiac output, and increased minute ventilation requirements secondary to increased alveolar dead space.
Right-sided heart catheterization defines the severity of pulmonary hypertension and the degree of cardiac dysfunction.
Patients with preoperative PVR greater than 1000 dynes⋅s⋅cm −5 have a greater operative mortality rate, but a markedly increased preoperative PVR does not contraindicate surgical treatment.
Postsurgical complications include: reperfusion pulmonary edema and persistent pulmonary hypertension.
Riociguat is the first medication approved by the US Food and Drug Administration for treating certain patients with CTEPH.
Balloon pulmonary angioplasty is an alternative approach to PTE in patients believed to have surgically inaccessible chronic thromboembolic disease.
Reperfusion pulmonary edema and airway bleeding are two of the most difficult complications of PTE to manage.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a form of pulmonary hypertension (PH) that is characterized by complete or partial obstruction of the pulmonary vascular bed as a result of a recurrent or residual intraluminal organized fibrotic clot leading to increased pulmonary vascular resistance (PVR), severe PH, and eventually right-sided heart failure. Its incidence is difficult to estimate because of the uncertainty regarding the frequency of acute pulmonary embolism (PE) and the percentage of patients in whom emboli fail to resolve. The incidence reflects a wide range, from less than 1% to as high as 9% of patients with acute PE.
Screening for CTEPH in patients with PH or unexplained dyspnea is of paramount importance because this form of PH is potentially curable with pulmonary thromboendarterectomy (PTE), also known as pulmonary endarterectomy. The success of the operation centers on endarterectomy of the organized fibrous thrombus in the intima and part of the medial layers of the pulmonary vascular tree. Lung transplantation is another potential option, but it is usually not a choice for patients with CTEPH because of the risk of death while on the waiting list, a shortage of organ supply, the expense, the risk of immunosuppressive agents, infection, and rejection.
Classifications of PH began in 1973 at the World Health Organization (WHO) conference and have since undergone multiple revisions as the appreciation of the disease and treatment of PH has evolved. Currently PH is divided into five distinct subgroups of patients sharing specific features ( Box 20.1 ).
Group I: Pulmonary arterial hypertension (PAH) and other subtypes of PAH
Group II: Left-sided heart disease
Group III: Respiratory disease and hypoxemia
Group IV: Chronic thromboembolic pulmonary hypertension
Group V: Miscellaneous causes
Further classification of PH defines the presence of precapillary (groups I, III, IV, and V) or postcapillary (group II) patterns. CTEPH is precapillary PH, as assessed by right-sided heart catheterization characterized by a mean pulmonary artery pressure (mPAP) greater than 25 mm Hg with normal pulmonary capillary wedge pressure lower than 15 mm Hg and an elevated PVR greater than 300 dynes⋅s⋅cm −5 . Postcapillary PH secondary to left-sided heart disease is the most frequent form of PH, and it is characterized by mPAP greater than 25 mm Hg and pulmonary capillary wedge pressure greater than 15 mm Hg with normal PVR. Differentiating pulmonary arterial hypertension (PAH) from pulmonary venous hypertension in group II is important given the high prevalence of left-sided heart disease. Echocardiography is an essential tool for initial screening and assessment for PH ( Table 20.1 ).
| Completed? | Action Item | Notes |
|---|---|---|
| □ | Record estimated PASP |
|
| □ | Evaluate RV size and function |
|
| □ | Evaluate for signs of elevated PVR |
|
| □ | Estimate volume status |
|
| □ | Evaluate severity of TR |
|
| □ | Evaluate for pericardial effusion |
|
| □ | Evaluate for causes of PH (left-sided heart disease, shunt lesions) |
|
| □ | Differentiate PAH from PVH |
|
Acute or recurrent PE is thought to be the inciting event in the development of CTEPH. Incomplete resolution of the embolus followed by thrombus organization and fibrosis leads to partial or complete vessel obstruction. In addition, vascular remodeling in the distal pulmonary arteries (pulmonary arteriopathy) also may contribute to the increased PVR, and it is the cause of residual PH seen in some patients after otherwise successful PTE. Unresolved PE in the proximal pulmonary arterial tree causes vascular obstruction in two ways: canalization of the clot leading to multiple small endothelized channels separated by bands and webs or fibrin clot organization or absent canalization leading to dense fibrous connective tissue that completely occludes the arterial lumen. This fibrous plug is firm and adherent to the arterial wall, and the surgical challenge is to remove enough of the fibrous plug as one unit to reduce the vascular resistance without disrupting the arterial wall.
