Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Integral to the system of pulmonary gas exchange are mechanisms to maintain matching of pulmonary perfusion and alveolar ventilation, known as ventilation / perfusion matching . The goal is that alveolar oxygen and pulmonary capillary blood have intimate contact to optimize diffusion of O 2 and CO 2 . Requisite features of the efficient gas-exchange apparatus include sustained effective ventilation to replenish the oxygen stores in alveolar gas, free diffusion of both oxygen and carbon dioxide across the alveolar-capillary barrier, and sustained blood flow through the capillaries. Evolution has produced a situation in which gas exchange must switch sites from the placenta to the lung rapidly after birth. Strictly in terms of O 2 exchange, this switch represents a substantial improvement in the efficiency of the gas-exchange apparatus, from the circulatory countercurrent pattern of the human placenta to the alveolar-capillary interface in the lungs. Placental gas exchange is associated with a gradient from maternal uterine artery partial pressure of oxygen ( P O 2 ) of approximately 85 mm Hg at sea level to a fetal umbilical venous PO 2 of approximately 40 mm Hg, a gradient of approximately 45 mm Hg. In normal adult lungs the gradient from alveolar oxygen tension (approximately 110 mm Hg in room air) to arterial blood oxygen tension (approximately 100 mm Hg) is approximately 10 mm Hg; this gradient is referred to as the alveolar-arterial difference in O 2 (AaDO 2 ). The mechanisms for maintaining the matching of alveolar ventilation (
), usually referred to as
, and pulmonary perfusion (
), usually referred to as
, and the factors that influence changes in the intrapulmonary distribution of
and
and thereby the matching of
to
(
matching) in neonates are the topics of this chapter.
A distinct feature of postnatal gas exchange to which the lung must accommodate is the different frequency at which
and
occur. The pulmonary circulation maintains exclusively unidirectional flow, although at varying velocity, through the pulmonary microvasculature. By contrast, the inhalation of gases is periodic, occurring normally at a rate in neonates that is 20% to 50% of the cardiac rate during health and may be closer to 75% with pulmonary disorders. These different rates of ventilation and perfusion must be accommodated in achieving and maintaining optimal pulmonary gas exchange a continuous process. To help sustain sufficient O 2 and CO 2 flux between gas and blood, an adequate alveolar gas volume or functional residual capacity (FRC) must be established shortly after birth and sustained thereafter. Development of the FRC occurs in the process of transition from fetal to neonatal life as the fluid-filled fetal lung empties of liquid and refills with gas. Once established, this gas volume serves as an intrapulmonary reservoir for oxygen allowing for the continuous process of pulmonary gas exchange.
The newborn infant is particularly vulnerable to the development of arterial hypoxemia for several reasons. At the time of birth there is a rapid switch from the fetal circulatory pattern to the adult pattern, i.e., a rapid increase in pulmonary blood flow coupled with the onset of alveolar ventilation. If this does not occur, then the infant will maintain extra-pulmonary shunting of blood away from the lung across the patent ductus arteriosus (PDA) and/or foramen ovale leading to arterial hypoxemia. There are also postnatal characteristics of the neonatal lung that lead to this vulnerability to arterial hypoxemia. First, the partial pressure of oxygen in arterial blood ( Pa o 2 ) of normal newborns is low compared with that of adults. , Second, the FRC is relatively low given the compliant chest wall, resulting in less intravascular oxygen reserve during periods of no oxygen movement into the lungs (i.e., during apnea). Third, the FRC in the neonatal lung is close to the airway closing volume. Atelectasis, or airway closure, is more likely to develop, especially considering the relative paucity of channels of collateral ventilation in the newborn. Finally, the metabolic demand for oxygen in infants is greater on a per-kilogram basis than in adults. Therefore, the infant more quickly depletes oxygen stores in the blood and in resident alveolar gas in attempting to maintain aerobic metabolism. These factors are accentuated in premature infants, who are more susceptible to apnea because of their immature respiratory drive and because of lung segment collapse at the end of expiration due to extremely compliant chest walls.
