Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pulmonary embolism (PE) and deep vein thrombosis (DVT) are common problems in patients with cancer owing to local and humoral effects of the tumor as well as to the effects of therapy. The diagnosis of venous thromboembolic disease is challenging because of nonspecific clinical signs, symptoms, and laboratory evaluations. Computed tomography (CT) angiography has taken a central role in the diagnosis of PE. CT provides the opportunity to identify PEs and to assess the severity of involvement and hemodynamic effects. Treatment of venous thromboembolism in cancer patients generally follows the standards used for nononcology patients. However, treatment can be complicated in patients with cancer because of drug interactions and continued stimulus for thrombus formation. PE remains a challenging problem in the management of patients with cancer.
PE results from the migration of thrombi formed in the systemic venous system, usually from the pelvis or lower extremities, into the pulmonary arterial system. These thrombi form as a result of abnormal veins, abnormal blood flow, or abnormalities of the coagulation system, all of which are frequently present in patients with cancer. Central venous catheters are a significant risk factor for thrombus formation, with up to 4% of patients with central venous catheters developing catheter-related thrombus. The majority of upper-extremity DVTs are associated with catheters. Abnormalities in the coagulation system that predispose to venous thrombus formation include various coagulation factor abnormalities and cancer-associated hypercoagulability. Chemotherapeutic agents such as methotrexate, doxorubicin, thalidomide, and hematopoietic stem cell–stimulating agents can promote thrombus formation. Venous thromboembolism rates are highest in patients on chemotherapy, with an incidence as high as 28% in patients with malignant gliomas. The frequency of presentation of venous thromboembolism is more closely related to the frequency of the tumor in the population, with most seen in patients with lung, colon, and prostate cancer.
Cancer patients are also at greater risk for poor outcomes after PE. For example, they are four to eight times more likely to die after a PE event. The 1-year survival for patients with cancer and venous thromboembolism is approximately one-third that of patients with cancer without venous thromboembolism.
DVTs occur most often in the pelvic and lower extremity veins. The fragments that detach and embolize are typically tubular in shape and can vary from less than 1 mm in diameter to up to 2 cm in diameter. The larger-diameter thrombi may tend to be longer and may have branching components.