The natural history of PE in most patients is complete resolution of the thromboembolic event with restoration of normal blood flow and hemodynamics. However, in some patients embolic resolution is incomplete, resulting in the development of CTEPH. The mechanism by which thromboembolic material remains unresolved is not fully understood. A variety of factors may play a role. The volume of the embolic substance may simply overwhelm the lytic system, with total occlusion of a major arterial branch preventing the lytic material from reaching and dissolving the embolus completely. The emboli may be made of substances such as well-organized fibrous thrombus that cannot be dissolved by normal mechanisms. Some patients may have tendencies to thrombus formation, a hypercoagulable state, or abnormal lytic mechanisms. Larger perfusion defects at diagnosis, idiopathic thromboembolic disease, high PA pressure (PAP) at the time of presentation, and a history of multiple PEs are risk factors for development of CTEPH after an acute PE.
Other identified risk factors include: ventriculoatrial shunts, infected pacemakers, splenectomy, previous venous thromboembolism, recurrent venous thromboembolism, blood group other than O, lupus anticoagulant or antiphospholipid antibodies, thyroid replacement therapy, or a history of malignant disease. Despite being a risk factor for venous thromboembolism, the prevalence of hereditary thrombophilic states (deficiencies of antithrombin III, protein C, and protein S, and factor II and factor V Leiden mutations) is similar to that in normal control subjects or in patients with idiopathic PH. In contrast, lupus anticoagulant or antiphospholipid antibodies can be found in up to 21% of patients with CTEPH, and increased levels of factor VIII were identified in 41% of patients with CTEPH. Finally, small, preliminary studies suggest the possibility of structural and functional abnormalities of fibrinogen in patients with CTEPH that perhaps confer resistance to fibrinolysis.
A history of a previous acute thromboembolic event is not present in 25% to 30% of patients diagnosed with CTEPH. Thus a high index of suspicion is important for the diagnosis of CTEPH in any patient presenting with exertional dyspnea and exercise intolerance, even without evidence of previous PE. Early in the disease process, patients may go through a “honeymoon period” in which signs and symptoms of PH are not obvious. Symptoms appear when the right ventricle is unable to increase contractility sufficiently to augment left ventricular (LV) preload and cardiac output (CO) during exercise. Progressive exertional dyspnea is often the initial symptom of CTEPH, and, unfortunately, it is often attributed to more common medical conditions such as obstructive lung disease, obesity, or deconditioning. Exertional dyspnea results from increased PVR limiting CO and increased breathing requirements because of increased alveolar dead space.
As the disease progresses and the right side of the heart fails, patients may develop ascites, early satiety, epigastric or right upper quadrant fullness, edema, chest pain, and presyncope or syncope. Other symptoms may include nonproductive cough, hemoptysis, and palpitations. Left vocal cord dysfunction and hoarseness may arise from compression of the left recurrent laryngeal nerve between the aorta and an enlarged left main PA. Early in the disease process the physical examination may be normal or may reveal an accentuated pulmonic component of the second heart sound. Pulmonary flow murmurs or bruits heard over the lung fields are caused by turbulent blood flow through partially occluded or recanalized thrombi. These flow murmurs are heard in 30% of patients with CTEPH and are not found in idiopathic PH.
