Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Prion (pronounced pree-ahn ) diseases (PrDs) are a group of uniformly fatal neurodegenerative diseases caused by the transformation of an endogenous protein, PrP (prion-related protein), into an abnormal conformation (misfolded protein) called the prion . The term prion is derived from the term proteinaceous infectious particle and was named by Stanley Prusiner, who discovered prions ( ). For many years, prion diseases were mistakenly thought to be due to “slow viruses,” in part owing to the transmissibility of the diseases and the long incubation period between exposure and symptom onset ( ; ). Research by Prusiner and others, however, determined that the infectious agent did not contain nucleic acid, a component of viruses. Furthermore, treating prion-contaminated material with methods that inactivated viruses and other microorganisms did not prevent these diseases from being experimentally transmitted; yet methods that denatured or destroyed proteins prevented transmission, strongly supporting the theory that the causative agent was a protein ( ; ). The identification of the gene-encoding human PrP ( ), PRNP , and mutations of this gene in patients with familial prion disease ( ; ) further helped support the prion hypothesis. In 1997, Prusiner received the Nobel Prize in Physiology or Medicine for his work on identifying the prion ( ). Through animal models, identification of prion gene mutations causing prion disease in humans, and in vitro production of prions with transmissibility, it essentially has been proven that the prion protein is necessary and sufficient to cause prion disease ( ; ). Although PrDs occur in animals and humans, this chapter focus on human PrDs, discussing animal prion diseases only relevant to human disease.
Perhaps one reason many find PrDs so fascinating is that they are unique in medicine because they can occur in three ways in humans: spontaneously (sporadic), genetically, and through transmission (acquired) ( ). Approximately 85% of human prion diseases are sporadic, 15% are genetic, and fewer than 1% are acquired (e.g., iatrogenic) ( ; ; ; ). Sporadic prion disease, or sporadic Jakob-Creutzfeldt disease (sJCD), is thought to occur spontaneously. Genetic prion diseases (gPrD) are due to a mutation in PRNP and historically have been classified into three forms based on clinical and pathological features: familial JCD (fJCD), Gerstmann-Sträussler-Scheinker disease (GSS), and fatal familial insomnia (FFI). As noted in the gPrD section, however, this classification is somewhat antiquated. Although acquired (infectious) prion diseases are the least common form of human prion disease, they are perhaps the most notorious, in part owing to their occurrence through inadvertent transmission of prions from animals to humans and from human to human. Because the genetic and acquired forms of human prion disease are less common, they will be discussed in less detail in this chapter than the much more common form, sJCD.
The incidence of human prion diseases is about 1–1.5 per million per year in most developed countries, with some variability from year to year and between countries ( ; ; ; ; ; ; ). Thus, annually there are about 6,000 human prion cases worldwide, including about 400–500 in the United States ( ; ). The incidence of cases can vary from year to year, particularly in countries with smaller populations in which a small fluctuation in cases can have a big impact on incidence ( http://www.eurocjd.ed.ac.uk/surveillance%20data%201.html ; ; ; ; ; ). The peak age of onset of sJCD occurs around a unimodal, relatively narrow peak of about 68 years ( ; ; ). Because sJCD tends to occur within a relatively narrow age range, a person’s lifetime risk of dying from sJCD is estimated to be about 1 in 5000–10,000, much higher than the incidence (which is across all age groups) of 1 in a million ( ; ).
The history of the nomenclature for JCD is quite interesting. In 1921 and 1923, Alfons Jakob published four papers describing five unusual cases of rapidly progressive dementia. He stated that his cases were nearly identical to a case described earlier by his professor Hans Creutzfeldt in 1920. This disease was referred to for many decades as Jakob’s disease or Jakob-Creutzfeldt disease until Clarence J. Gibbs, a prominent researcher in the field, started using the term Creutzfeldt-Jakob disease (CJD) because the acronym was closer to his own initials ( ). It turns out that the cases Jakob described were very different than Creutzfeldt’s case, and that only two of Jakob’s five cases actually had the disease that we now call JCD or CJD (prion disease), whereas Creutzfeldt’s case did not ( ). Therefore, the name for prion disease should be Jakob’s disease or possibly Jakob-Creutzfeldt disease . Unfortunately many continue to use the term CJD, either for historical reasons or because the term JC disease (JCD) can be easily confused with progressive multifocal leukoencephalopathy (PML) caused by the JC virus. In this chapter, we will use the more historically accurate terms Jakob-Creutzfeldt disease and JCD . Prion diseases also have been historically called transmissible spongiform encephalopathies (TSEs) due to two properties common to many prion diseases: transmissibility and, on neuropathology, spongioform changes. We will not use the older term TSE because some gPrDs might not be transmissible ( ) and not all human prion diseases have spongiform changes (now called vacuolation due to fluid-filled vesicles in the dendrites) on pathology ( ; ).
