Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
With an increase in outdoor activities, changing weather patterns, and the growing epidemic of homelessness in our country, issues pertaining to hypothermia remain in the forefront. Hypothermia is not only a common diagnosis in rural areas but has also become more commonplace in urban centers across the nation secondary to inadequate housing or lack of preparation for cold weather changes. It is also important to note that numerous cases of accidental hypothermia (AH) are reported each year in areas typically considered warm weather locales such as Florida, Texas, California, Alabama, and even the Sahara desert. Every year, many recreational and elite athletes participate in outdoor sporting events. The higher the environmental stress, the greater the potential for failure in performance and the development of hypothermia. The high-altitude expeditions on Mt. Denali in 2003, Mt. Hood in 2006, and Mt. Everest in 1996, 2014 and 2015, are reminders that even well-protected, acclimatized individuals can succumb to cold-related fatalities.
Neonates are at particular risk of hypothermia due to large surface area-to-mass ratio, deficient subcutaneous tissue and inefficient shivering. Neonates also lack behavioral defense mechanisms. Acute neonatal hypothermia is common after emergency delivery or resuscitation and has been reported in cases of infant abdandonment. Optimal treatment of hypothermia remains controversial. It is a well-accepted practice to carry out resuscitation of these individuals for extended periods. The medical literature contains numerous anecdotal reports of profoundly hypothermic individuals who are successfully resuscitated and discharged neurologically intact, the longest being in cardiac arrest for 8 hours and 40 minutes. Despite these spectacular reports of survival, both morbidity and mortality from hypothermia are common. Between 1972 and 2002, 16,555 deaths in the United States were attributed to hypothermia, which equates to 689 deaths per year. The United States Centers for Disease Control and Prevention (CDC) reported that in the United States during the years of 1999–2011, there were an average of 1301 deaths from environmental cold exposure per year. The actual number of patients seen in emergency departments (EDs) with hypothermia is unknown. Poverty, homelessness, alcoholism, and psychiatric illnesses are commonly associated conditions. Ethanol intoxication is the most common cause of excessive heat loss in urban settings. This chapter critically reviews approaches and procedures appropriate to the management of several categories of hypothermic patients. The recommendations combine treatment efficacy with safety. Before describing procedures and making recommendations, essential terms are defined and the pathophysiology of hypothermia is briefly reviewed.
Accidental hypothermia has been defined as an unintentional decrease in core (vital organ) temperature to below 35°C (< 95°F). Victims of hypothermia can be separated into the following categories: mild hypothermia, 35°C to 32°C (95°F to 90°F); moderate hypothermia, lower than 32°C to 30°C (< 90°F to 86°F); and severe hypothermia, colder than 30°C (< 86°F). Other factors that may be useful in separating groups of patients with AH include the presence of underlying illness, altered neurologic state on arrival at the ED, hypotension, and the need for prehospital cardiopulmonary resuscitation (CPR). Shivering has been observed down to 31°C and may not cease until 30°C. Additionally, there are case reports of patients being verbally responsive at 25°C and having signs of life at temperatures less than 24°C. A hypothermia outcome score has been developed that incorporates some of these factors and may permit comparison of outcomes in patient groups treated with different modalities.
Risk factors for the development of AH include burn injuries, extremes of age, ethanol intoxication, dehydration, major psychiatric illness, trauma, use of intoxicants, significant blood loss, sleep deprivation, malnutrition, and concomitant medical illnesses. Risk factors for the development of hypothermia indoors include advanced age, coexisting medical conditions, being alone at the time of illness, being found on the floor, and abnormal perception or regulation of temperature. Unlike healthy exposed outdoor enthusiasts, such as skiers or mountaineers, hypothermia in urban populations is most often associated with conditions that impair either thermoregulation or the ability to seek shelter. In the majority of studies of urban hypothermia, death has been attributed to the severity of the underlying disease.
Because signs and symptoms may be vague and nonspecific, mild to moderate hypothermia may easily be overlooked in the ED. A common error is failure to routinely obtain an accurate core temperature in all patients at risk. The diagnosis is frequently delayed because of false reliance on standard oral temperatures. Symptoms such as confusion in the elderly and combativeness in intoxicated patients might not initially be recognized as symptoms of hypothermia. Hypothermic patients frequently will not feel cold or shiver, particularly the elderly population, who have impaired thermoregulatory responses because of their advanced age. Paradoxical undressing, a cold-induced psychiatric dysfunction, has been described in confused patients in whom a sensation of heat develops at lowered body temperatures. It occurs as a result of constricted blood vessels near the surface of the body that suddenly dilate. In many cases these patients are mislabeled as psychotic, thereby leading to further delays in appropriate treatment.
Because of the nonspecific nature of the symptoms of hypothermia, accurate assessment of temperature is a necessity when considering this diagnosis. It is of paramount importance not only for confirmation of the diagnosis but also for guidance in further diagnostic and therapeutic decisions. Any thermometer that does not record temperatures in the hypothermic range is inappropriate for evaluating significant hypothermia. Standard glass/mercury thermometers generally cannot record temperatures lower than 34°C (< 93.2°F), although some models are available that record temperatures as low as 24°C (75.2°F) (Dynamed, Inc., Carlsbad, CA). An electronic probe with accompanying calibrated thermometer is recommended when monitoring this vital sign. Examples of thermometers with accompanying accuracy at various temperature ranges are shown in Fig. 65.1 .