Late in the disease process, patients experience exertion-related syncope and resting dyspnea. The physical signs are far from uniform, and the physical examination may be surprisingly unrewarding if right ventricular (RV) failure is not yet present, even in patients with severe dyspnea. Physical findings of RV failure such as jugular venous distension, RV lift, fixed splitting of the second heart sound, murmur of tricuspid regurgitation (TR), RV gallop, hepatomegaly, ascites, and edema may appear in later stages of the disease. Most patients are hypoxic, with room-air arterial oxygen tension in the range of 65 mm Hg. This hypoxia is a result of ventilation/perfusion (V̇/Q̇) mismatch and low mixed venous oxygen saturation. Marked hypoxemia at rest implies severe RV dysfunction or the presence of a considerable right-to-left shunt, typically through a patent foramen ovale (PFO). Carbon dioxide tension is slightly reduced with metabolic compensation (reduced bicarbonate). Dead-space ventilation is increased, along with V̇/Q̇ mismatch, although these features correlate poorly with the degree of pulmonary vascular obstruction.
Basic pulmonary function studies do not provide specific clues to the diagnosis of CTEPH, and these tests are most useful in evaluating patients for coexisting parenchymal lung disease or airflow obstruction. Twenty percent of patients with CTEPH exhibit a mild-to-moderate restrictive defect, often a result of parenchymal scarring from previous lung infarction. Similarly, a modest reduction in single-breath diffusing capacity of the lung for carbon monoxide (D lco ) may be present in some patients with CTEPH. A normal value does not exclude the diagnosis, and severe reduction in D lco indicates that the distal pulmonary vascular bed is significantly compromised, thus making an alternative diagnosis likely.
The chest radiograph may be unremarkable in the early stages of CTEPH. However, as the disease progresses with the development of PH, the proximal pulmonary vascular bed enlarges. In some patients with chronic thromboembolic disease of the main or lobar PAs, this central PA enlargement can be asymmetric. This is not a radiographic finding in those patients with PH resulting from small-vessel disease. As the right ventricle adapts to the rise in PVR, radiographic signs of chamber enlargement such as obliteration of the retrosternal space and prominence of the right heart border can be observed. Relatively avascular lung regions can be appreciated if an organized thrombus has compromised blood flow to that area. In these poorly perfused lung regions, peripheral alveolar opacities, linear scarlike lesions, and pleural thickening may be found as a result of parenchymal injury and infarction.
Transthoracic echocardiography (TTE) is a frequently used screening modality in patients with suspected PH. It often provides the first objective indication of the presence of elevated PAPs or RV compromise. Current technology allows for estimates of systolic PAP (using Doppler analysis of the velocity of TR), along with CO and RV performance. Enlargement of the right heart chambers and the resultant TR, flattening or paradoxical motion of the interventricular septum, and encroachment of an enlarged right ventricle on the LV cavity resulting in impaired LV filling are findings in patients with significant PH. Contrast echocardiography using intravenous agitated saline may show the presence of an intracardiac shunt, as a result of a PFO or another previously undetected septal defect. The echocardiogram is also useful in excluding LV dysfunction, valvular disease, or congenital heart disease, which may cause PH. In some patients with suspected CTEPH, TTE showing normal or minimally elevated PAPs at rest can sometimes demonstrate a substantial rise in PAP or dilatation of the right ventricle with exertion.
The next step in the evaluation of patients for CTEPH is the acquisition of a V̇/Q̇ scan. For those patients with diagnosed PH, and for patients with dyspnea of unclear origin and suspected pulmonary vascular disease, the V̇/Q̇ scan is the recommended screening test for CTEPH. In CTEPH, at least one, and more commonly several, segmental or larger mismatched perfusion defects are present ( Fig. 20.1 ). In those patients with small-vessel pulmonary vascular disease, perfusion scan results either are normal or exhibit a “mottled” appearance characterized by nonsegmental defects. The greatest value of a V̇/Q̇ scan as a screening study is that a relatively normal perfusion pattern excludes the diagnosis of surgical CTEPH. Investigators have also observed that the magnitude of perfusion defects exhibited by patients with CTEPH with operable disease may understate the degree of pulmonary vascular obstruction determined by angiography. Therefore CTEPH should be considered, and an evaluation for operable disease is warranted, even if the V̇/Q̇ scan demonstrates a limited number of mismatched perfusion defects or regions of relative hypoperfusion (“gray zones”).