Once detached, emboli flow through the venous system until they encounter an impeding structure or reach a vessel that is too small to permit their passage, commonly a pulmonary arterial branch. Emboli may be captured en route to the pulmonary arterial system in trabeculations within the right atrium, in a congenital Chiari network located at the base of the right atrium, or while passing through a patent foramen ovale. Thrombi that pass through the foramen ovale can embolize to systemic structures, including the brain.
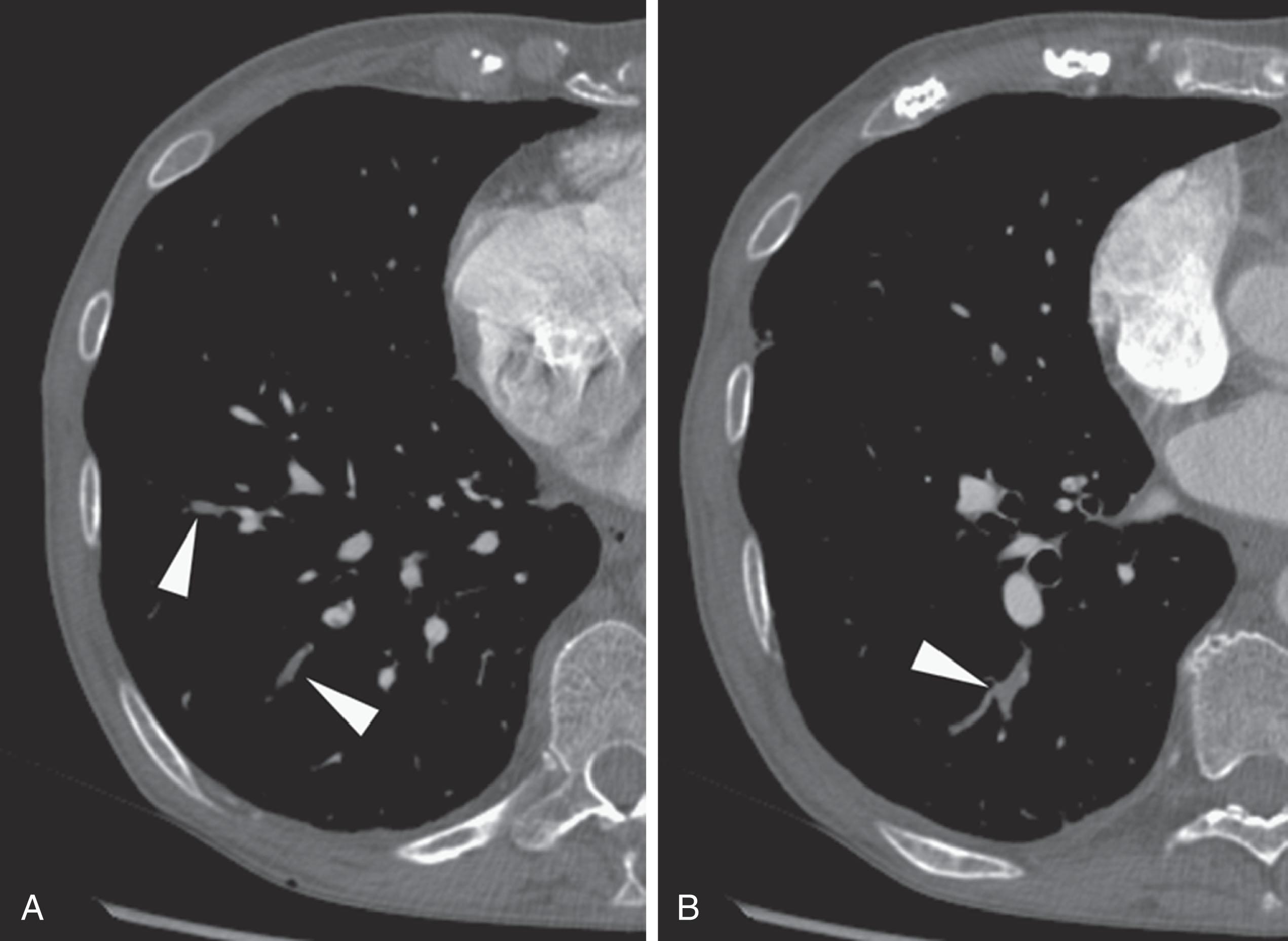
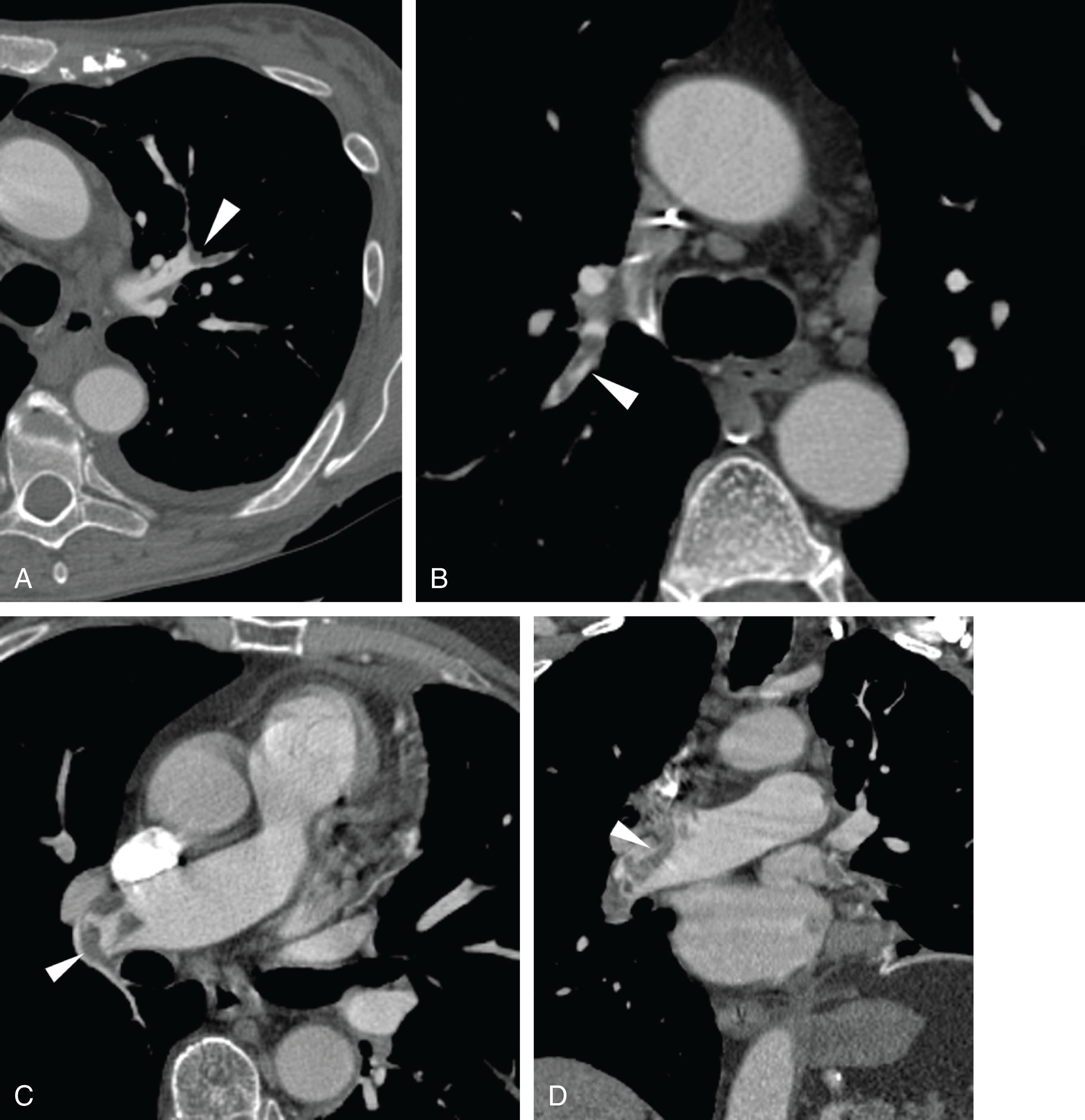
Emboli that reach the lungs become lodged at arterial branch points or may reach an arteriole of lesser diameter than the embolus ( Fig. 42.1 ). Larger and more elongated emboli will commonly be captured at branch points and may extend into multiple lobes and segments of the pulmonary arterial tree ( Fig. 42.2 ). The largest PEs may become lodged across the main pulmonary artery bifurcation and are called saddle emboli ( Fig. 42.3 ). Usually, there is a small bandlike component extending across the pulmonary artery bifurcation, with larger components of thrombus extending into the right and left pulmonary arteries and lobar or segmental branches.