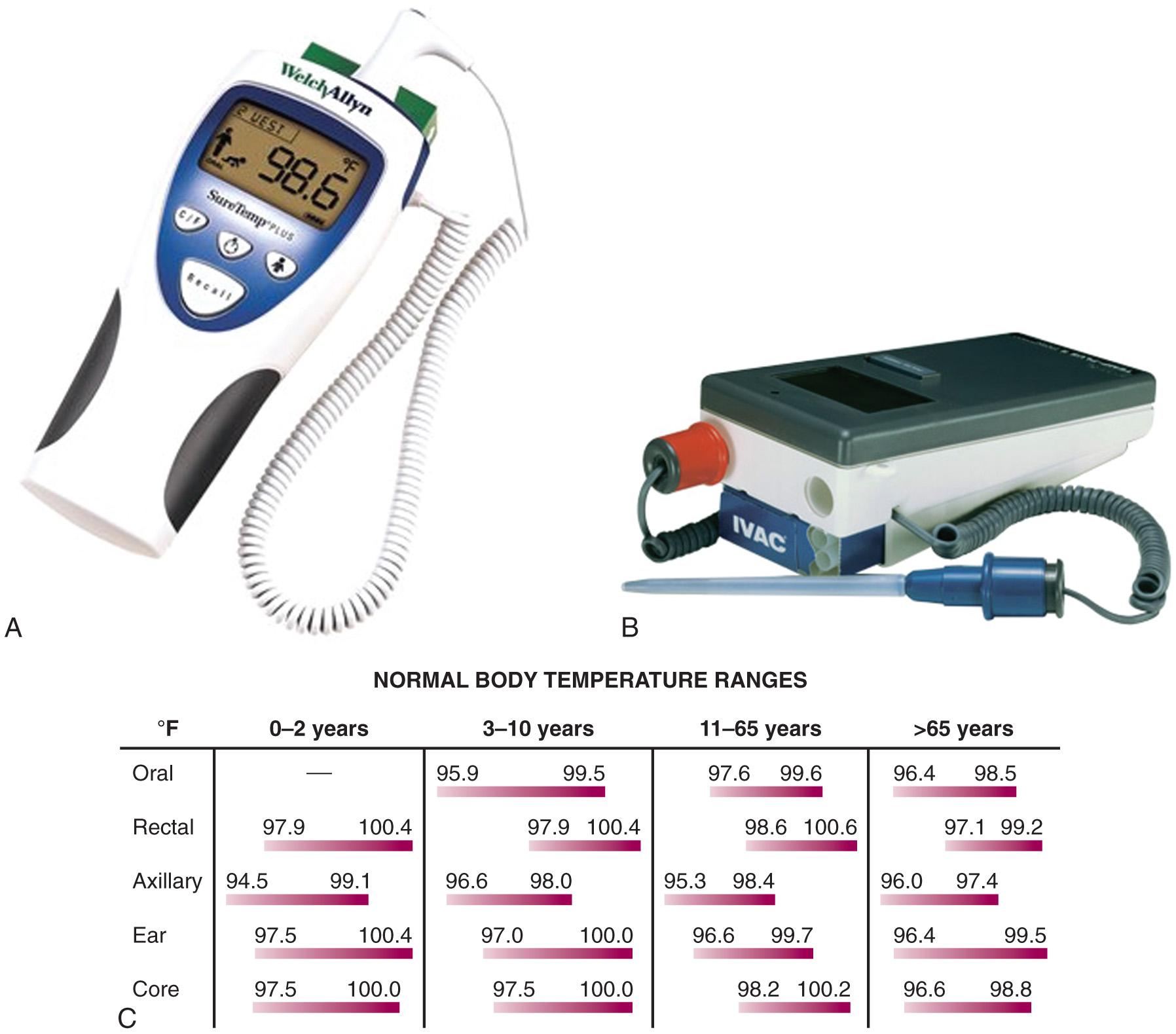
Core temperature is traditionally estimated with a rectal probe, but due to large gradients within the body, rectal temperature often lags behind core temperature by up to 1 hour, reading higher than esophageal temperature during cooling and lower than esophageal temperature during rewarming. Esophageal probes may be used, although they may be affected by warm humidified air therapy. Other possible sites for measurement of temperature include the tympanic membrane, nasopharyngeal tract, and urinary bladder. Fresh urine temperature can closely approximate core temperature. “Deep forehead” temperatures measured with a Coretemp thermometer (Teramo, Tokyo) have also demonstrated excellent accuracy and approximation of core temperatures. For continuous monitoring purposes, rectal or bladder probes are preferred. Infrared tympanic temperatures have demonstrated excellent correlation with core temperatures. However, studies show that although easier to use and faster, infrared tympanic temperatures can be inaccurate at extremes of temperature by underestimating higher temperatures and overestimating lower temperatures. When a rectal probe is used, insert it at least 15 cm beyond the anal sphincter and verify its position frequently. One should remember that temperature gradients exist in the human body and therefore consistency of monitoring at one or more sites is mandatory. A chart and formula that convert centigrade to Fahrenheit temperatures will assist the clinician in assessing the severity of hypothermia (see Fig. 65.1 C ) .
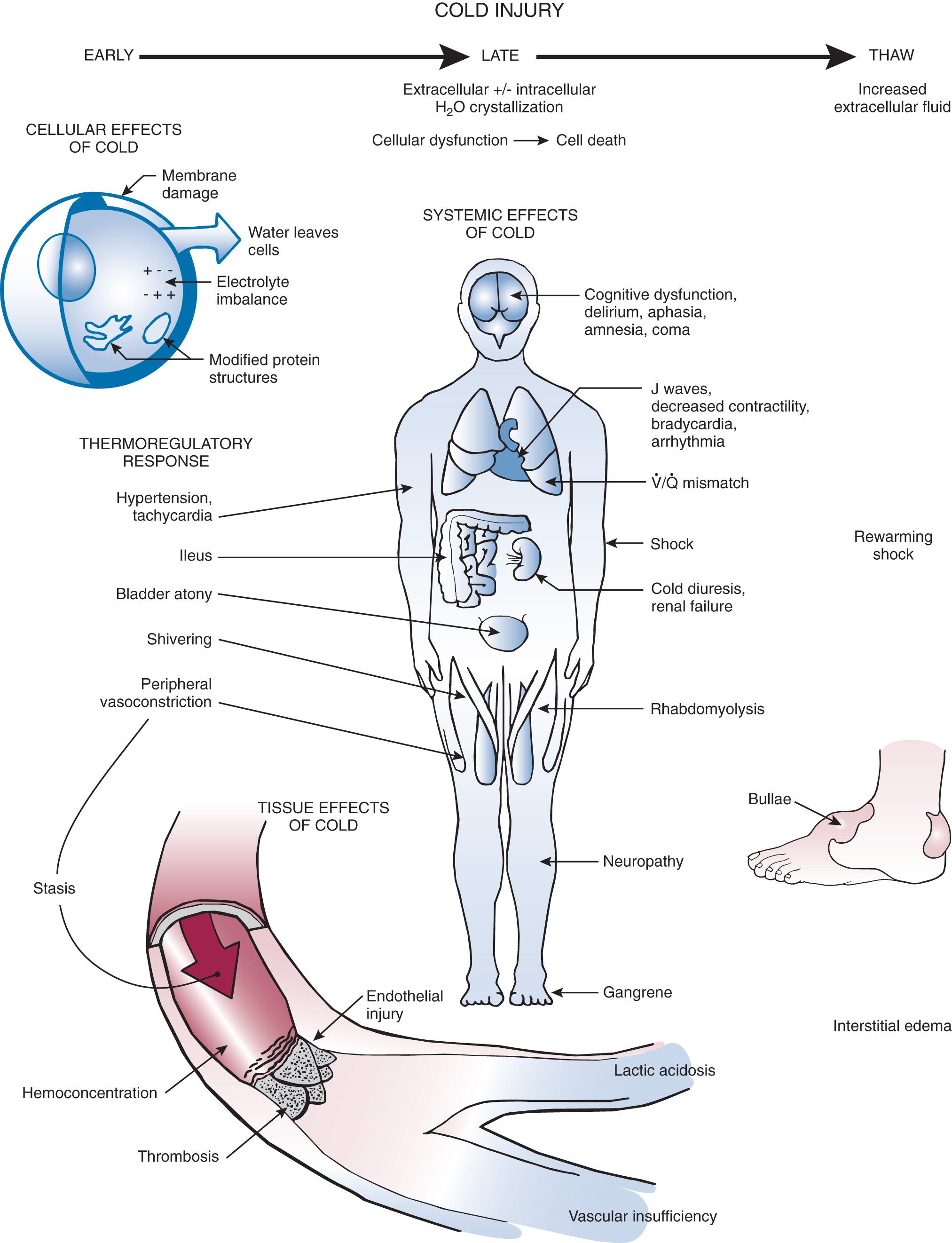
AH results from failure of the body's thermoregulatory responses to generate enough heat to compensate for heat losses. These thermoregulatory responses include shivering, tachycardia, tachypnea, increased gluconeogenesis, peripheral vasoconstriction, and shunting of blood to central organs ( Fig. 65.2 ). As core temperature drops despite these compensatory mechanisms, the patient becomes poikilothermic and cools to ambient temperature.