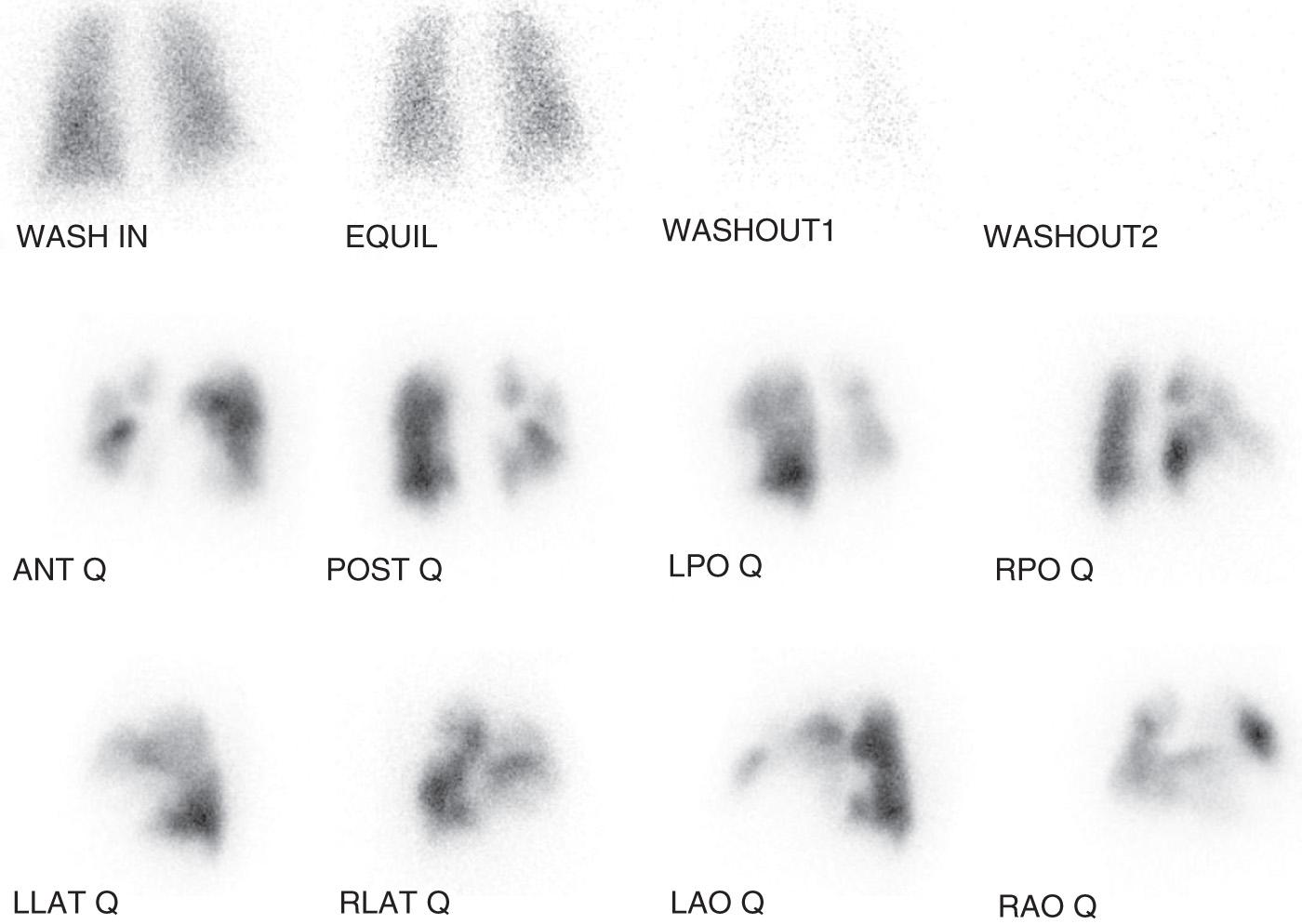
Although an abnormal perfusion scan finding is observed in patients with CTEPH, this finding lacks specificity. Several other disorders affecting the proximal pulmonary vessels may result in scan findings similar to those in CTEPH, and, as such, further diagnostic imaging is necessary. Depending on imaging modality availability, and interpretive expertise, conventional catheter-based angiography—computed tomography pulmonary angiography (CTPA)—and magnetic resonance imaging (MRI) are most valuable methods for defining large-vessel, pulmonary vascular anatomy and providing the necessary diagnostic information for the confirmation of CTEPH.