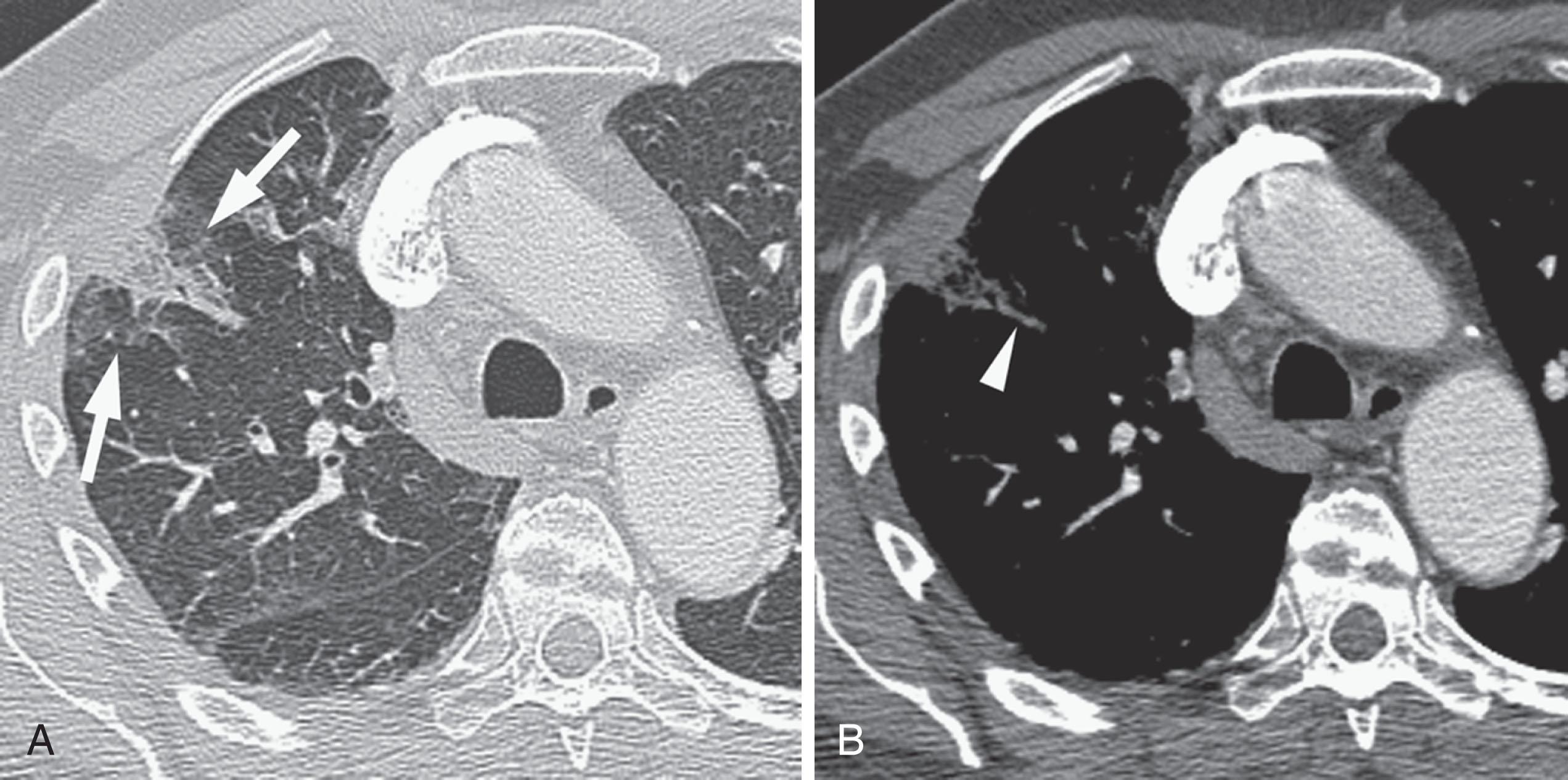
If the embolus completely occludes a segment of the pulmonary arterial system, it can result in pulmonary infarction. This is relatively uncommon because of the parallel blood supply from the bronchial arteries. Pulmonary ischemia or infarction results in hemorrhage and edema within the affected portion of lung. The involved area typically extends out in a wedge-shaped pattern from the occluded arteriole and reaches the pleural surface ( Fig. 42.4 ). The hemorrhagic phase is followed by necrosis and coagulation of the underlying lung architecture. The infarct typically evolves over several days to weeks and may resolve completely or result in a small area of scarring.
Emboli are typically elongated “casts” of the vein of origin.
Emboli tend to become lodged at branch points or become wedged in smaller-caliber arteries.