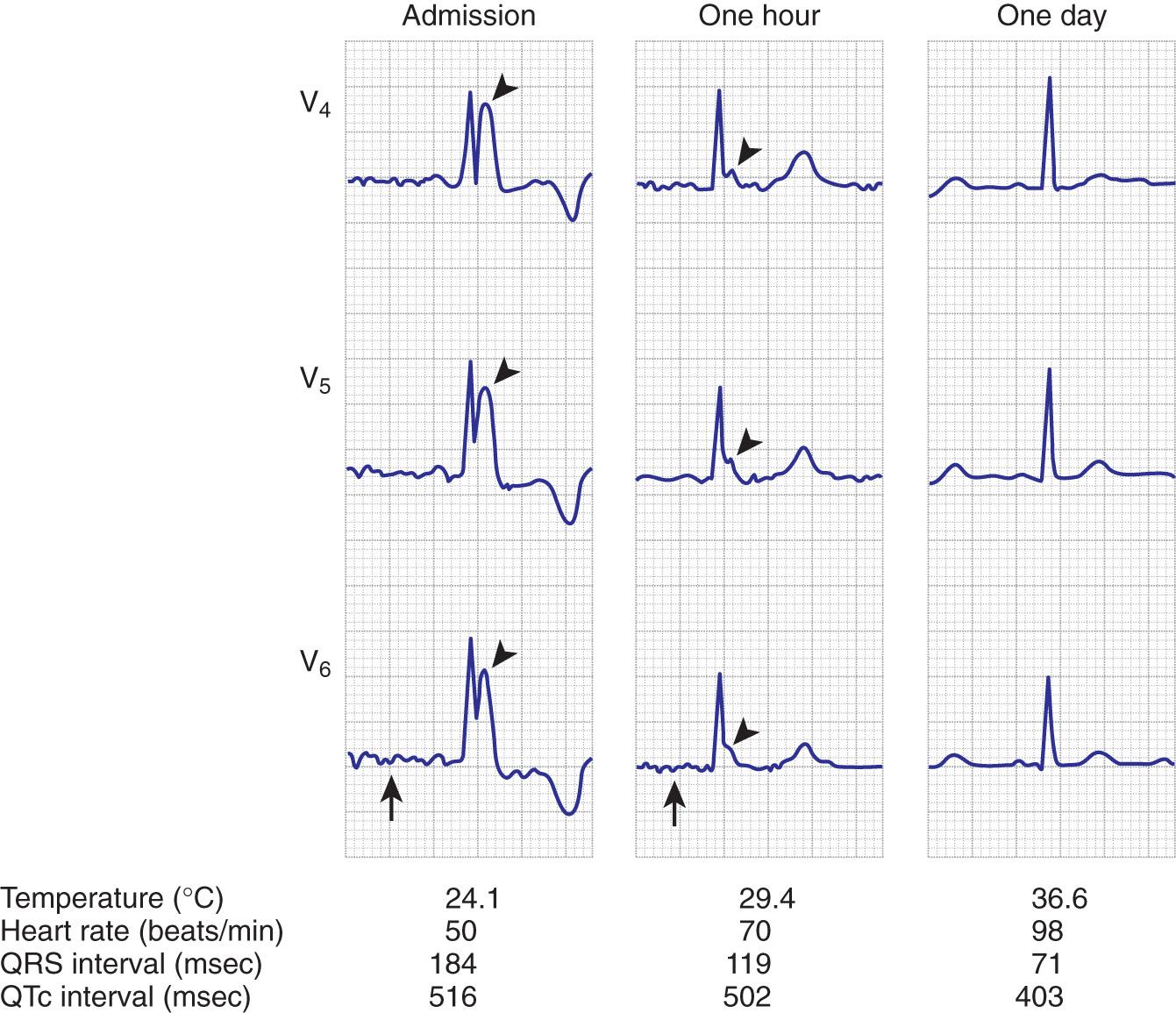
Four methods of heat loss affect the body: radiation, conduction, convection, and evaporation. Radiation involves transfer of heat from a warmer body to a cooler environment and accounts for approximately 60% of heat loss in a normothermic individual. Conduction refers to loss of heat from direct contact with a cooler surface. These losses are most profound with immersion hypothermia. Convection occurs when cool air currents pass by the body and this accounts for 15% of heat loss, especially with a wind chill factor. Evaporation refers to significant loss of heat through sweating and insensible water loss. With hypothermia, the enzymatic rate of metabolism decreases twofold to threefold with each 10°C (18°F) drop, and cerebral blood flow decreases 6% to 7% per 1°C (1.8°F) drop. Signs and symptoms of hypothermia vary according to the core temperature. The overall functioning of all organ systems is impaired by the cold. The greatest effects are seen in the cardiovascular, neurologic, and respiratory systems ( Table 65.1 ). As core body temperature drops below 33°C (< 91.4°F), the patient becomes confused and ataxic. The initiation of involuntary motor activity (shivering) prevents the reduction in core temperature. Shivering thermogenesis in skeletal muscle operates on acute cold stress. In a malnourished patient, the mechanism may be rendered ineffective secondary to reduced muscle mass. Shivering stops at approximately 32°C (89.6°F), and shivering artifact on an electrocardiogram has been associated with increased survival of individuals with severe hypothermia. Atrial fibrillation occurs frequently as the temperature continues to drop and the patient loses consciousness. A J wave on the electrocardiogram often appears before ventricular fibrillation ( Fig. 65.3 ). Though classically considered pathognomonic for hypothermia, the J or “Osborne” wave has no prognostic or predictive value in cases of hypothermia. Studies have found that Osborne waves are present in 36% of AH survivors and in 38% of nonsurvivors. , Ventricular fibrillation may occur below 29°C (< 84.2°F) and becomes common as the core temperature drops to 25°C (77°F). The electroencephalogram flattens at 19°C to 20°C (65.2°F to 68°F). Asystole commonly develops at 18°C (64.4°F) but has been seen at higher temperatures. Initial core temperature does not necessarily correlate with patient outcome. The lowest recorded temperature in a survivor of AH is 9°C (43.7°F).