Before further discussion, it is important to understand what a prion is. Just as the nomenclature for human prion diseases is complicated, unfortunately so is the terminology for the biology of prions. The normal prion protein (PrP) is referred to as PrP C , in which the C stands for the normal cellular form. Prion proteins, PrP C , can be transformed into prions, an abnormal, “infectious” form of PrP often called either PrP Sc or PrP Res ( Sc refers to the abnormally shaped PrP found in scrapie—the prion disease of sheep and goats—and Res refers to the fact that prion being partially resistant to digestion by proteases (enzymes that digest proteins). In this chapter we use the term PrP Sc .
PrP C and PrP Sc essentially have identical amino acid sequences (except in gPrD; see later) but different three-dimensional structures, with the former mainly consisting of α-helical structure with little or no β-sheet structure and the latter mainly having β-sheet structure ( ; ), possibly stacked as a solenoid ( ). Prions are characterized by the intrinsic ability of their structures to act as a template and convert the normal physiological PrP C into the pathological disease-causing form, PrP Sc . Per the current prion model, when PrP C comes in contact with PrP Sc , PrP C changes shape into that of PrP Sc . Thus, PrP Sc acts as a template for the misfolding of PrP C into PrP Sc . It is believed that it is the accumulation of prions, PrP Sc , in the brain that leads to nerve cell injury and death ( , ), although some data suggest that it is the transformation of PrP C into PrP Sc , and not the accumulation of PrP Sc , that causes neuronal injury and subsequent disease ( ). Sporadic JCD can present quite variably despite all cases having PrP Sc with an identical amino acid sequence; one reason for this is that there are different strains of PrP Sc , each with slightly different biological and physicochemical properties. Such prion strain diversity contributes clinicopathologically to variabilities in tissue tropism, host affinity, and clinical presentations ( ; ; ).