The measurement of P a O 2 provides an excellent approximation of the efficiency of the lung as a gas-exchanging organ. One can use the P a O 2 and some assumptions to calculate the idealized alveolar (A) to arterial (a) oxygen gradient (or AaDO 2 ) by first calculating the P A O 2 by solving the alveolar air equation:
where R is the respiratory exchange ratio. P A CO 2 is assumed to be approximately equal to P a CO 2 and P I O 2 is calculated from measured barometric pressure and body temperature, and assumes that inspired gas is 100% saturated with water vapor ( PH 2 O = 47 mm Hg) on reaching the acinar space ( P I O 2 = F I O 2 × [ PB – PH 2 O ]). Thus, at sea level, breathing ambient air, the P I O 2 = 150 mm Hg. As an example, the P A O 2 for a normal adult breathing ambient air at sea level and assuming a P A CO 2 of 40 mm Hg and an R of 0.8 would be P A O 2 = 150 − 35/0.8 + [0.21 × 40 × (1 − 0.8)/0.8] = 108 mm Hg. Thus, if the P a O 2 is 100 mm Hg, then the AaDO 2 = 108 mm Hg − 100 mm Hg = 8 mm Hg. Neonates have larger AaDO 2 values than do adults during ambient air breathing, and the AaDO 2 can be as high as 40 to 50 mm Hg shortly after birth, even allowing for R values close to 1.0 in neonates (presumably based on relatively high utilization of carbohydrates). AaDO 2 may remain in the 20 to 40 mm Hg range for days after birth in term nondistressed infants , even with Sp o 2 values greater than 90% (remember that the oxygen–hemoglobin dissociation curve for fetal hemoglobin is shifted to the left compared to adult hemoglobin).
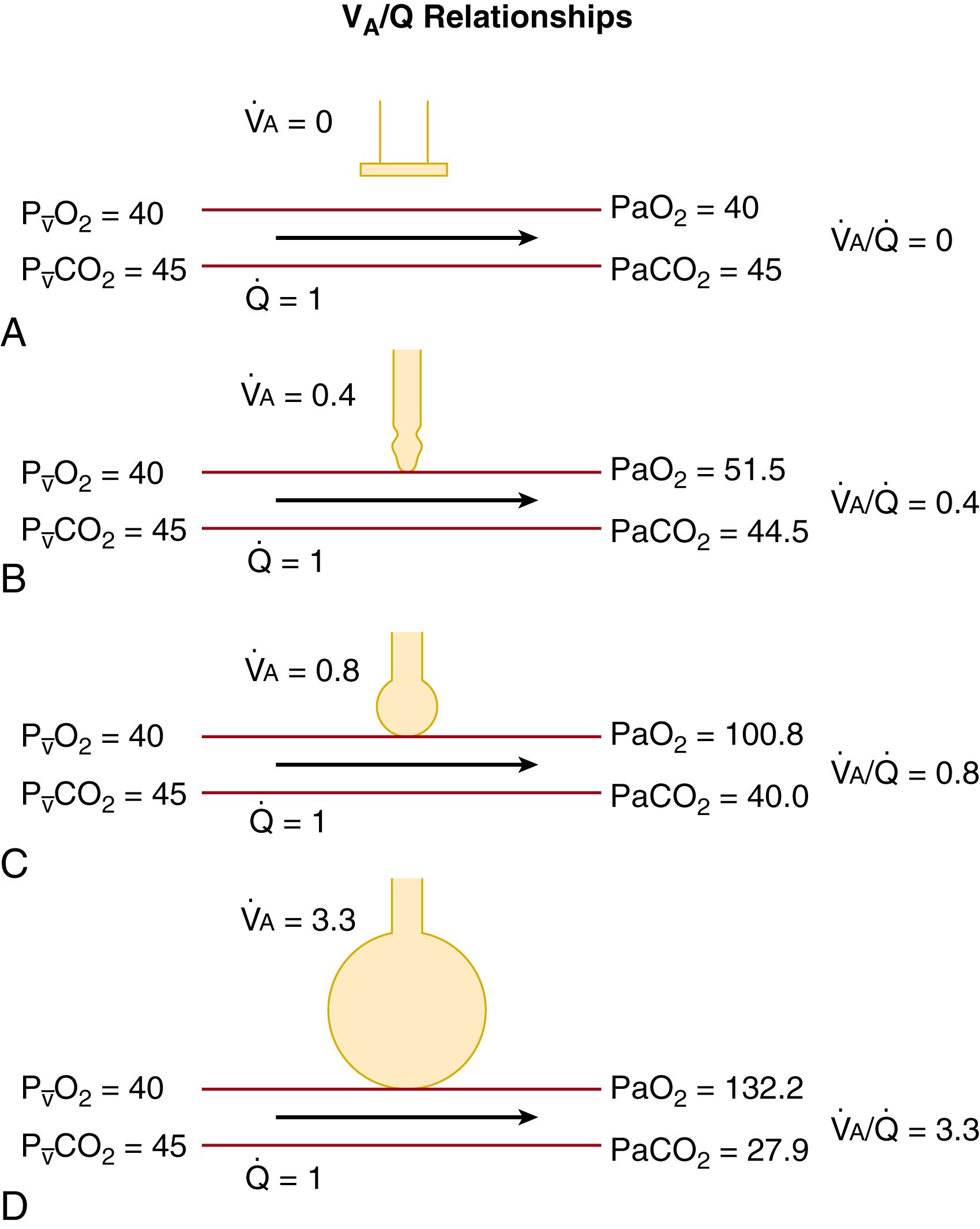
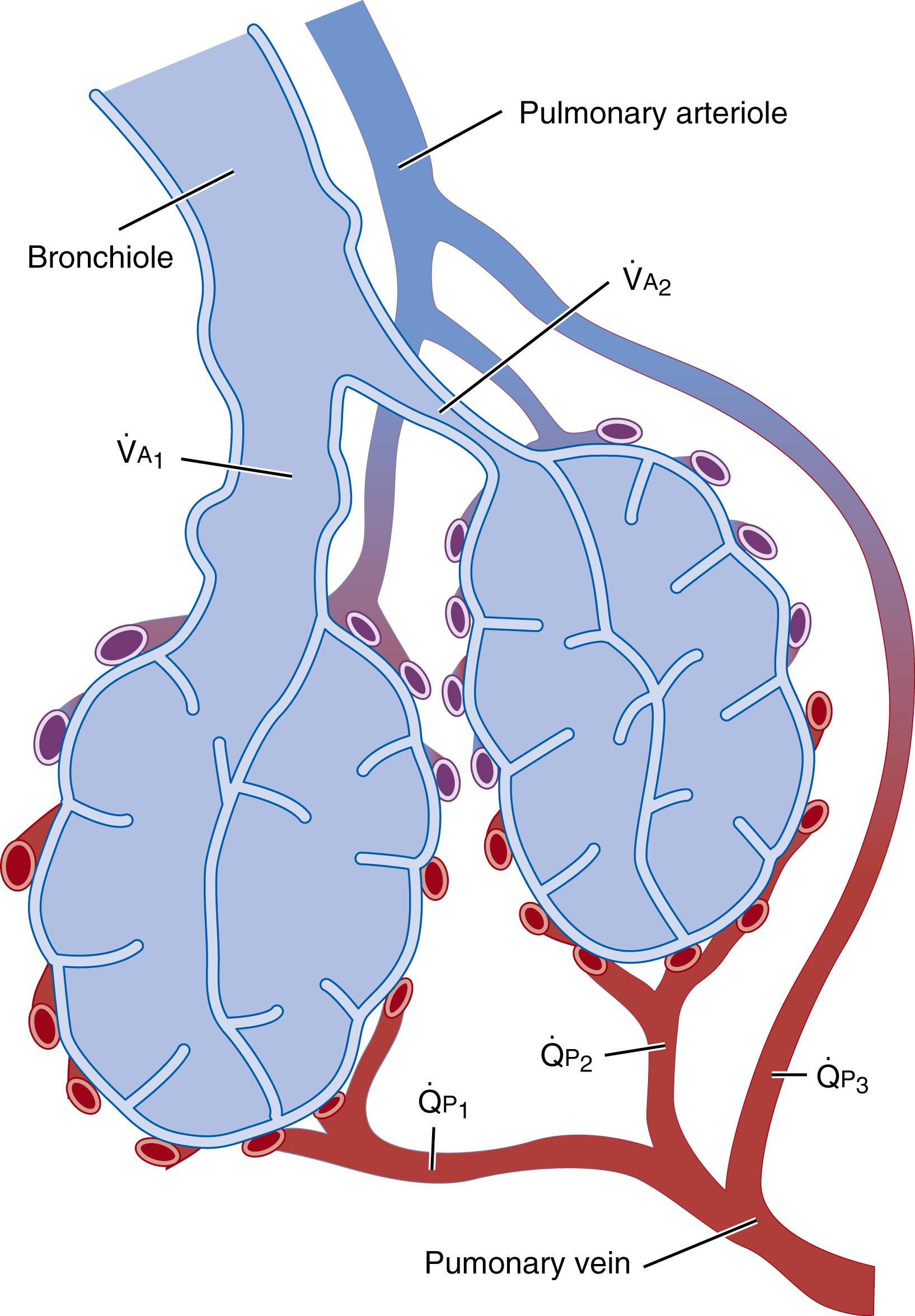