| CORE TEMPERATURE (°C) | CARDIOVASCULAR SYSTEM | RESPIRATORY SYSTEM | CENTRAL NERVOUS SYSTEM |
|---|---|---|---|
| 30–34 | Tachycardia Increased afterload Increased systemic blood pressure |
Tachypnea Increased minute ventilation |
Lethargy Mild confusion Loss of fine motor coordination |
| 30–34 | Progressive bradycardia Decreased cardiac output Hypotension Lengthening of cardiac conduction Atrial/ventricular dysrhythmias |
Increased bronchial secretions Diminished gag reflex Depressed cough response | Delirium Slowed reflexes Muscle rigidity Abnormal EEG findings |
| < 30 | Spontaneous ventricular fibrillation Osborne waves at 25°C | Respiratory rate decreased to 5 breaths/min | Areflexia Coma Fixed pupils Rigidity EEG silent at 19°C |
Several guidelines for the treatment of AH exist. The State of Alaska Cold Injuries Guidelines were last updated in 2014 and include a section on AH. Additionally, in 2014, the Wilderness Medical Society developed practice guidelines for out-of-hospital care of AH. Treatment of hypothermia can be divided into prehospital care and ED management.
In the prehospital setting, focus primarily on removing the patient from the current environment to prevent further decreases in core temperature. Studies have shown that oral temperatures are sufficiently accurate for field use; however, infrared tympanic thermometers may not be reliable in the prehospital setting. Handle these patients with special care and anticipate the presence of an irritable myocardium because aggressive measures can inadvertently trigger cardiac dysrhythmias. Hypovolemia and a large temperature gradient often exist between the periphery and the core in a hypothermic patient. Avoid aggressive field management and prolonged transport times. After removing the patient's wet clothing, wrap the patient in dry blankets or sleeping bags. Field rewarming is a misnomer because adding significant heat to a hypothermic patient in the field is extremely difficult. Studies have shown that for mild hypothermia, resistive heating (e.g., warming blankets) can be used safely in the prehospital setting. Resistive heating augments thermal comfort, increases core temperature by approximately 0.8°C/hr (33.4°F/hr), and reduces patient pain and anxiety during transport. In one study, resistive heating more than doubled the rewarming rate when compared with passive insulation and did not produce an afterdrop. With longer transport times, use active rewarming methods limited to heated inhalation and truncal heat application. Place insulated hot water bottles near the patient's axilla or groin. The Res-Q-Air device (CF Electronics, Inc., Commack, NY) is lightweight, portable, and delivers heated humidified air or oxygen at temperatures ranging from 42°C to 44°C (107.6°F to 111.2°F) and down to ambient conditions of −20°C (−4°F). In more remote settings, another option is to use a modified forced-air warming system in the field. The Portable Rigid Forced-Air Cover is heated with a Bair Hugger heater/blower (Augustine Medical, Inc., Eden Prairie, MN). It covers the patient's trunk and thighs and can adapt to various transport vehicle power sources.
Immobilize patients with potential traumatic injuries to the spine or extremities before transport. Pay continuous attention to airway maintenance. Initiate fluid resuscitation with intravenous (IV) crystalloid, preferably 5% dextrose in normal saline (D 5 NS). Alternatively, give warmed oral glucose-containing drinks to a patient who is awake and alert. Most hypothermic patients are dehydrated because fluid intake is reduced and cold causes diuresis. Avoid using lactated Ringer's solution because it can theoretically decrease the metabolism of lactate by cold-induced hepatic dysfunction. If possible, use warmed IV fluids because they are generally well tolerated. If available, use a flameless heater, which is currently being used by military medical units and provides an easy and expedient means of warming fluids in the prehospital setting.
Intubate unresponsive patients, but recognize that there is no universal agreement on when to intubate a hypothermic patient who has detectable vital signs. Pulse oximetry is not usually helpful because vasoconstriction limits blood flow to the periphery and readings may be inaccurate or not possible. Prehospital providers should assess for pulses and initiate CPR at a standard rate of 100 compressions per minute if no pulses are palpable after 1 minute. Delayed or intermittent CPR can be associated with good outcomes with neurologically intact survivors after prolonged chest compressions, but should be used only if evacuation efforts necessiate. Some authors suggest that pulseless victims with core temperatures below 32°C (<89.6°F) should be transported with continuous CPR. Other authors believe that it is unnecessary to perform CPR on a patient who has any perfusing cardiac rhythm because it may precipitate ventricular fibrillation. There is no universally accepted standard for intubation or CPR in hypothermic patients with detectable vital signs.
Definitive prehospital determination of cardiac activity requires a cardiac monitor. Cardiac arrest is a common misdiagnosis because peripheral pulses are difficult to palpate when extreme bradycardia is present along with peripheral vasoconstriction. Some authors report that asystole is a more common rhythm than ventricular fibrillation. In the field, differentiating between ventricular fibrillation and asystole may be impractical. Successful defibrillation has been reported at 20°C but attempted defibrillation is often unsuccessful until the core temperature is above 30°C. If the defibrillation attempt is unsuccessful, initiate active rewarming while continuing CPR defibrillation. These attempts can be given occasionally during the rewarming process.
Transport cold, stiff, cyanotic patients with fixed and dilated pupils because the treatment dictum for prehospital personnel remains, “No one is dead until warm and dead.” Some patients are actually cold and dead. It is useful and humane if they can be safely identified. Because human physiologic responses are variable, it is difficult to predict outcome. A succinct summary of the prehospital care of a hypothermic patient is rescue, examine, insulate, and transport. In the majority of cases, evacuation, resuscitation, and transport should be initiated, provided that doing so does not endanger prehospital responders.
There are no universally established standards of care regarding the use of specific techniques for rewarming a hypothermic patient or cooling a hyperthermic patient. This chapter describes all potentially useful modalities. Many are not applicable for general use in the ED, whereas others are safe, beneficial, and easily accomplished in the general ED setting. Some invasive procedures, however, such as cardiopulmonary bypass and irrigation of the peritoneal or thoracic cavity, may be overly aggressive or of anecdotal or theoretical benefit only. Exactly when to institute any given intervention is best determined by the resources available, the initial scenario, and clinical judgement individualized for each patient.