Emboli can become entrapped in transit through the right heart chambers or pass through a patent foramen, causing systemic embolism.
Wedge-shaped consolidation peripheral to an occluded vessel represents pulmonary ischemia or infarction and involutes over time.
DVT may be completely asymptomatic or may present with a variety of nonspecific symptoms, including asymmetrical extremity swelling or an erythematous tender mass along the venous structure. PE may also be asymptomatic or cause nonspecific symptoms such as chest pain or dyspnea. The acute onset of dyspnea is the most suggestive symptom and may be accompanied by a sense of impending doom. Emboli that cause right heart strain can produce systemic edema and abnormalities at cardiac auscultation.
Clinical history is helpful in suggesting PE. Any recent period of prolonged immobilization, active cancer, or use of drugs associated with thrombosis should raise a suspicion of PE in patients with new dyspnea.
Patients with PE often have nonspecific laboratory abnormalities such as elevated d -dimer levels, abnormal coagulation measurements such as prothrombin time, partial thromboplastin time, and the International Normalized Ratio, as well as elevated erythrocyte counts and hemoglobin levels and abnormalities of platelet number or function. These are typically abnormal related to the cause of thrombus formation, rather than a result of the thrombus or embolus itself. Arterial blood gas may show an increased difference between alveolar and arterial oxygen concentration, an increased A-a gradient, and pulse oximetry is often abnormal. These findings are nonspecific and indicate only an abnormality of pulmonary oxygenation, which may be the result of pneumonia, embolism, or tumor, among other possibilities.
PEs may be detected on routine imaging of cancer patients without suspicion of PE. In patients without cancer, PEs are detected in up to 1% of outpatients and 3% of inpatients. Among patients with cancer, 3% to 4% of outpatients and over 6% of inpatients will have unsuspected PE at routine CT scanning. Unfortunately, up to 75% of these, including large central emboli, will not be seen at clinical interpretation, particularly in patients with complex presentations of their underlying malignancy. The significance of subsegmental or smaller PE is unknown. Some studies have shown recurrence rates in patients with isolated subsegmental PE similar to that of negative CT pulmonary angiography and much less than for patients treated for PE. Other studies have shown that anticoagulation for unsuspected PE improves survival. Further study is needed to determine the appropriate treatment of isolated subsegmental PEs in particular and unsuspected, asymptomatic PEs in general.
The size of PEs and the extent of involvement of the pulmonary arterial tree can impact the clinical symptoms and long-term outcome of these patients. More severe PEs are more likely to be associated with right heart strain and more likely to require aggressive intervention, including intensive care unit admission. Evaluation of pulmonary embolic disease, therefore, involves an assessment of the amount of thrombus and of any secondary effects.
Several scoring systems for the extent of pulmonary embolic disease have been proposed. These vary from simple assessment of the most proximal level of involvement to detailed scoring of the number and severity of obstructed vessels, and correlate with short-term outcomes and the use of intensive care unit admissions. However, the risk of death from an acute episode of PE primarily depends on the hemodynamic state of the patient. Patients with cardiac shock have a high risk of death, normotensive patients with right heart dysfunction have an intermediate risk, and patients without heart dysfunction do not have a high risk. Whereas cardiac shock is diagnosed clinically, right heart dysfunction may be evaluated with imaging.
