Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Appreciation is extended to Drs. J. Duncan, P. Eide, M. Wagshul, G. Silverberg, P. McAllister, and M. Pollay for helpful discussions on chapter material. Acknowledgement is due to N. Johanson and J. Johanson for text editing and figure construction.
The choroid plexus secretes most of the ventricular cerebrospinal fluid (CSF) and regulates its pH/ions as well as its volume and pressure.
Cephalocaudad CSF volume transmission (bulk flow) from lateral ventricles to basal cisterns delivers vitamins, neurotrophic factors, and peptides to multiple brain regions.
The choroid plexus–CSF mediates central neuroendocrine and immune actions by regulating transport of hormones and immune molecules/leukocytes across the blood-CSF interface.
Chemical sensors in the choroid plexus epithelium respond to changes in neurochemical and immune molecules in CSF by secreting trophic molecules to heal injured brain regions.
To control intracranial pressure, fluid formation by the choroid plexus decreases compensatorily in response to increasing titers of CSF neurotransmitters and fluid-regulating neuropeptides.
Nocturnally increased CSF flow promotes glymphatic flushing of brain interstitial waste to lymph drainage sites; chronic glymphatic blockage predisposes to neurodegeneration.
Noninvasive MRI assessments of aqueductal flow and brain compliance to predict shunting outcome also help evaluate CSF hydrodynamic changes in neural diseases.
In aging, idiopathic normal pressure hydrocephalus (iNPH), and Alzheimer disease, dwindling CSF formation and ventriculomegaly cause harmful stagnation of CSF turnover rate.
To maintain healthy fluid dynamics in older adult patients, novel biomedical strategies are needed to maintain viable choroid plexus–CSF flow routes and brain capillary function.
CSF secreted by the choroid plexus dynamically affects the neuronal milieu. Diverse choroidal regulations mediate homeostasis of fluid composition, volume, and pressure. One of several transport interfaces in CNS, the choroid plexus–CSF performs work to complement the blood-brain barrier (BBB), ependyma, and pia mater ( Fig. 42.1 ). Known as the blood-CSF barrier (BCSFB), the choroidal epithelium carries out abundant transport/secretory actions that aid the brain. Epithelial cell ultrastructure ( Fig. 42.2 ) reveals a high capacity of organelles to form fluid and adapt to changes in CSF neurochemistry.
Over a lifespan, adverse physico-chemical stressors and diseases (e.g., hydrocephalus, stroke, and Alzheimer disease [AD]) strike the CSF-brain nexus. Sensors/receptors in the choroid plexus detect elevated intracranial pressure (ICP) and biochemical/biophysical harm to the peripheral nerve, spinal cord, and injured brain. , Adjustive responses by choroid plexus elements then help repair injury and restore milieu balance at distant regions of the nervous system. Breakdown of the homeostatic reserve of the choroid plexus in senescence and neurodegeneration jeopardizes the ability of the CSF to maintain brain well-being. This chapter discusses the neurosurgical aspects of adult choroid plexus–CSF secretions and flow, relative to brain fluid balance in health and disease.
The master generator of ventricular CSF is the choroid plexus (see Figs. 42.2 to 42.4 ). Continuously produced CSF implements stability of CNS extracellular fluid ions and volume. During the day and at night, the choroid epithelia churn out fluid. Ventricular CSF formation is ∼0.4 mL/min/g choroid plexus for several mammals and is somewhat less for humans. Nascent CSF is more than a passive ultrafiltrate of plasma. Active secretion by choroidal epithelium is exquisitely modulated so that ICP is stable when CSF absorption is normal. One-tenth of the choroidal blood flow of ∼4 mL/min/g , becomes new CSF in the ventricles. Volume relationships among plexus blood flow, choroid cell size, and fluid output are regulated by hormones.
Multisource evidence indicates that 60% to 70% of CSF is formed by plexuses in the lateral third and fourth ventricles. It is rarely challenged that the choroid plexus is the cardinal source of ventricular CSF. Current , and historical , overwhelming evidence points to the choroidal tissue as the main origin of CSF. , Clinical cauterizations of choroid plexus lower ICP, further evidence that ventricular CSF originates largely from choroidal tissues. A lavish net turnover of fluid at the blood-CSF interface is energized by high blood flow to the plexus, substantial activities of Na + -K + -ATPase , and carbonic anhydrase, and numerous ion transporters. In sum, the choroid plexus is operationally geared for great fluid turnover.