Treatment priorities in the ED setting are to prevent further decreases in core body temperature; establish a steady, safe rewarming rate; maintain stability of the cardiopulmonary system; and provide sufficient physiologic support. Adjust the rate of rewarming and the techniques used according to the degree of hypothermia and the severity of the patient's clinical condition ( Table 65.2 ). Anticipate and prevent complications.
| CORE TEMPERATURE (°C) | METHOD AND TECHNIQUES |
|---|---|
| > 32 |
|
| ≤ 32 |
|
In a pulseless, apneic patient, initiate CPR and continue until the core temperature is above 34°C (> 93.2°F). Profound hypothermia results in coma, hyporeflexia, fixed and dilated pupils, severe bradycardia, and often an unobtainable blood pressure. With severe hypothermia, a pulse might not be palpable and measurement of blood pressure might require the use of a Doppler device. If available, use ultrasound to detect the presence of cardiac wall motion. Follow the heart rate and rhythm with electrocardiographic monitoring. In patients who have anything more than minimal impairment, perform arterial blood gas analysis frequently to determine oxygenation, ventilation, and acid-base status. If feasible, establish large-bore IV lines. Avoid central lines if possible because insertion of such lines may exacerbate the myocardial irritation. Give maintenance IV fluids. Warm all IV fluids to 40°C to 42°C (104°F to 107.6°F), but be aware that the usual volumes administered will not contribute significant heat calories. With long standard IV tubing, the heated IV fluids may actually cool to room temperature before entering the patient's IV site.
With a mild to moderate reduction in core temperature, the level of mentation correlates with the severity of the AH, associated illnesses, or both. Noteworthy exceptions are alcoholics and diabetics, who can be in a coma at higher core temperatures because of concomitant hypoglycemia. Perform bedside glucose measurements on patients when they arrive in the ED. A high correlation exists between alcohol consumption and the development of hypothermia, especially in colder climates. A review of 68 cases of hypothermic deaths in Jefferson County, Alabama, found that a significant number of cases involved middle-aged men who had consumed alcohol. In other urban cases of AH reviewed, the majority were alcoholics. This study noted glycosuria in patients, even when low serum glucose values were evident, and described a renal tubular glycosuria in patients with AH. Such glycosuria may worsen or cause hypoglycemia. Glycosuria in AH is no guarantee of an adequate serum glucose concentration. This supports the routine use of supplemental IV glucose unless a normal serum glucose value can be quickly ensured. Consider administering IV thiamine (100 mg) and a trial dose of 0.4 to 2 mg of IV naloxone (Narcan, Adapt Pharma, Inc. Radnor, PA) in a comatose patient. Although failure to rewarm spontaneously has been noted in victims with hypothyroidism and other endocrine deficiencies, reserve the use of thyroid hormones and corticosteroids for patients with suspected thyroid and adrenal insufficiency, respectively.
The thermoregulatory vasoconstriction caused by hypothermia significantly decreases subcutaneous oxygen tension. Good correlation exists between the incidence of wound infection and subcutaneous oxygen tension. As core temperatures decrease from 41°C to 26°C (105.8°F to 78.8°F), neutrophil function is significantly impaired. In animal models, hypothermia appears to decrease leukocyte sequestration within the brain parenchyma, thus offering some resistance to meningitis. Although antibiotics are not routinely indicated for victims of uncomplicated mild hypothermia, some authors advocate the routine empirical initiation of broad-spectrum antibiotic therapy on admission of severely hypothermic patients. In this setting, detection and treatment of the underlying cause, such as infection, may be more critical than treatment of the hypothermia.
Hypothermia affects virtually every organ system because of generalized slowing of the body (see Fig. 65.2 ). Management goals depend on the severity of the hypothermia, but in all cases the primary goal is to increase core temperature and prevent further loss. In a patient with mild hypothermia, a conservative approach to rewarming is generally advocated. Overly aggressive methods may be more harmful to the patient by causing worsening hypotension, a paradoxical decrease in core temperature, and cardiac dysrhythmias. Other complications may include bleeding and infection of surgical incisions. The optimal rewarming rate remains unclear and varies with each case. Standard rewarming rates are a 0.5°C/hr to 2.0°C/hr (0.9°F/hr to 3.6°F/hr) rise in temperature in an otherwise stable patient ( Table 65.3 ). Carefully consider and individualize invasive therapy to the severity of the hypothermia and the condition of the patient. Avoid overtreating and overusing invasive techniques in an otherwise stable hypothermic patient. In patients with severe underlying problems such as hypoglycemia, hyperglycemia, sepsis, adrenal crisis, drug overdose, or hypothyroidism, treat these conditions appropriately in addition to treating the hypothermia. Long-term outcome may depend more on treatment of the underlying illness than on treating the hypothermia.
| PASSIVE EXTERNAL | ACTIVE EXTERNAL | INHALATION OF WARM AIR | PERITONEAL LAVAGE | BLADDER LAVAGE | |
|---|---|---|---|---|---|
| 1st hr | 1.4 | 1.5 | 1.5 | 1.5 | 1.3 |
| 2nd hr | 1.4 | 2.4 | 2.0 | 2.5 | 1.7 |
| 3rd hr | 1.8 | 2.0 | 1.9 | 3.2 | 1.8 |
The cornerstone of the effectiveness of passive external rewarming relies on the body's ability to restore normal body temperature through its own mechanisms for heat production. Stop further heat loss with insulation and manipulation of the environment. Give warm fluids containing glucose to patients who are fully alert. For patients with mild AH, remove wet clothing and then provide passive external rewarming with blankets. The technique is simple, but the patient must be capable of generating enough body heat for this method to be successful. Give warmed IV fluids to counteract the cold-induced diuresis. Internal heat generation is required for rewarming, and this effect will be relatively slow. In an otherwise stable patient, aggressive intervention with drugs and invasive monitoring might be more harmful than beneficial. Patients who cannot shiver, those who are hypotensive, or those who are intoxicated or malnourished may not have this capability. Survival rates with passive external rewarming have ranged from 55% to 100%.
For patients in the moderate or severe category of hypothermia, a more aggressive approach may be warranted. The options available are active external rewarming and active core rewarming. Active core rewarming techniques can be further divided into less invasive and more invasive techniques. The aggressiveness of therapy depends more on the patient's underlying health, hemodynamic status, and response to initial therapy than on the initial temperature.
The application of heat to the skin of a hypothermic patient has been termed active external rewarming .
Although there is some suggestion that active external rewarming of profoundly hypothermic patients by immersion may be associated with an increase in mortality over other treatments, other studies suggest that this technique is highly effective for mild hypothermia. Use it selectively and limit it to the trunk. Other forms of active external rewarming are increasingly being used in the ED as adjunctive care of moderately hypothermic, otherwise healthy individuals. Vasoconstriction limits the ability to increase core temperature with techniques that primarily warm the skin.