AaDO 2 quantifies the degree of venous admixture plus alveolar-capillary membrane diffusion disequilibrium. However, neither AaDO 2 nor the calculated ratio of arterial-to-alveolar oxygen tension ( P a O 2 / P A O 2 ) discriminates between these components, and both are insensitive to situations with both intrapulmonary (or extrapulmonary) shunt and pulmonary parenchymal diseases. Venous admixture includes both intrapulmonary shunt (perfusion of pulmonary capillary blood past non-ventilated lung regions before joining the stream of pulmonary venous blood, see Fig. 64.1A and
in Fig. 64.2 ) and perfusion of low
areas (see Fig. 64.1B and
Fig. 64.2 ), in which alveolar ventilation is insufficient to restore P A O 2 to the value predicted from the “idealized” P A O 2 calculated earlier. Pulmonary end-capillary to alveolar space equilibrium can be predicted to occur for oxygen and carbon dioxide in low
areas because P A O 2 is low and only a small rise in pulmonary P a O 2 occurs as blood traverses the pulmonary microcirculation ( Fig. 64.1B ). Depressed pulmonary end-capillary PO 2 is associated with disproportionately depressed oxygen content because of the shape of the oxygen-hemoglobin curve, leading to an exaggerated depression of the mixed arterial PO 2 as measured in the left atrium or systemic arteries (assuming no extrapulmonary right-to-left shunts). Therefore, overall pulmonary gas exchange is the flow-weighted and alveolar ventilation–weighted sum of gas exchange occurring across all of the lungs’
regions (see Fig. 64.2 ). Note that the rise in P a O 2 in the high
region (see Fig. 64.1D ) and the decrease in P a CO 2 do not equal levels of alveolar gas partial pressures that would be found if no regional perfusion existed. Lung units with both low
and normal
relationships are depicted in Figs. 64.1 and 64.2 . Also shown is a pulmonary arterial to pulmonary venous connection bypassing any aerated area (see Fig. 64.1A and