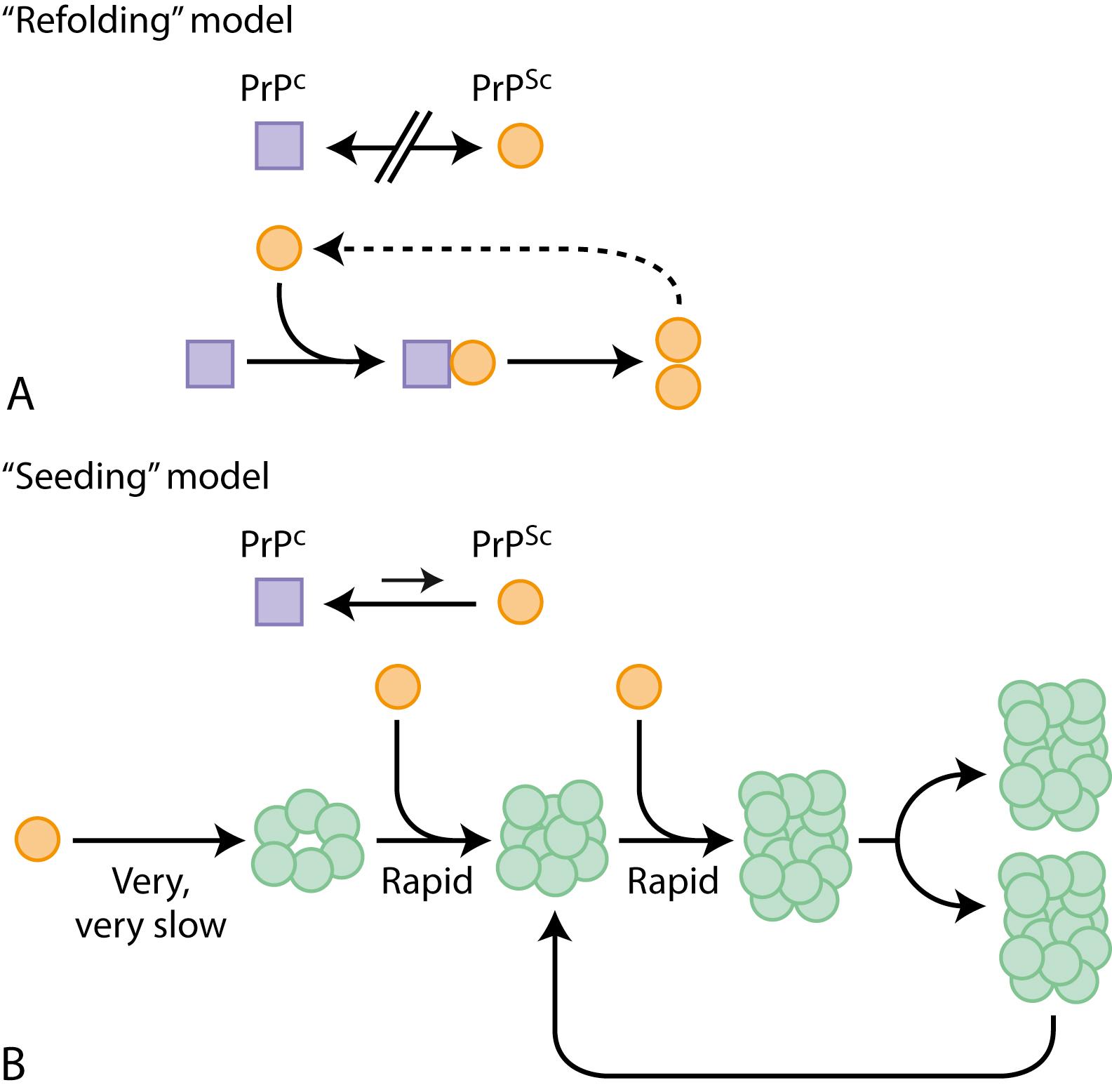
Although it is not known how prions spread throughout the brain, at least two models have been proposed: a refolding model and a seeding model, which are not mutually exclusive ( Fig. 94.1 ). Importantly, mice that are devoid of PrP C can neither be infected with nor replicate prions ( ; ; ). Furthermore, when an explant of neuronal tissue overexpressing PrP C is explanted into a PrP C knockout mouse and inoculated with prions, the explant shows extensive PrP Sc accumulation and neurodegeneration, but host brain tissue shows no toxicity despite containing PrP Sc derived from the graft ( ; ). These studies provide strong evidence that PrP C is necessary for prion disease. Furthermore, propagation capacity of different prion strains is shown to be partly related to surrounding cellular cofactors ( ).
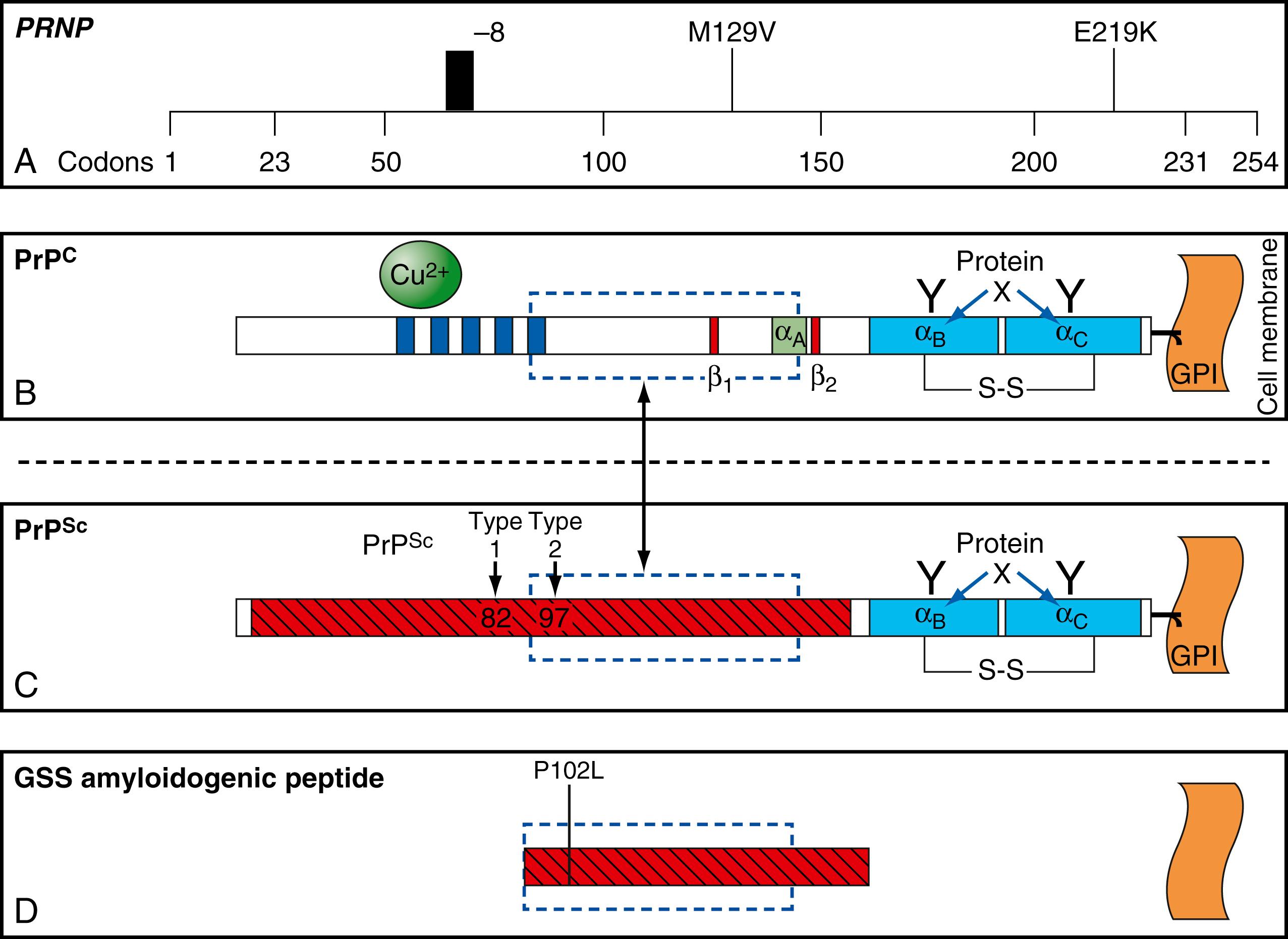
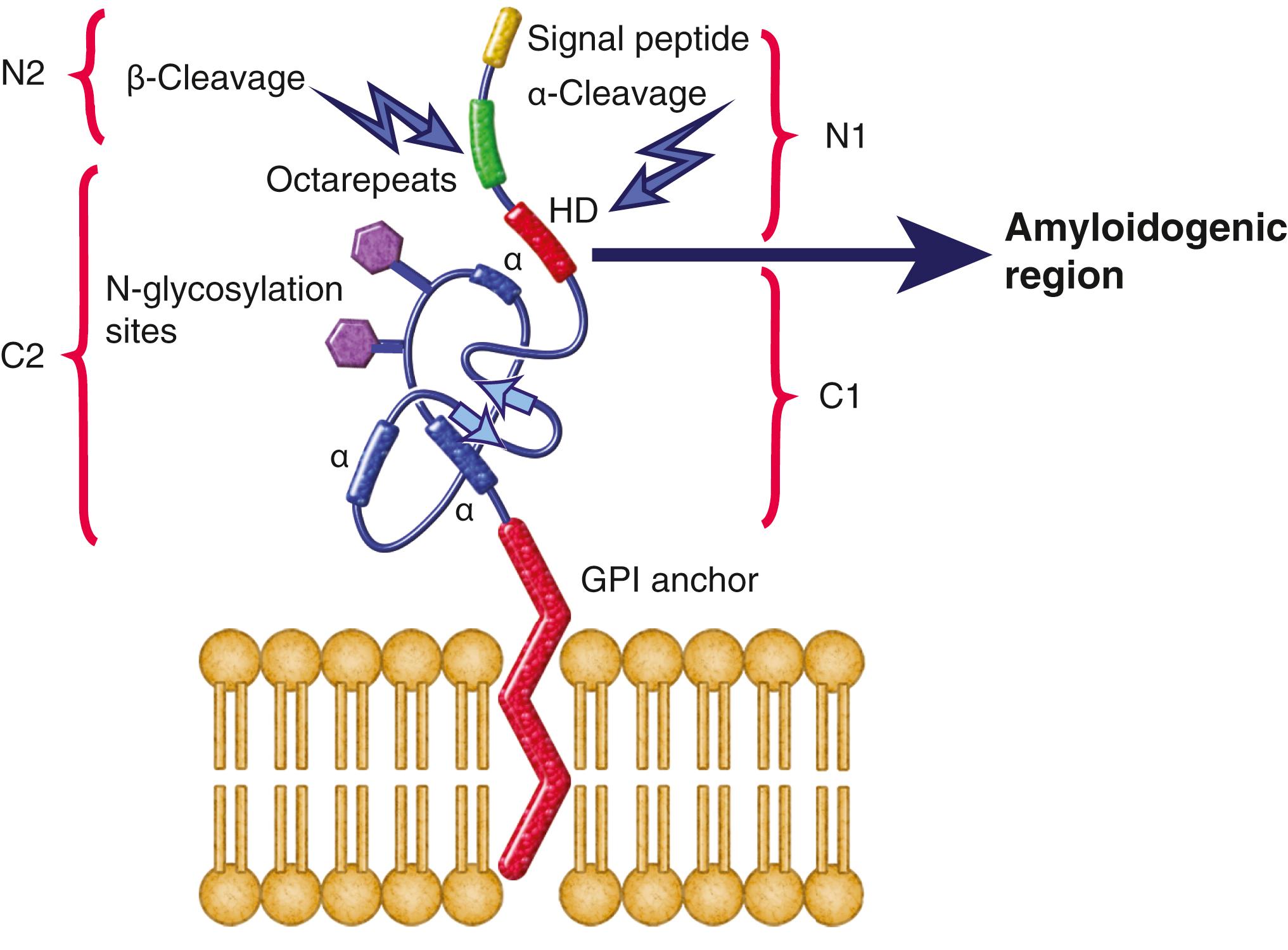
The function of PrP C is still not entirely known ( ; ; ). It is evolutionary conserved, so it probably plays an important role in neuronal development and function ( ). In humans, it is encoded by PRNP, located on the short arm of chromosome 20 ( ; ). PrP C protein typically consists of a highly conserved central hydrophobic segment (HD) and a C-terminal hydrophobic region that is commonly attached to the outer cell membrane by a glycosylphosphatidylinositol (GPI) anchor and an amino terminal flexible tail ( ; ; ) ( Figs. 94.2 and 94.3 ). PrP C is primarily membrane bound and resides primarily on nerve cell membranes and on other cells in the body, including lymphocytes. Mice that have had both copies of the open reading frame (ORF) of their PrP gene, Prnp , deleted (PrP −/− ) have a normal lifespan and appearance ( ; ). Furthermore, conditional knockout mice, in which the gene is not removed until after the mouse has already developed, also appear normal and unaffected by gene removal ( ). Although the mice essentially were clinically asymptomatic, deeper phenotyping revealed several abnormalities, as discussed below.
PrP C binds to many proteins and cellular constituents. Animal and cell models have suggested a variety of possible functions of PrP C , including cell signaling, adhesion, proliferation, differentiation, and growth ( ; ; ; ). Studies with various Prnp knockout mice models of mixed genetic backgrounds found they develop peripheral nerve demyelination ( ); have increased susceptibility to ischemic brain injury ( ; ); altered sleep and circadian rhythm ( ; ); altered hippocampal neuropathology and physiology, including deficits in hippocampal-dependent spatial learning and hippocampal synaptic plasticity ( ; ); and olfactory dysfunction ( ). The octarepeat peptide regions of PrP C have even been implicated in playing an involved role in multidrug resistance of gastric cancer cells ( ). There also have been several studies suggesting PrP C binds β-amyloid (Aβ), a major protein in Alzheimer disease (AD), and might play a role in the pathogenesis of AD ( ; ). Some studies in mice suggest that PrP C mediates the toxic effects of Aβ oligomers and might be necessary for memory deficits to occur in AD ( ; ). In fact, in one study infusion of anti-PrP C antibodies ameliorated cognitive deficits in an AD mouse model ( ). This possible role of PrP C in binding to Aβ and causing dysfunction in AD is still controversial, however. In one study, with human APP (J20) crossed onto a PrP C -deficient background still had the neurological impairment that was present in the J20 mice, suggesting PrP C might not be a major mediator of Aβ-induced impairment ( ).