On the other hand, net formation of fluid at the BBB (see Fig. 42.1 ) is much slower than at the choroidal BCSFB. Actually, substantial bidirectional H 2 O exchange at the BBB may be faster than choroidal CSF production ; however, this cerebral capillary H 2 O exchange flux is not a true net fluid secretion by capillaries. Such primary fluid-secretory capacity difference between CNS transport barriers manifests in that BBB Na + transport (linked to H 2 O transfer) is insensitive to acetazolamide (Diamox) inhibition of carbonic anhydrase, in contrast with inhibitable Na + transport by the ICP-sustaining choroid plexus fluid generator. Thus evidence is lacking that cerebral capillaries form a CSF-like HCO−3 -rich fluid, the active secretion of which helps set intraventricular pressure. Additionally, the choroid plexuses greatly affect ventricular CSF volume and composition.
A hallmark function of CSF is BCSFB regulation of CSF ions. Perturbed levels of ions in plasma are minimally transferred to CSF as a result of (1) choroid plexus permeability barriers (tight junctions) and (2) active ion transporters (efflux reabsorption of ions that leak into CSF). Neurochemical stability, essential to synaptic functional integrity, is affected by ion fluxes at the blood-CSF interface (choroid and arachnoid membranes). Stable cation and anion composition characterizes CSF, even in neurodegeneration. Brain extracellular ion concentration stability, particularly CSF K + , promotes efficient neurotransmission.
Several multivalent ion transporters in choroid epithelium actively maintain steady CSF levels. Extracellular Ca ++ and Mg ++ affect neuronal excitability; these divalent cations are actively translocated by the plexus to attain CSF concentrations, respectively, below and above those in plasma. A dozen different choroidal Zn ++ transporters provide substantial CSF zinc for glutamatergic neurons. Because of impeded diffusion at CNS barriers, an actively regulated supply of multivalent ions in CSF fluid formation mediates a gamut of neuronal modulations.
To preserve sound ICP and volume, the CNS relies on a battery of choroidal and extrachoroidal fluid-regulating mechanisms. Orderly CSF percolation depends on precisely controlled solute and H 2 O fluxes at several transport interfaces separating blood, CSF, and brain. Extrachoroidally, the ependyma (see Fig. 42.1 ), glia, leptomeninges, and parenchyma have complementary roles, perhaps responsible for one-third of healthy intracranial fluid formation. For the past half century, the pharmacologic manipulation and isolated preparations of choroid plexus have consistently demonstrated fluid formation. Similar direct evidence for extra-choroidal fluid production is less extensive. The many systematic studies of choroid plexus allow CSF investigators to regard this tissue as primary in ventriculocisternal flow dynamics.
The choroidal secretory epithelium also affects parenchyma/interstitium by supplying pulsatile CSF movement for “priming” the cerebral glymphatic flow. Disturbed CSF flow in aging and from mass blockages compromises extensive exchange between large-cavity CSF and brain interstitial fluid (ISF). Accordingly, a diminished CSF formation rate in senescence and disease disrupts pressure gradients and drainage flow routes in the CNS.
CSF dynamics affect central metabolism; thus it is necessary to assess optimal CSF formation rate in health. Adult humans normally form choroidally derived CSF at ∼0.35 mL/min. , Nocturnally elevated CSF production likely modifies cerebral metabolism and CSF clearance of catabolites during sleep. Sleep disorders diminish excretion of brain waste products at night.
CSF formation fluctuates in neurologic disease. Neurosurgeons face situations in which the choroid plexus forms excess or inadequate fluid volume. Inflammation of choroid plexus–CSF by excess tumor necrosis factor or from CSF hemorrhage increases the CSF formation rate. Thus in certain patients, altered barrier transport and CSF dynamics may be controlled by reducing choroid plexus–CSF inflammation. , With hypersecreting choroidal papillomas, , surgical excision often reduces ICP. With a hyposecreting choroid plexus in normal pressure hydrocephalus (NPH) and AD, the stagnated CSF turnover rate , injures the brain by retaining toxic proteins/catabolites in ISF. Severely impaired choroid plexus–CSF dynamics harm cranial neurons (e.g., in hydrocephalus and neurodegeneration), thereby requiring surgical/medical remediation.