in Fig. 64.2 ), which in the neonate could represent shunt through the ductus arteriosus, the foramen ovale, connections between pulmonary and bronchial arteries, or through the Thebesian circulation (venous drainage from the myocardium directly to the left ventricle). In the preterm infant with surfactant deficiency, this could also represent blood flowing past atelectatic alveoli, which have collapsed due to increased surface tension. Indeed, one reason that preterm infants with surfactant deficiency respond to exogenous surfactant therapy with an increase in P a O 2 (or Sp o 2 ) is that
matching is improved as atelectatic alveoli are reinflated and ventilated.
Although the AaD o 2 measured in ambient air provides an overall summary of pulmonary oxygen exchange efficiency, it provides little information about pulmonary reserves or about the cause of an increased AaDO 2 . A surrogate measure of pulmonary gas exchange that is related to the AaDO 2 , the oxygenation index (OI) combines P a O 2 , F I O 2 , and lung distending pressure as measured by the mean airway pressure (MAP), where OI = (MAP × F I O 2 × 100)/ P a O 2 . The OI is commonly used in the neonatal intensive care unit (NICU), in particular in term infants, to help guide decisions related to need for advanced therapies. Although the OI is helpful in estimating AaDO 2 or the degree of
mismatch, it also does not discriminate between intrapulmonary and extrapulmonary causes of hypoxemia.
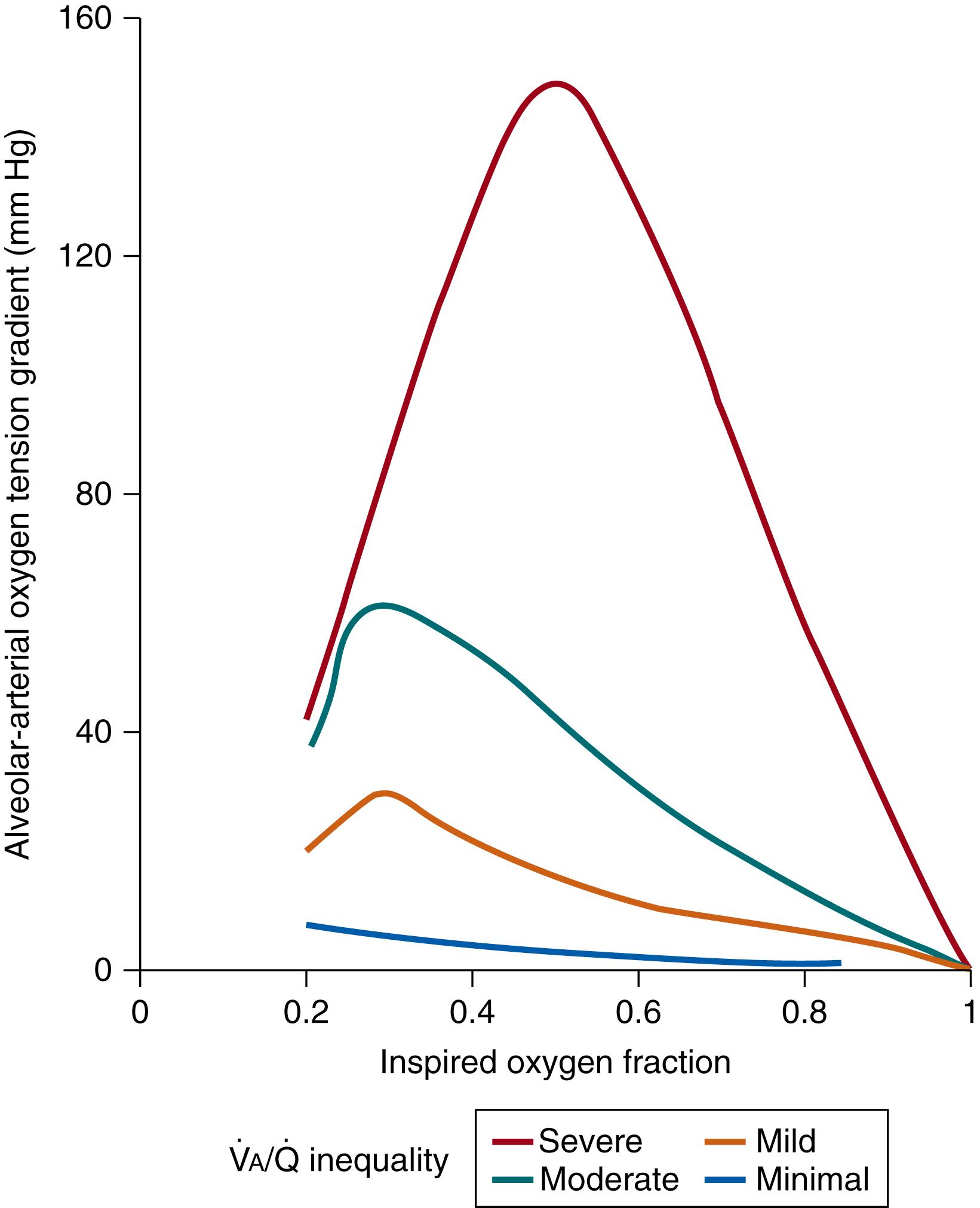
The effect of low
areas on AaDO 2 can be eliminated by breathing 100% oxygen to remove nitrogen from any open but underventilated lung regions ( Fig. 64.3 ). However, breathing 100% oxygen is not sustainable as a method to improve
matching, since absorption atelectasis tends to develop, which would paradoxically increase the contribution of the intrapulmonary shunt and thereby result in an increase in