For reasons that were unclear at the time, many phenotypes identified in certain Prnp knockout mice were not reproducible in knockout mice with different genetic backgrounds, resulting in confusion regarding the physiological role of PrP C in transgenic knockout mice models ( Prnp -/-) ( ). One possible reason is that some reported findings were caused by knocking out genes adjacent to Prnp rather than Prnp itself; this is because the experiments were done with mice of mixed genetic backgrounds, which may harbor variable Prnp -flanking genes that can lead to poorly controlled Mendelian segregation of these polymorphic alleles. This led to systematic genetic confounds and in some cases incorrect conclusions regarding the function of PrP C , such as the inhibition of macrophage phagocytosis ( ; ; ; ; ; ). Using Prnp knockout mice with pure genetic backgrounds that do not possess flanking genes ( ; ), investigators found few of the prior putative phenotypes reported in Prnp knockout mice of impure genetic backgrounds ( ; ). Thus, genotype-phenotype relationships in impure mice strains need to be interpreted with caution and earlier experiments investigating the role of PrP C with impure mice models need to be replicated with Prnp mice with pure genetic backgrounds ( ; ; ). Phenotypes that remain in Prnp knockout mice with pure genetic background include chronic demyelinating peripheral neuropathy, suggesting a role for PrP C in myelin maintenance ( ), altered circadian rhythm and sleep pattern ( ), and altered synaptic plasticity ( ). Given the findings from Prnp mice with pure backgrounds, it seems that PrP C interacts with other membrane proteins, can regulate transport and regulation of these proteins, can modulate their functionality, and can even signal distinct biological pathways via its N-terminal tail cleavage products and scavenge Aβ amyloid aggregates ( ).