Ions penetrate the CNS in large part across the choroid plexus. , Control of brain fluid balance therefore starts with knowledge of choroidal transport mechanisms. Fluid secretion into the ventricles is mediated by an array of ion transporters asymmetrically positioned at the blood- and CSF-facing membranes. Structurally and functionally, the choroidal epithelium resembles the proximal tubule. Renal-like organs are designed to transfer copious volumes of fluid.
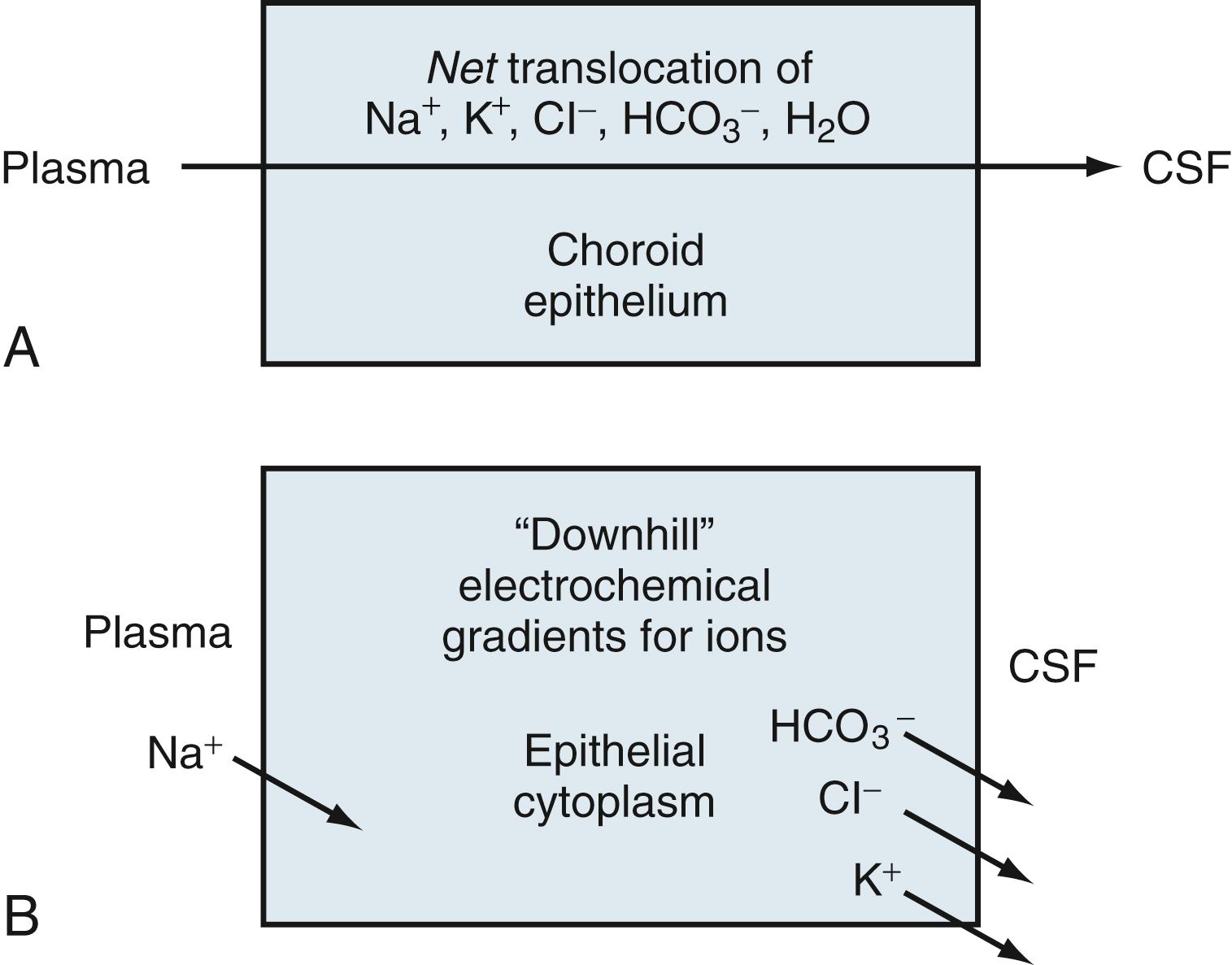
CSF production is directly proportional to net transfer of Na + and Cl – from the blood to the ventricles. Reduced Na + and Cl – transport across the choroid plexus into the CNS attenuates CSF formation. , The driving force for ion movements across choroidal membranes is an energetically downhill concentration or electrochemical gradient. At the external-limiting membranes, the gradient direction for Na + , Cl – , K + , and HCO−3 is given in Fig. 42.3 . Na + entry into the choroid epithelium is downhill (gradientwise) from the plasma across the basolateral membrane.