mismatch and a decrease in P a O 2 . Furthermore, prolonged exposure to 100% oxygen causes severe central nervous system and pulmonary toxicities. Interestingly, as shown in Fig. 64.3 , the AaDO 2 tends to increase as the F I O 2 is increased from 0.21, and then as the F I O 2 continues to increase, slowly decreases until with an F I O 2 of 1.00 there is no AaDO 2 (see Fig. 64.3 ). The greater the degree of
mismatch, the larger the increase in AaDO 2 with increasing F I O 2 . There is evidence that increased shunt with oxygen breathing occurs in premature infants and neonatal lambs but not in adult dogs. These differences have been attributed to different degrees of development of pathways of collateral ventilation. Hence, neither P a O 2 nor the ratio of arterial-to-alveolar PO 2 alone provides detailed knowledge of the state of the
relationships in the lung. The unique shape of the oxygen and carbon dioxide hemoglobin dissociation curves make precise quantification of exact patterns of
heterogeneity difficult to determine. Use of multiple inert gas techniques to directly measure
have not completely eliminated the uncertainty in
distribution. Furthermore, there is no clinically useful way of measuring
distribution in the neonatal lungs due to the size of infants, although some experimental techniques have been reported. There has been an attempt to model various
distributions using readily available bedside measurements such as Sp o 2 , arterial blood gases, and other means. However, all of these models have their own limitations and therefore there is no particular method that has come into widespread clinical use. Thus, Sp o 2 and/or P a O 2 are used in the NICU as surrogate measures of
matching, despite all of the weaknesses described above.