Prion disease is generally regarded as a gain of function disease for at least two reasons: (1) deletion or reduction (e.g., hemizygous) of Prnp in mice results in mice that are normal or largely asymptomatic; and (2) the prion protein expression level in mice models is directly associated with the rate of disease progression ( ; ; ; ). Nevertheless, some of the roles for PrP C suggest that dysfunction in prion diseases might be due not only to pathogenicity of misfolded PrP C into prions but also to loss of normal function of PrP C ( ).
Post-translational modification of prion protein and the GPI anchor may play a role in the pathogenesis of prion diseases. Different proteolytic processing of the prion protein generates protein fragments that have variable functions. For instance, the hydrophobic domain (HD) region in PrP C (amino acid 106–126) (see Fig. 94.3 ) is considered to have amyloidogenic properties ( ). In the normal state, α-cleavage occurs in this region, causing a loss of the amyloidogenic tendency and generating both a tethered C terminal protein (C1), which helps maintain myelin integrity, and a free N terminal fragment (N1), which has anti-apoptotic activity. In contrast, β-cleavage, which occurs more frequently in prion disease, generates a membrane-bound C terminal protein (C2) that retains the amyloidogenic core, and a free N terminal fragment (N2), the latter of which is speculated to have antioxidant instead of anti-apoptotic properties. It is believed that a higher-stress environment favors β-cleavage that tends to reduce oxidative stress but promote prion aggregation ( ; ; ). When Prnp − / − transgenic mice that retain the region encoding the C1 protein (i.e., less amyloidogenic) were inoculated with prions, they remained asymptomatic and showed resistance to protease-resistant PrP accumulation ( ). As aforementioned, these studies involved transgenic mice with mixed genetic backgrounds and further study replications using pure genetic mice models is warranted. Regarding the role of the GPI anchor attaching PrP C to the cell membrane, anchorless transgenic mice, which encode PrP C without the GPI anchor, remained asymptomatic when inoculated with PrP Sc but still showed diffuse amyloid PrP Sc plaques in the brain ( ). Another version of anchorless transgenic mice, however, had spontaneous late-onset neurological symptoms and GSS-like pathology (i.e., amyloid plaques) ( ). The former anchorless PrP C study suggests that PrP Sc deposition alone might not be sufficient to cause neurotoxicity, but both studies suggest the GPI anchor might be involved in prion disease pathophysiology.
As noted above, nomenclature in prion diseases can be confusing, with the terms JCD or CJD used to refer to just sporadic JCD or to all human prion diseases interchangeably. To reduce confusion in this chapter, we refer to all human prion diseases as prion disease (PrD), whereas sJCD will be used to refer only to sporadic JCD. The rarity of sJCD and the fact that its incidence is similar (∼1–1.5/million) in most countries with appropriate surveillance suggest that it is unlikely to be due to an environmental cause and likely arises as a rare stochastic event in otherwise healthy persons, possibly through the spontaneous transformation of the prion protein PrP C into PrP Sc or through a somatic mutation that results in the formation of a prion protein that is more susceptible to changing into PrP Sc ( ; ; see , for a discussion on possible origins of sJCD).