Active external rewarming is most beneficial when the heat supplied by the external source is greater than the loss of rewarming heat incurred by the cessation of shivering. In more remote wilderness settings where more aggressive warming techniques are precluded because of the lack of equipment or personnel, active external rewarming with body-to-body contact may be the only option. The rewarming contribution of body-to-body contact appears to be limited, however.
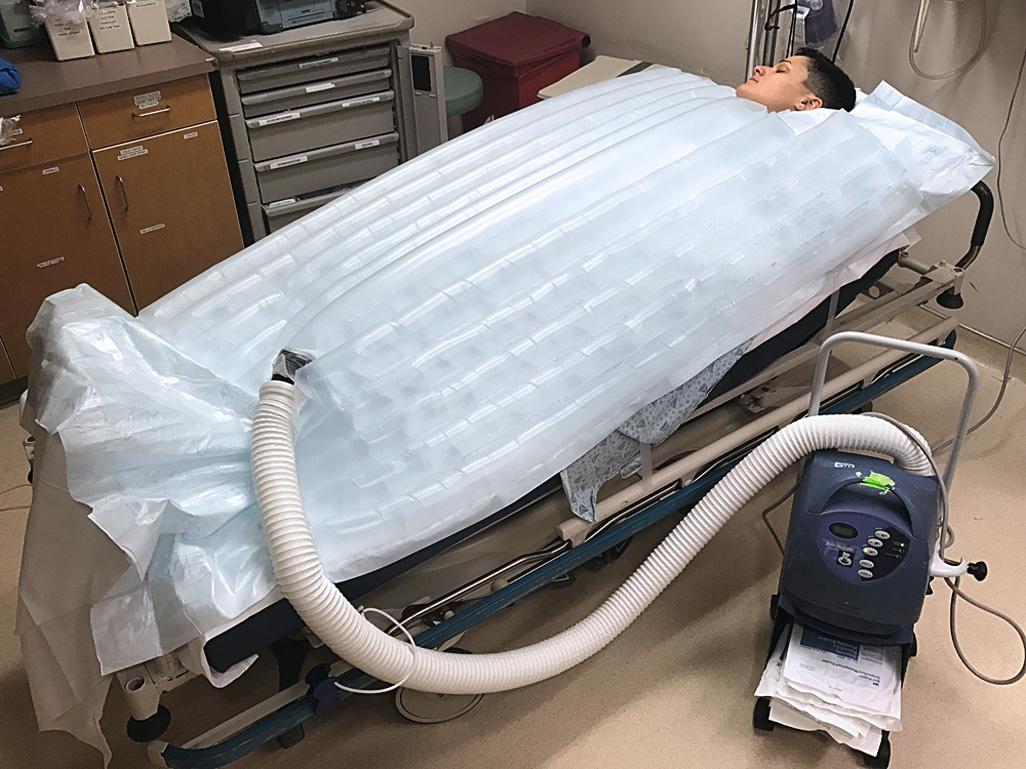
Traditionally, immersion therapy has used a heated (40°C to 42°C [104.0°F to 107.6°F]) water tank of the type present in most burn units. Generally, immerse a hypothermic patient entirely except for the extremities and head, but immersion of the extremities may hasten rewarming. A major drawback is the inability to closely monitor patients undergoing immersion. Alternatively, use a warm water–filled heat exchange blanket (e.g., Blanketrol, Cincinnati Sub-Zero Products, Cincinnati, OH) for conduction warming. Intraoperative studies have demonstrated excellent results. A forced warm air convection system (Bair Hugger, Augustine Medical, Eden Prairie, MN; Snuggle Warm Convective Warming System, Sims Level 1, Inc., Rockland, MA) has been used for postsurgical rewarming. This approach has also been used successfully for ED-based AH therapy ( Fig. 65.4 ). Rewarming by warm air convection permits continued monitoring in the ED and is better tolerated than immersion because of the less rapid development of vasodilation in peripheral tissues. As an alternative for noninvasive active external rewarming, there are case reports describing rewarming hypothermic patients with a device called Arctic Sun (Medivance, Inc., Artic Sun, Temperature Management System, Louisville, CO), a system also used to induce therapeutic hypothermia. The system circulates warm water through gel pads placed on areas of the body with large surface areas (e.g., the trunk and thighs). Case reports exist showing rewarming rates from 0.63°C to 2.5°C per hour, with one patient rewarmed from an initial temperature of 22°C.
Because profound fluid shifts can occur with conduction warming, give the patient supplemental IV fluid warmed to 40°C (104.0°F; Hotline Fluid Warmer, Sims Level 1, Inc., Rockland, MA) at a rate sufficient to generate a urinary output of 0.5 to 1.0 mL/kg per hr. Give an initial fluid bolus of 500 mL of D 5 NS. Note that blood pressure is not an accurate means of gauging fluid resuscitation because serious hypothermia is always accompanied by “physiologic” hypotension. Because patients requiring mechanical ventilation have rarely been subjected to tank immersion, it cannot be recommended for hypothermic patients who require intubation. Rewarming rates ranging from 0.9°C to 8.8°C (1.6°F to 15.8°F) per hour have been reported with immersion therapy.
A heat exchange blanket allows the patient to receive other treatments that may be difficult or impossible to carry out in a tub, such as defibrillation, CPR, or more invasive warming techniques. Place the heating blanket and overlying cloth sheet underneath the patient. Set the blanket temperature to 40°C to 42°C (104.0°F to 107.6°F), and initiate the measures described in the section on Passive Rewarming Techniques. Forced-air rewarming (convection) uses a blanket cradle to create an environment through which heated air is blown. Access to the patient is quite good with this system because the overlying blankets can be raised temporarily to evaluate the patient or perform procedures. Experience with mild immersion-induced hypothermia in volunteers suggests that the forced-air technique warms at a rate comparable to that of vigorous shivering, but with less metabolic stress and less afterdrop.
Arteriovenous anastomoses rewarming (AVR) involves immersion of the distal end of the extremities (hands, forearms, feet, and lower part of the legs). Advantages include rapid rewarming rates. A study of AVR immersion at temperatures of 45°C and 42°C (113.0°F and 107.6°F) in healthy volunteers demonstrated rewarming rates of 9.9°C/hr (±3.2°C/hr) for the former and 6.1°C/hr (±1.2°C/hr) (43.0°F ± 34.2°F/hr) for the latter. There was also a decrease in postcooling afterdrop. AVR is well tolerated by patients because of the rapid rise in core temperature and the shortened period of shivering.
There is concern that surface warming with accompanying vasodilation may produce relative hypovolemia in a hypothermic patient. Other complications described with the active external rewarming method include core temperature afterdrop and rewarming acidosis. In core temperature afterdrop , colder peripheral blood is transported to the warmer core organs, thereby further reducing core temperature. In rewarming acidosis , colder blood and lactic acid return to the core organs and worsen the acidosis. To limit these complications in patients with moderate hypothermia, some authors advocate using active external warming only after active internal techniques have been initiated. Others suggest that core afterdrop is inevitable regardless of the rewarming method, as temperature will temporarily decrease in any object with a warm core and cool periphery, due to conductive properties.