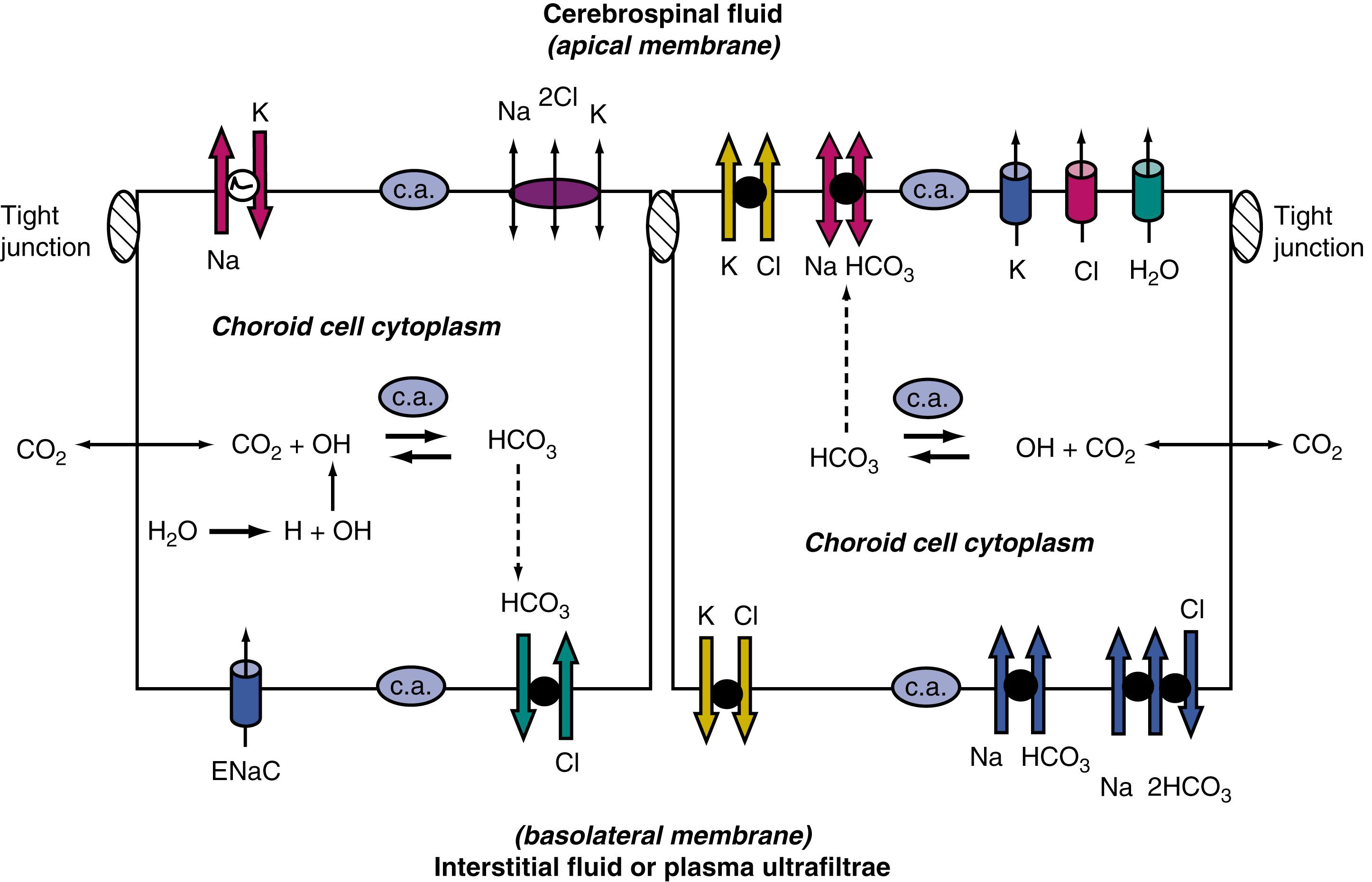
On the other side of the cell, K + , Cl – , and HCO−3 move downhill across the apical membrane into the CSF. Basolaterally and apically, these downhill ionic movements are set up by uphill active transport (requiring chemical energy as ATP) via the primary Na + pump ( Fig. 42.4 ). Active Na + pumping into the CSF keeps choroid cell Na + concentration relatively low, thereby establishing a basolateral inward-driving force on Na + transport from the plasma into the epithelium.