Sporadic JCD is typically a very rapid disease with a median survival of about 4.5–6 (range 1–130) months ( ; ) and mean survival of about 8±11 months ( ). About 85% of patients die within 1 year from onset of symptoms ( ), and ∼ 50%–60% die in less than 5–6 months ( ; ). The median age of onset is 60–67 years old, with a range from 12 to 95 ( ; ; ) ( Table 94.1 ). Occurrence of sJCD at young (20s–40s) ( ; ; ) or old (>80) ages is uncommon ( ). Patients younger than 20 years of age are extremely rare, although a few have occurred, including a 13-year-old in the United States ( ) and an unpublished case of a 12-year-old girl with sJCD in Spain (biopsy-proven with no PRNP mutation; M. Geschwind, personal communication). Sporadic JCD individuals with younger age of onset were reported to have longer survival and higher tendency to present with non-cognitive features including affective disorder, behavioral change, or sleep illnesses ( ; ).
| Characteristic | sJCD | vJCD | fJCD | iJCD | FFI | GSS | Kuru |
|---|---|---|---|---|---|---|---|
| Average age at onset (years) | 67 | 28 | Variable among kindreds, 23–55 | All ages | 50 | 40 | All ages |
| Average duration of disease (months) | 8 | 14 | Variable among kindreds, 8–96 | 12 | 18 | 60 Variable among kindreds, 60–240 |
11 |
| Average incubation periods (range) | N/A | 17 years (12–23 years); blood transfusion, 7 years (6.5–8 years) | N/A | Neurosurgical, 18 months (12–28); dura graft, 6 years (1.5–23 years); hGH, 5 years (4–36 years) | N/A | N/A | 12 years (5–50 years) |
| Most prominent early signs | Cognitive and/or behavioral dysfunction | Psychiatric abnormalities, sensory symptoms (later dementia, ataxia, and other motor symptoms) | Cognitive and/or behavioral dysfunction | Cognitive dysfunction, ataxia | Insomnia, autonomic instability | Ataxia, tremor, extrapyramidal symptoms | Ataxia, tremor |
| Cerebellar dysfunction (%) | >40 | 97 | >40 | >40 | No | 100 in P102L mutation, less common in most other mutations | 100 |
| DWI/FLAIR MRI positive | Yes, >92% | Yes, pulvinar sign | Yes for most mutations | Variable; some positive in deep nuclei or cerebellum | Unclear | Variable; most negative | N/A |
| PSWCs on EEG | Yes, 65% | No (rarely at end stage) | Yes | Yes | No | No | N/A |
| Amyloidosis | Sparse plaques in 5%–10% | Severe in all cases | Sporadically seen | Sporadically seen | No | Very severe | 75% of cases |
| Presence of PrP Sc in the lymphoreticular system | No | Yes | No | Yes | No | No | Unlikely |
Symptoms of sJCD vary widely but typically include cognitive changes (dementia), behavioral and personality changes, difficulties with movement and coordination, visual symptoms, and constitutional symptoms ( ; ; ). Cognitive problems are often among the first symptoms in sJCD and typically include mild confusion, memory loss, and difficulty concentrating, organizing, or planning. Motor manifestations of sJCD include extrapyramidal symptoms (bradykinesia, dystonia, tremor), cerebellar symptoms (gait or limb ataxia), and later in the disease, myoclonus (sudden jerking movements). Whereas the cognitive and motor symptoms are often obvious, other common early symptoms may be subtle. These include behavioral or psychiatric symptoms (i.e., irritability, anxiety, depression, or other changes in personality) and constitutional symptoms (i.e., fatigue, malaise, headache, dry cough, lightheadedness, vertigo, etc.). Visual symptoms typically present as blurred or double vision, cortical blindness, or other perceptual problems; they are due to problems with processing of visual information in the brain and not due to retinal or cranial nerve abnormalities. Other symptoms such as aphasia, neglect, or apraxia (inability to do learned movements) due to cortical dysfunction might also occur and can be presenting features. Sensory symptoms such as numbness, tingling, and/or pain are less well-recognized symptoms and are probably under-reported, given the magnitude of the other symptoms in sJCD ( ; ; ; ). sJCD can sometimes be classified based on the initial presenting symptoms (within the first few weeks of onset), into cognitive, Heidenhain (visual presentation), affective (mood disorders presentation), classic (cognitive symptoms and ataxia), and Brownell-Oppenheimer (ataxia presentation) variants, with cognitive and cerebellar symptoms being the most common initial presentations ( ; ; ; ). Each of these sJCD variants can have distinctive disease courses as well as electroencephalogram (EEG) and magnetic resonance imaging (MRI) findings ( ; ). Classic and visual variants often progress most rapidly with the shortest survival time. Affective variants tend to have a younger age of onset and longest disease duration. In contrast, Oppenheimer-Brownell variants usually have an older onset age and lack presence of periodic sharp-wave complexes on EEG or basal ganglia hyperintensity on brain MRI ( ). Clinical symptomatology and course appear to vary, in part, based on the molecular classification of sJCD based on the prion type and a PRNP polymorphism (see later discussion) ( ). Sporadic JCD usually progresses rapidly over weeks to months from the first obvious symptoms to death. The end stage is usually an akinetic-mute state (no purposeful movement and not speaking) ( ). Most patients with prion disease die from aspiration pneumonia.