CPR and other advanced cardiac therapy and monitoring are impossible with immersion rewarming. Until studied further, active external rewarming should be considered only in a clinically monitored setting for mildly hypothermic patients who can protect their airways. When using a heating device, also monitor the potential for burns in areas that have the greatest contact with the heating source.
There is evidence that active core rewarming may decrease mortality from severe hypothermic exposure when compared with other techniques. In the face of circulatory failure, often the best chance of survival is treatment with extracorporeal circulation (ECC) and warming of the blood. Several methods have been described, including the use of warm humidified air through an endotracheal (ET) tube or mask, peritoneal lavage, gastric or bladder lavage with warm fluid, thoracic tube lavage, cardiopulmonary bypass, AVR, peripheral vascular extracorporeal warming, hemodialysis, and thoracotomy with mediastinal lavage. These techniques transfer heat actively to the body core and achieve varying rewarming rates. The specific techniques and some of the advantages and disadvantages for each procedure follow.
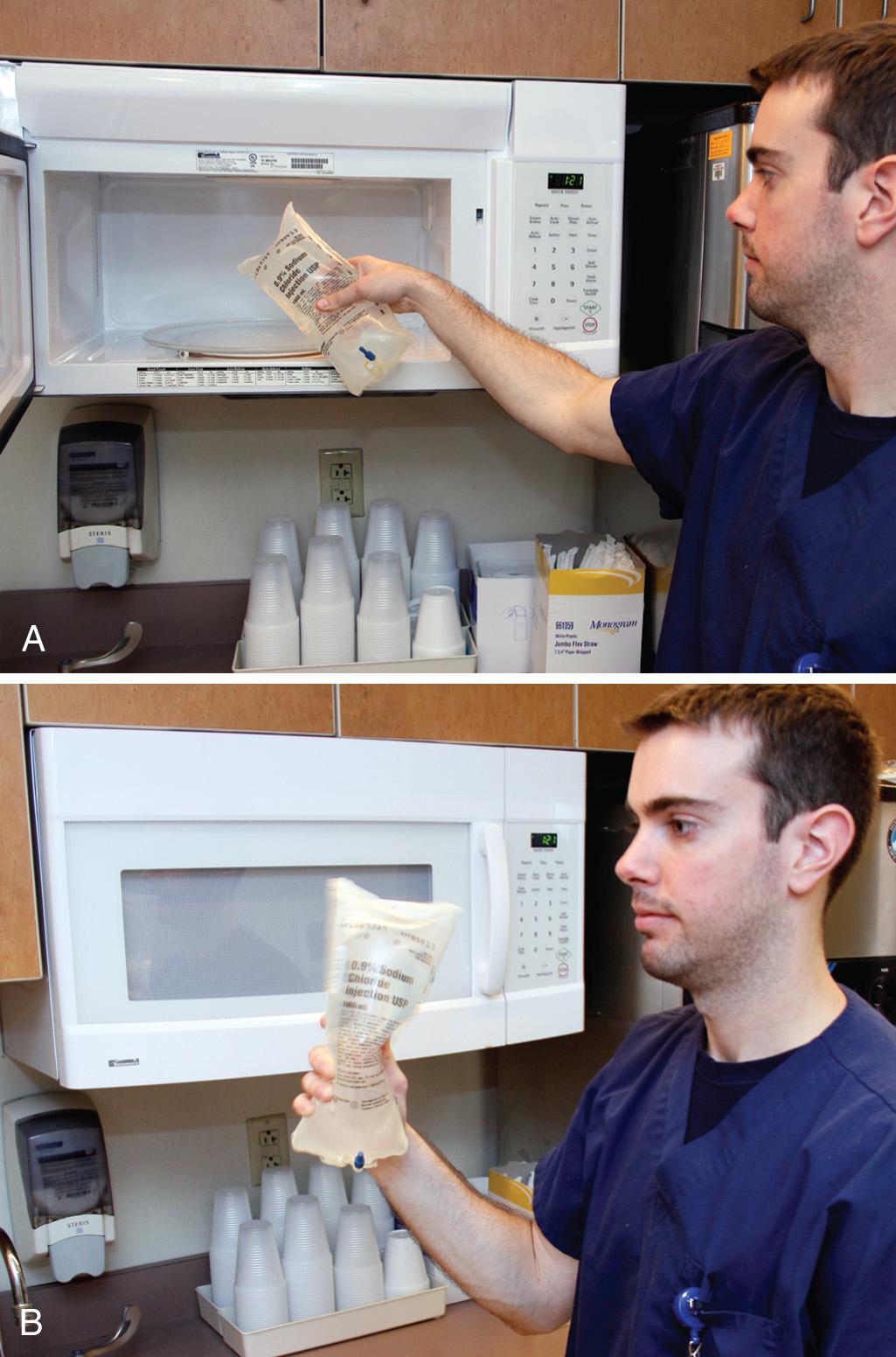
Under ideal circumstances, keep saline in a standard warming device. When large amounts of saline are required for such procedures as peritoneal lavage, warm 1-L saline bags rapidly in a standard microwave oven ( Fig. 65.5 ). Although devices vary, a 650 W microwave oven has been demonstrated to warm 1 L of room-temperature non–dextrose-containing saline from 21.1°C to 38.3°C (70°F to 101°F) in 120 seconds on the high setting. At midcycle (i.e., after 60 seconds), interrupt the heating with agitation, and repeat the agitation at the end of the cycle before infusion. Fluids containing dextrose should not be warmed in this manner, as glucose caramelizes at 60°C. Fluids in glass bottles and blood products are also not safe to be warmed with this method.
The use of warm humidified oxygen to treat hypothermia has been well established. Average rates of rewarming of 1°C/hr (33.8°F/hr) via mask and 1.5°C/hr to 2.0°C/hr (34.7°F/hr to 35.6°F/hr) via ET tube with heated aerosol at 40°C (104.0°F) can be obtained. Faster rewarming rates may be accomplished with a maximum safe aerosol temperature of 45°C (113°F). Core rewarming with this technique occurs through the following mechanisms. The warmed alveolar blood returns to the heart and warms the myocardium. The heated, humidified air delivered to the alveoli also warms contiguous structures in the mediastinum by conduction. Warming the inhaled air or oxygen eliminates a major source of heat loss.