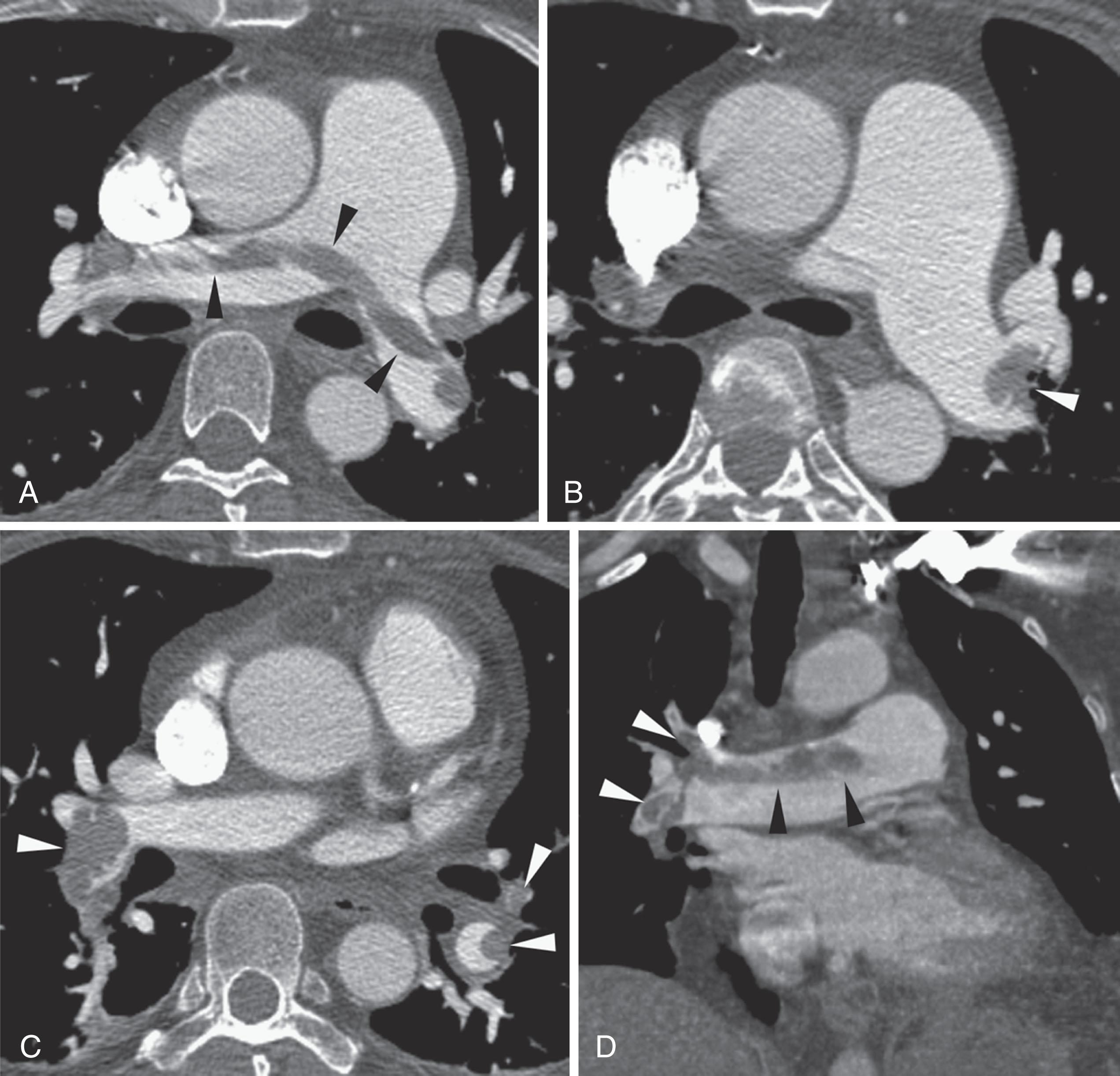
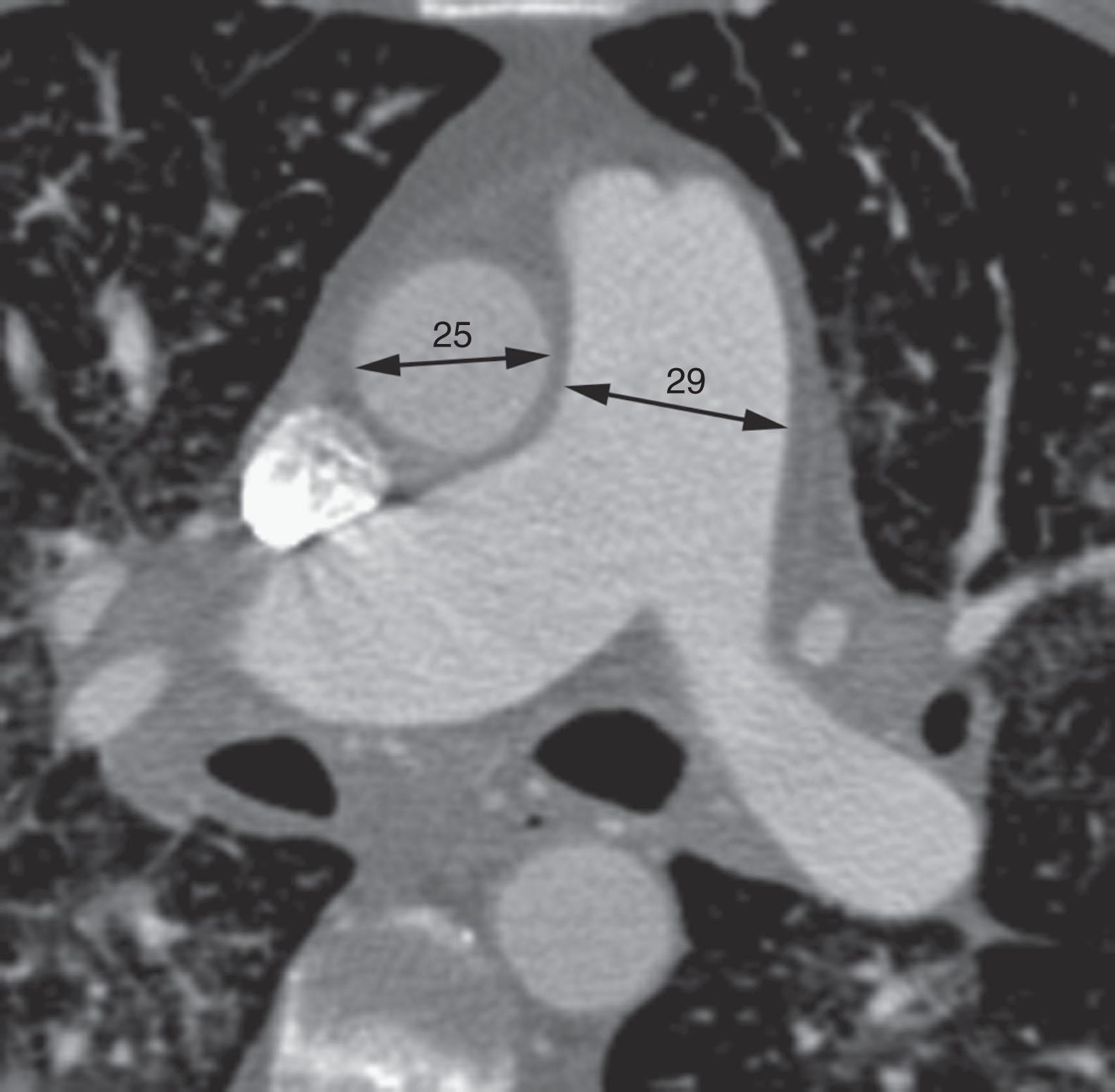
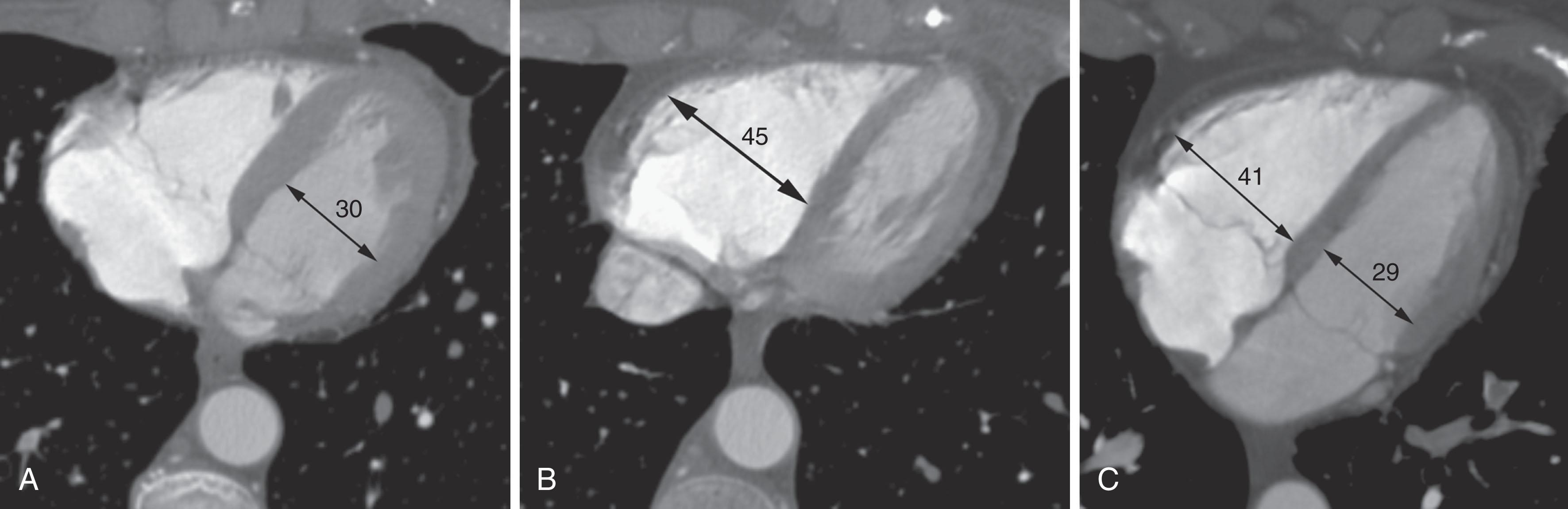
Right heart dysfunction as a result of PE is represented in the development of pulmonary hypertension, right heart strain, and right heart failure. Assessment of these parameters at echocardiography or CT is predictive of survival to hospital discharge and the need for intensive care unit admission. At CT, pulmonary artery diameter greater than 30 to 33 mm or greater than the aortic diameter ( Fig. 42.5 ) is suggestive of pulmonary hypertension. A right ventricle to left ventricle diameter ratio greater than 1.0:1 to 1.5:1 suggests right heart strain or right heart failure ( Fig. 42.6 ). Secondary signs of right heart strain and failure are less specific but include reflux of contrast material into the inferior vena cava (IVC) and hepatic veins and evidence of systemic edema, including hepatic congestion and subcutaneous edema.
These various methods of assessing the amount and severity of pulmonary arterial embolic disease and the effects of right heart strain are useful in identifying patients in need of more aggressive therapy and monitoring. They are not as helpful in predicting recurrent embolic disease. Therefore, identifying and ameliorating the inciting factors are important in the patient’s long-term management.
Scoring systems reflect the number of lung segments involved by emboli and predict the need for intensive management.
Right heart strain, reflected in right ventricle or pulmonary artery dilatation, also predicts survival and need for more intensive management.
Identifying inciting factors is critical in long-term management.
Traditionally, imaging for PE was previously done with chest x-ray and ventilation-perfusion (V/Q) scanning. Unfortunately, these methods are nonspecific and often do not provide a final diagnosis. Imaging now focuses on CT pulmonary angiography, which allows direct and noninvasive imaging of the pulmonary arterial tree. Imaging for DVT is predominantly done with ultrasonography. Venous-phase imaging in the lower extremity veins after CT pulmonary angiography has also been performed, but its radiation dose is high, especially relative to the nonionizing ultrasound examination.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here