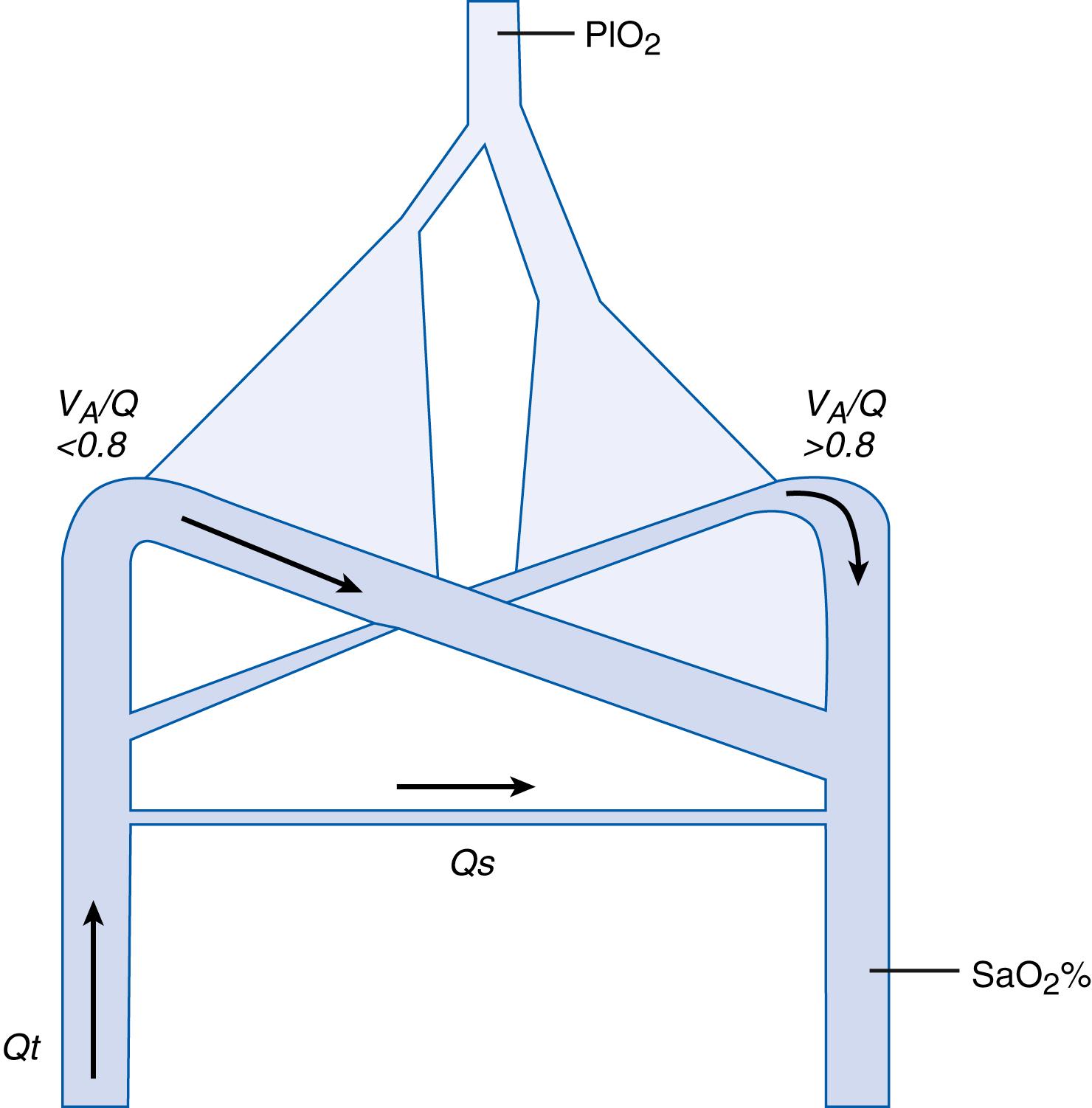
In an effort to separate the effects of intrapulmonary shunt from regions of low
, Roe and Jones and Smith and Jones have created algorithms in which simultaneous measurements of Sp o 2 were plotted against the calculated P I O 2 value. Using these graphs, one can differentiate shunt from low
areas. The method assumes that changes in the shape of P I O 2 versus Sp o 2 curves, or changes in the position of the curves, are reflective of changes in shunt or in low
areas, or both. ( Fig. 64.4 ). Thus, the degree of right shift of the curves away from the oxyhemoglobin dissociation curve is an index of reduced
matching. This technique may translate into individualization of approaches to improving gas exchange in the two different circumstances or in the circumstance in which both significant shunt and significant low
areas contribute to arterial hypoxemia. One limitation of the technique is the need to measure Sp o 2 and P I O 2 data points quickly. The measurement of Sp o 2 in the NICU has its own limitations. Another limitation of the technique proposed , is that the low
area is not further quantifiable. The importance of the low
area, which is treated as a single value, may not be differentiated among different regions with variably low
areas (<0.8). As with other techniques, this assessment assumes standard hemoglobin concentration and pH. The model uses a three-compartment gas-exchange model: namely, shunt, a normal
region, and a low
region, as shown in Fig. 64.5 . Differentiation of shunt from low
at the bedside could contribute to both improved understanding of the cause of hypoxemia and consequently to more specific therapy.