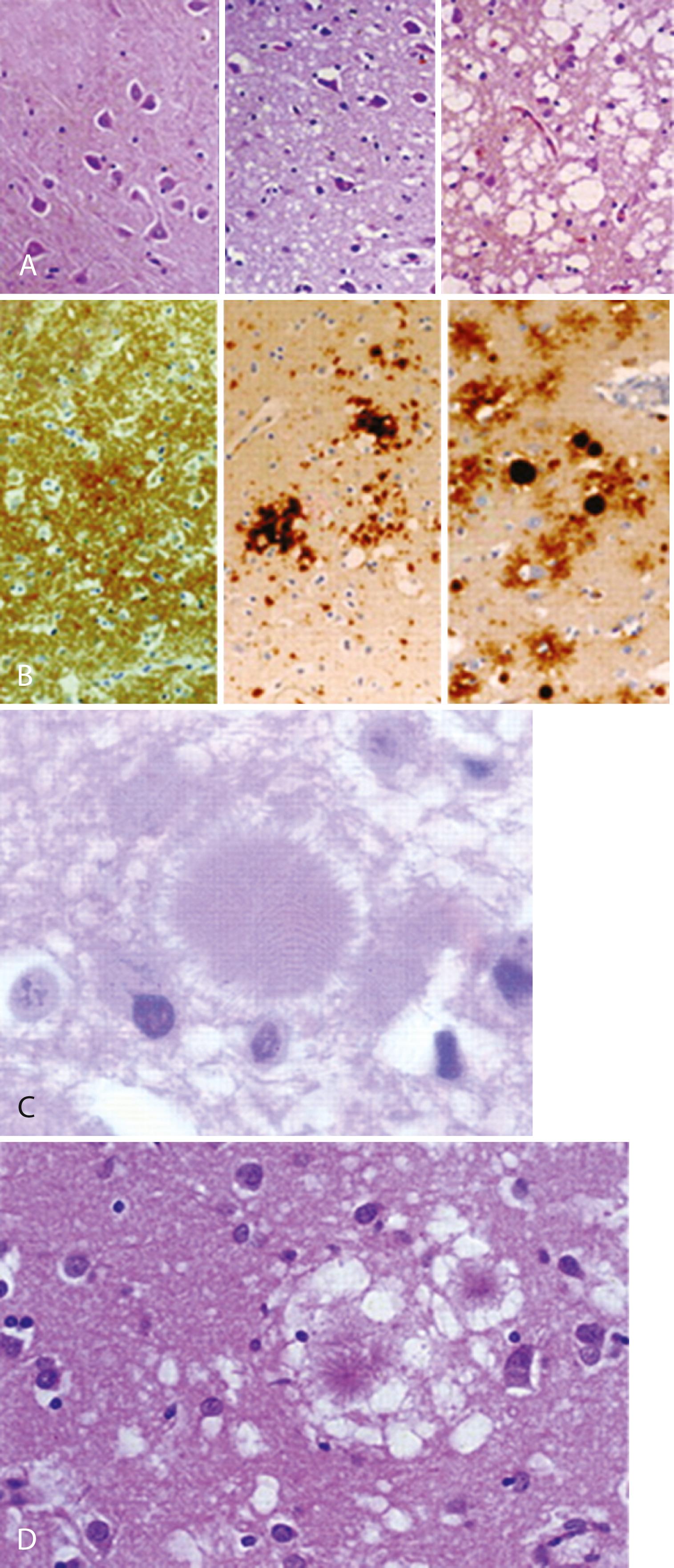
Typical neuropathological features of sJCD include neuronal loss, gliosis (proliferation of astrocytes), vacuolation (i.e., spongiform changes), and deposition of PrP Sc ; except for PrP Sc deposition, the other features are found in many other neurodegenerative and other neurological conditions and are not specific for JCD ( ) ( Fig. 94.4 ). PrP Sc deposition followed by mild vacuolation (spongiform change), and gliosis are early features of prion diseases ( ; ), with later more severe vacuolation, gliosis, and neuronal loss ( ). Some pathologists are of the opinion that the term vacuolation is the more appropriate term to describe the spongiform changes, as these are not holes but rather fluid-filled vesicles formed in distal dendrites near synapses ( ). Clinical and other features of several prion diseases are summarized in Table 94.1 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here