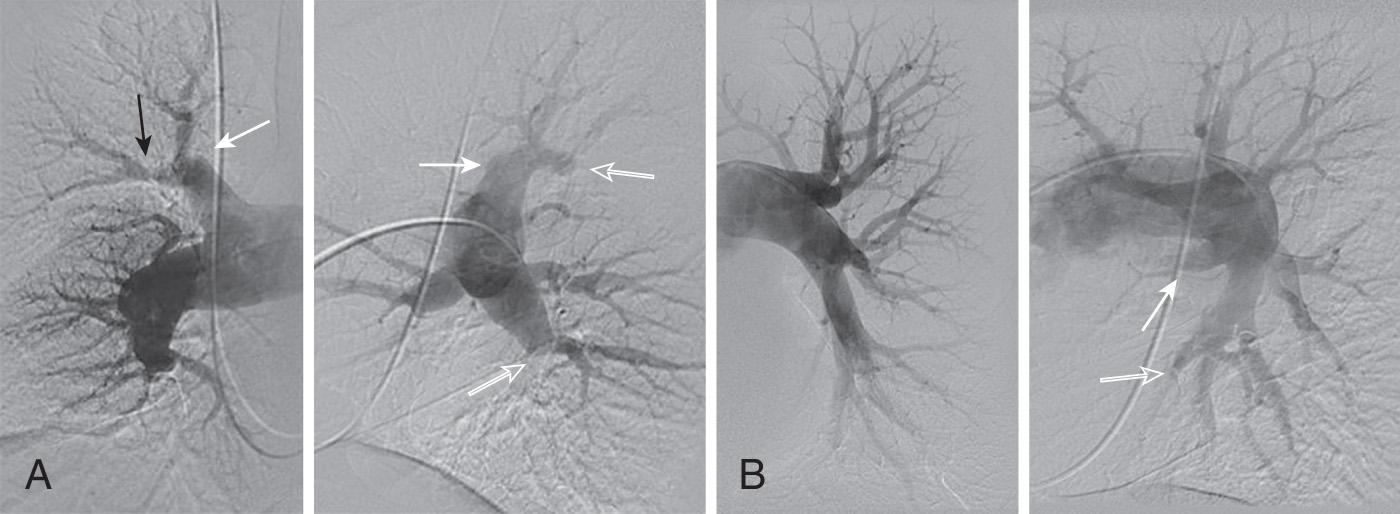
Catheter-based pulmonary angiography has traditionally been considered the gold standard for confirming the diagnosis of CTEPH and assessing the proximal extent of chronic thromboembolic disease in evaluating patients for PTE. In most circumstances, a properly performed pulmonary angiogram, including lateral projections, provides sufficient information on which to base a decision regarding chronic thrombus location and surgical accessibility. The angiographic appearance of CTEPH is distinct from the well-defined, intraluminal filling defects of acute PE. The angiographic patterns encountered in CTEPH reflect the complex patterns of organization and recanalization that occur following an acute thromboembolic event. Several angiographic patterns have been described in CTEPH, including “pouch defects,” PA webs or bands, intimal irregularities, abrupt and frequently angular narrowing of the major PAs, and complete obstruction of main, lobar, or segmental vessels at their point of origin. In most patients with CTEPH, two or more of these angiographic findings are present, typically involving both lungs ( Fig. 20.2 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here