![Fig. 64.4, The effects of arterial oxygen saturation (S a O 2 ) with changing inspired oxygen pressure P I O 2 (heavy lines ), with either (A) increasing shunt from 0% to 20% (downward arrow) or (B) reducing ventilation–perfusion ratio ( V˙/Q˙ V˙/Q˙ from 0.8 to 0.4 [rightward arrow] ). The oxyhemoglobin dissociation curve is the long dashed line, which plots blood S a O 2 versus P a O 2 . Fig. 64.4, The effects of arterial oxygen saturation (S a O 2 ) with changing inspired oxygen pressure P I O 2 (heavy lines ), with either (A) increasing shunt from 0% to 20% (downward arrow) or (B) reducing ventilation–perfusion ratio ( V˙/Q˙ V˙/Q˙ from 0.8 to 0.4 [rightward arrow] ). The oxyhemoglobin dissociation curve is the long dashed line, which plots blood S a O 2 versus P a O 2 .](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PulmonaryGasExchangeintheDevelopingLung/3_3s20B9780323712842000641.jpg)
When P A O 2 is low because of diminished alveolar ventilation (i.e., insufficient replacement of alveolar oxygen from inspiratory gas relative to the rate of removal by pulmonary capillary blood), the alveolar partial pressure of nitrogen ( P A N 2 ) rises because during “no flow” states (end-expiration and end-inspiration) the sum of gas partial pressures in the alveolar spaces will equal atmospheric pressure. P A H 2 O and P A CO 2 are relatively constant; therefore, when P A O 2 is diminished, P A N 2 must be increased. If the elevated P A N 2 is sustained, there will be increased absorption of nitrogen into pulmonary capillary blood and development of an arterial-to-alveolar nitrogen gradient (aAD N 2 ), which has been used to identify the presence of low
lung regions. Perfusion of non-ventilated lung units produces no increase in P A N 2 , because the mixed venous and end-capillary partial pressures are the same for O 2 , CO 2 , and N 2 , as well as for any other inert gas solution (see Fig. 64.1A ). An aADN 2 gradient can develop because of the presence of either a small area of very low
or a larger region of low
, as long as
is less than 1.0. Use of aADN 2 to indirectly estimate shunt (
= 0) may result in an overestimation of shunt, because of the sigmoidal shape of the oxygen-hemoglobin dissociation curve (i.e., small reductions in P A O 2 can disproportionately depress the arterial oxygen content on the steep part of the curve).
On the basis of measurements of AaD o 2 and aADN 2 in healthy newborn infants Krauss and colleagues , inferred that a relatively small low
region occurred after 1 to 2 days of postnatal life. Furthermore, the calculated aADN 2 in infants with respiratory distress syndrome (RDS) showed little contribution from low
areas. This observation suggests that the large venous admixture occurring with RDS results from intrapulmonary and/or perhaps extrapulmonary shunt.
Arterial-to-alveolar differences for carbon dioxide (aADCO 2 ) reflect areas of the lungs that are poorly perfused but have a large dead space because they receive a substantial fraction of minute ventilation (
). Dead space in the lung is defined as areas that are ventilated but do not participate in gas exchange. There is normally a part of the tidal volume that fills the parts of the respiratory apparatus not involved in gas exchange, but involved in getting air from the atmosphere to the alveoli, including the upper airways, trachea, and conducting airways, and this is referred to as the anatomic dead space. Physiologic dead space includes the anatomic dead space plus those areas of the lung that are ventilated but not perfused, or ventilated and very poorly perfused (high
areas). Mismatching of
as a cause of CO 2 retention has been emphasized to explain the development of respiratory acidosis when minute ventilation is elevated above normal levels. A small aADCO 2 exists in some newborn infants, but when a normal FRC has been established, the small magnitude of the gradient implies only a modest presence of high
areas, except in premature infants where the aADCO 2 gradient remains somewhat larger, suggesting the presence of relatively more high
areas. Deficiencies in CO 2 exchange during respiratory disorders probably persist after the minute ventilation has been normalized (i.e., with mechanical ventilation) due to excess physiologic dead space ventilation.