The use of heated humidified air or oxygen is a simple technique that should be used routinely in all patients with hypothermia, regardless of severity. If the correct equipment is available, it can be used in the field and in the hospital. One must address the risk for burns during the inhalation of warm air in the field environment. Mouth-to-tube ventilation in an intubated hypothermic prehospital patient has the theoretical advantage of providing warm humidified air without special equipment. A ventilating rescuer can inhale oxygen and then expire it into the patient's ET tube to provide air with increased oxygen content. There are no contraindications to or reported complications from the use of warm humidified air for hypothermia, and there is no afterdrop.
Use a heated cascade nebulizer with a mask for patients with spontaneous respirations. Use a volume ventilator for intubated patients. Monitor the inspired air to maintain a temperature of approximately 45°C (≈113.0°F). Temperatures higher than 50°C (122°F) may burn the mucosa, and temperatures lower than 45°C (< 113°F) do not deliver the maximum heat. Humidify the air or oxygen and note that the heater module may need modification because many units have feedback mechanisms that shut off at a given temperature. It may be difficult to deliver oxygen at the recommended temperature because of equipment limitations. In many cases the air temperature is only 30°C (86°F).
Inhalation of warm humidified air or oxygen results in gradual rewarming of the core and should be the mainstay of all rewarming therapy. Studies have suggested that the rewarming rate of inhalation therapy is inferior to that of peritoneal lavage, thoracic lavage, and bath rewarming. Inhalation therapy can be combined with any and all other methods of rewarming and is relatively noninvasive and inexpensive. This therapy should be considered as the initial treatment of choice for hypothermic patients.
Peritoneal dialysis (lavage) is an attractive treatment for severe hypothermia because it is available in most hospitals and does not require any unusual equipment or training. Rewarming rates of 2°C to 3°C (3.6°F to 5.4°F) per hour, depending on the dialysis rate, can be achieved without sophisticated equipment that may delay therapy or require transfer of the patient to a tertiary care facility. This technique can also be used to help correct electrolyte imbalances. Peritoneal lavage is useful primarily in severe cases in combination with other rewarming techniques for patients without spontaneous perfusion, but has also been used alone in patients undergoing CPR in whom ECC was felt to be contraindicated or not available.
Rewarming by peritoneal dialysis was first used successfully in a patient in ventricular fibrillation with a temperature of 21°C (69.8°F). Since that time, there have been reports of successful rewarming with peritoneal lavage in stable, severely hypothermic patients and unstable hypothermic patients in cardiac arrest. Peritoneal lavage works via transfer of heat from lavage fluid to the peritoneal cavity. The peritoneal great vessels and abdominal organs provide a large surface area for exchange of heat. The use of warmed peritoneal lavage fluid is an effective approach to rewarming. There have been reports in the literature of success with rapid high-volume peritoneal lavage in pediatric patients. The technique involves the use of an infraumbilical “mini-laparotomy” incision followed by placement of a large silicone peritoneal dialysis catheter. The catheter is connected to a rapid infusion device with delivery of 1 L of warmed normal saline every 90 seconds.
Peritoneal dialysis is appropriate therapy in a severely hypothermic patient. In practice, it is often omitted if other measures appear to be successful. There are no universally established criteria for performing peritoneal lavage in hypothermic patients who have detectable vital signs. Though theoretically less effective than other techniques that directly warm the thorax in the setting of cardiac arrest, it has been used successfully in that situation. It is theoretically useful in hypothermic patients who have overdosed with a dialyzable toxin. Other less invasive methods, such as gastric or bladder lavage or warm nebulized air or oxygen inhalation, may be preferred in stable patients with temperatures higher than 26°C to 28°C (> 78.7°F to 82.4°F). Peritoneal dialysis should not be performed on patients with previous abdominal surgery. It should be used with extreme caution in patients with a coagulopathy.
We recommend using the Seldinger technique with a commercially available disposable kit (e.g., Arrow Peritoneal Lavage Kit, product no. AK-09000, Arrow International, Inc., Reading, PA) because of the ease of performance and minimal morbidity associated with this procedure.
In a noncritical patient, obtain a coagulation profile before the procedure, but in life-threatening situations, initiate the procedure immediately before laboratory studies. Place the patient in the supine position with a Foley catheter and nasogastric tube in place. After infiltrating with lidocaine, make an infraumbilical stab incision with a No. 11 scalpel blade, and place an 18-gauge needle into the peritoneal cavity directed toward the pelvis at a 45-degree angle. Insert a standard flexible J wire through the needle, and then remove the needle. Pass the 8-Fr dialysis catheter over the wire with a twisting motion, and then remove the wire.
Lavage rates of 4 to 12 L/hr can be achieved with two catheters. Warm the fluid with a standard blood warmer to 40°C to 45°C (104.0°F to 113.0°F). Use a standard 1.5% dextrose dialysate solution. Add potassium (4 mmol/L) if the patient becomes hypokalemic. Saline has also been used successfully. The rate should be at least 6 L/hr and preferably 10 L/hr.
The Seldinger method has a complication rate of less than 1%. A “mini-lap” performed via direct dissection may also be used but might have a higher complication rate. Further discussion of potential complications is provided in Chapter 43 .
Peritoneal dialysis is a useful method because it entails readily available fluid and can be done with a self-contained disposable kit. If a hospital also treats trauma victims, the lavage kit can be the same as that used for evaluation of abdominal trauma. If this technique is combined with warm nebulized inhalation, warming rates of 4°C/hr (7.2°F/hr) can be achieved. Peritoneal lavage rewarms the liver and restores its synthetic and metabolic properties.
Gastric or bladder irrigation offers some of the same advantages as peritoneal dialysis without invading the peritoneal cavity. Heat is delivered to structures in close proximity to the core. In the Multicenter Hypothermia Study, gastric/bladder/colon lavage had a first-hour rewarming rate of 1.0°C to 1.5°C/hr (33.8°F/hr to 34.7°F/hr) and a second-hour rewarming rate of 1.5°C/hr to 2.0°C/hr (34.7°F/hr to 35.6°F/hr) for severe hypothermia. In a multifactorial analysis of the Multicenter Hypothermia Study there was a trend toward improved survival in patients treated in this manner.
Although the amount of heat delivered with gastric lavage appears to be less than that delivered with peritoneal dialysis, it is somewhat easier to use and less invasive. When combined with other methods, gastric or bladder lavage provides significant warming. Serum electrolyte levels should be monitored if large volumes of tap water are used because dilutional electrolyte disturbances may occur. Children and geriatric patients might be more susceptible to electrolyte changes with tap water irrigation.
Warmed gastric or bladder lavage may be used as adjunctive therapy for moderate or severe hypothermia. It can be combined with other warming techniques when rapid rewarming is needed. Patients who are obtunded and lack protective airway reflexes should undergo ET intubation before gastric lavage to prevent aspiration of gastric contents. Refer to the appropriate chapters concerning nasogastric tube placement (see Chapter 40 ), gastric lavage (see Chapter 42 ), and urethral catheterization for specific contraindications to these procedures.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here