Epithelial transport polarity enables fluid formation. Polar distribution (sidedness) of specific active transporters and passive channels enables net fluid movement (blood to CSF; see Fig. 42.4 ). Basolateral (interstitial) and apical (CSF) transporters and channels thus mediate streaming of ions and H 2 O. Directionally in CSF formation, the fluxes are mainly from the choroid plexus interstitium to the epithelial parenchyma to the ventricles. Fig. 42.4 schematizes the primary and secondary active ion transporters. Apical channels allow passive diffusion of K + and Cl – into nascent CSF. Several cationic species participate in CSF production, such as K + , Mg ++ , and Ca ++ . However, fluid formation is primarily generated by net secretion of Na + , Cl – , and HCO−3 . Water osmotically follows ion transport across the apical membrane (see Fig. 42.4 ). Such transfers occur by stepwise (sequential) and parallel processes, which are described in the following sections.
Energetically, the pivotal initiating step in CSF formation is primary active transport of Na + from the choroidal epithelium to the ventricle. Na + -K + -ATPase activity empowers Na + pumping (see Fig. 42.4 ) by generating ATP. To stabilize choroid pH and epithelial volume , in CSF elaboration, the apical Na + efflux is balanced by continuous basolateral Na + influx with HCO−3 (via carrier slc4a10) and possibly by the epithelial Na + channel (ENaC; diffusion). Transport primacy of slc4a10 (Na + -dependent Cl – - HCO−3 exchange) is manifested by reduced ion transport into the choroid plexus–CSF (with decreased ventricular volume) when slc4a10 activity is disrupted.
As the main anion in CSF, Cl – is actively transported across the basolateral membrane in exchange for cellular HCO−3 . This pulls plasma Cl – into the epithelium, accumulating above electrochemical equilibrium. Under some conditions, intraepithelial Cl – diffuses into the CSF via the efflux arm of the Na + -K + -Cl – cotransporter. However the downhill diffusion of Cl – into the CSF via apical Cl – channels is likely also a prominent pathway by which Cl – accesses the ventricles to sustain fluid formation.
HCO−3 in the choroid plexus has a dual source. First, carbonic anhydrase catalyzes hydration of CO 2 to H + and HCO−3 in the choroid epithelium. In addition, HCO−3 is pulled from the plasma into the epithelium by Na + -coupled HCO−3 transport. On accumulation, the HCO−3 is available for release across the CSF-facing membrane via two mechanisms. In the first route, HCO−3 in the epithelium diffuses downhill via an anion channel into the CSF. In the second route, HCO−3 is transferred via an electrogenic Na + -coupled HCO−3 cotransporter at the apical membrane. , The critical maintenance function of this Na + - HCO−3 cotransport carrier to CSF is revealed by gene knockout, yielding a phenotype of collapsed ventricular volume and ICP as well as disrupted CSF ion homeostasis. HCO−3 -rich nascent CSF reflects facilitated movement of this labile anion into the ventricles as CSF is produced. Acidic products of brain metabolism and subarachnoid hemorrhage are countered in part by choroid plexus HCO−3 secretion.
CSF is 99% H 2 O. The watery medium of CSF enables multiple buffering, distributive, and excretory functions, thus it is significant to characterize H 2 O movement across the choroid plexus. Following Na + , Cl – , and HCO−3 transport into the CSF, H 2 O chases these osmotically active ions into the ventricles by diffusing down its chemical potential gradient through aquaporin 1 (AQP1) channels in the apical membrane. , AQP1 channel involvement in CSF formation is deduced from AQP1 knockout mice displaying substantially reduced fluid movement into the ventricles. As a result, ICP is lowered. The Na + -K + -2Cl – cotransporter (apical membrane) also facilitates the cell-to-CSF movement of H 2 O. Such transcellular H 2 O movement is potentially a drug target to modify CSF dynamics.
Steroid hormones as well as agents structurally related to acetazolamide, furosemide, and bumetanide modulate H 2 O traffic through AQP1 channels and via Na + -K + -2Cl – cotransport. , Rates of H 2 O delivery, arterial blood–to–ventricular CSF, are now assessable by tracer-free MRI; this enables quantification of how H 2 O movement across the BCSFB affects ventricular volume.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here