Trace quantities of inert gases can be used to quantitate the continuous distribution of
from shunt to dead space (
of 0 to
of infinity). Wagner and colleagues , described a technique that uses six inert gases (which encompass a wide range of solubility in blood, see Fig. 64.6A ) and in which retention of each gas in blood perfusing the lung can be expressed by the simple relationship
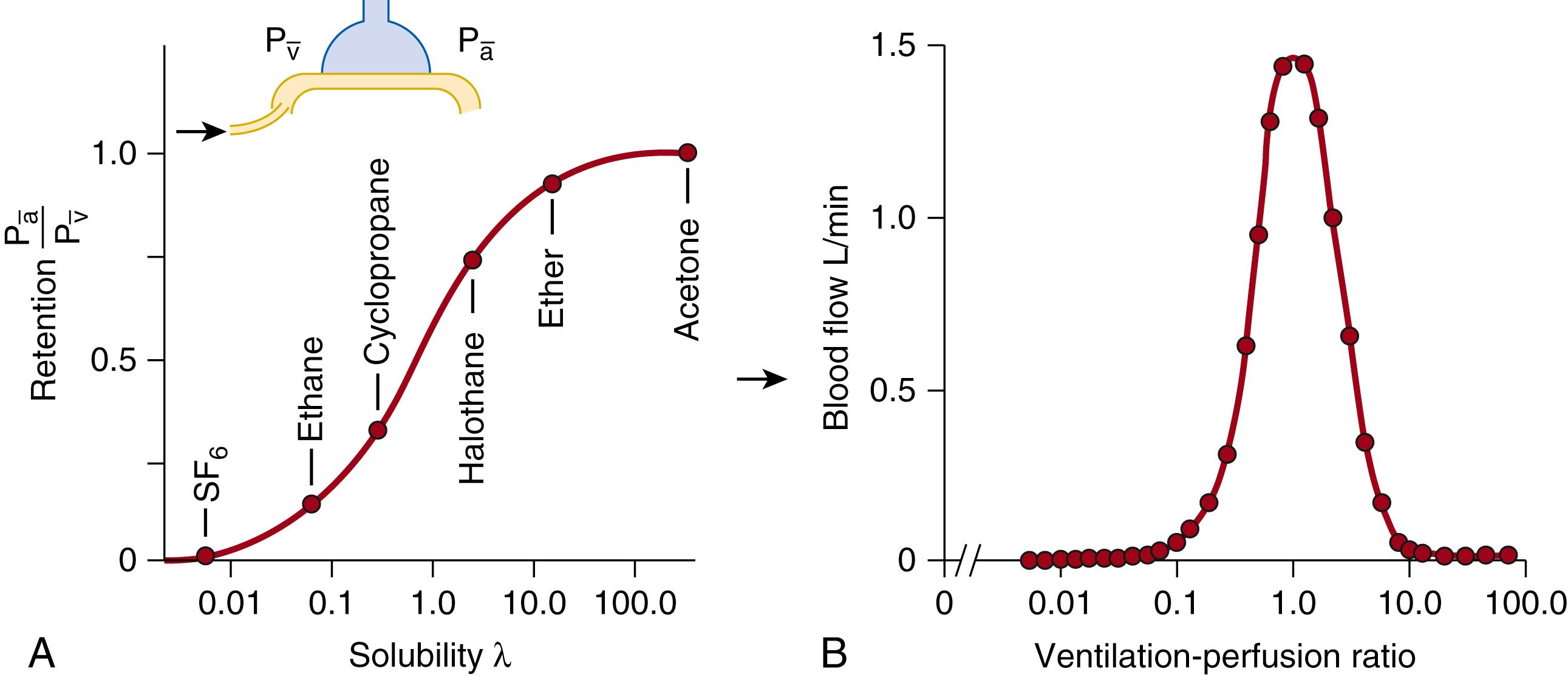
where λ is the Ostwald blood-gas solubility coefficient (unique for each gas) and P a , P v , and P alv are the partial pressures of the gas measured in arterial blood, mixed venous blood, and alveolar gas (in reality measured in mixed expired gas and corrected for dead space ventilation), respectively. The equation assumes that each gas demonstrates a linear dissociation between blood or plasma and air, behavior unlike that demonstrated by the respiratory gases O 2 and CO 2 . Under conditions of presumed steady-state gas exchange, which is always an approximation of in vivo situations, the multiple inert gas elimination technique (MIGET) allows quantification of shunt, dead space, and low and high
lung regions during both normal and experimental conditions (see Fig. 64.6B ). A major advantage of the technique is the quantification of shunt, separate from low
areas, without the need to resort to 100% oxygen breathing. The experimental data consist of the measured ratios of the mixed arterial to mixed venous and mixed expired to mixed venous partial pressure for each of the gases used (usually sulfur hexafluoride, ethane, cyclopropane, halothane, diethyl ether, and acetone) and the measured solubility of each gas ( Fig. 64.7 ). Limitations of the inert gas technique include the lack of online analysis capacity for assessment of
relations and the necessity of the assumption of steady-state gas exchange.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here