Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Percutaneous image-guided tumor ablation uses the cytotoxic properties of thermal energy or electric field strength to cause cell death.
The thermal ablative effect due to coagulation necrosis is generated by the Radiofrequency probe, which acts as the cathode in an electrical circuit closed by attaching grounds pads to the patient.
Cytotoxicity of Cryoablation results from denaturation of protein structures, and cellular membrane disruption caused by freezing and thawing.
Microwave frequency causes rapid molecular friction and significant rise in water temperature, leading to cellular death and coagulation necrosis.
Irreversible Electroporation mechanism is entirely electric and nonthermal, using pulses of high electrical current to disrupt the lipid cellular membrane with formation of multiple permanent nanopores leading to cell death.
Chemical ablation uses acetic acid and ethanol to destroy tissues, leading to immediate cytoplasmic dehydration, vascular thrombosis, protein denaturation, and consequent coagulation and ischemic necrosis.
Percutaneous image-guided tumor ablation with both thermal and nonthermal techniques has gained growing attention for the treatment of many focal neoplasms with a parallel continuous advance in energy delivery and technical developments. Percutaneous ablative therapy, delivered by using needlelike applicators connected to a generator, uses the cytotoxic effects of thermal energy, electric field strength, or chemicals to cause cell death.
This chapter will review the principles of percutaneous ablative therapies currently used in the field of interventional oncology, including thermal techniques (radiofrequency ablation [RFA], cryoablation, microwave ablation) as well as nonthermal techniques (irreversible electroporation and chemical ablations). High-intensity focused ultrasound (HIFU) and laser ablative therapies are beyond the scope of this chapter. The clinical practice of these percutaneous ablative therapies will be discussed in different chapters.
RFA is the most frequently used percutaneous ablative technique for many focal neoplasms, especially liver and lung tumors. Most RFA systems operate in a monopolar mode by using two different types of electrodes: interstitial electrodes inserted into the lesion and dispersive electrodes placed on the skin surface known as ground pads. , The interstitial electrode delivers energy to the tumor, creating a volume of high current density and localized heating around the electrode tip. The ground pad closes the electrical current circuit with dispersing energy over a large surface area to reduce the likelihood of thermal injury to the skin ( Figure 2-1 ).
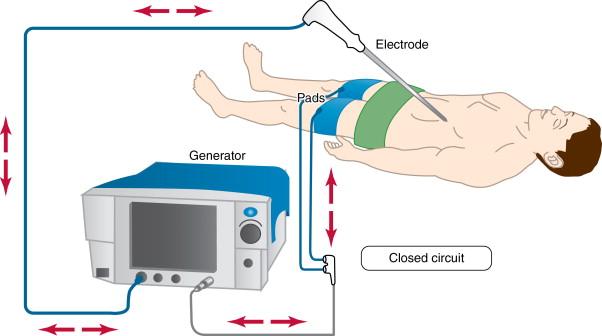
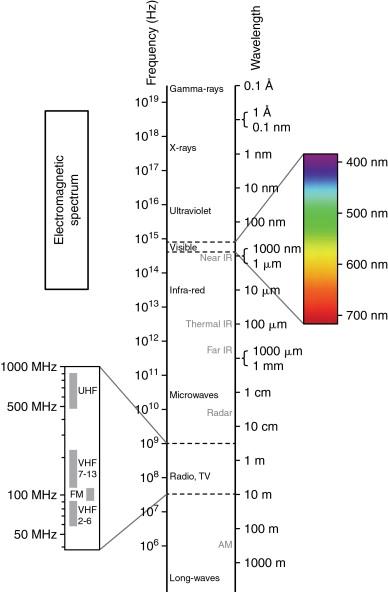
Radiofrequency (RF) waves range from 300 to 600 kHz in the electromagnetic spectrum, and it is followed in an increasing frequency by microwave, infrared, visible, ultraviolet, x-rays, and gamma rays ( Figure 2-2 ). Molecules close to the active component of the RF electrode, especially dipolar molecules like water, respond with motion to the alternating current produced by the RF generator. The charged molecules move in order to constantly realign their electric poles with the continuously alternating current. This motion causes friction and results in the production of heat. Heat is locally conducted from hotter to cooler tissues, and heat can be drawn away from tissue on the basis of continuous transfer to flowing blood in vessels larger than 3 mm in diameter, a process called “heat sink.” Tissues surrounding the electrode undergo coagulation necrosis because of a higher deposited energy flux in a small surface of the probe tip when compared to a lower energy flux in a large surface of the grounding pads. This thermal ablative effect is generated by the RF probe, which acts as the cathode in an electrical circuit closed by attaching grounds pads to the patient.
Cellular death occurs within a few seconds at 55°C. At 100°C, cell death is almost instantaneous, and it is accompanied by vaporization and microbubble formation. Nitrogen gas, released on cellular death at high temperatures, acts to insulate against the efficient conduction of heat to adjacent tissue. Clinically, this type of rapid tissue death caused by very high temperature is referred to as “charring.” Therefore, a slow RF application, over many minutes, is more effective and efficient than a quick temperature rise. Slower transfer of heat results in less charring and tissue desiccation; otherwise, these undesirable factors act to further insulate the probe, limiting further extension of the ablation zone. , The physical properties involved in the production and transfer of heat energy away from the electrode into more distant tissue limits the size of the ablation achievable with a single probe. RFA appears to perform best when tumors have a diameter of 3 cm or less. In addition, an additional ablative margin of 1 cm is preferable to ensure complete tumor coverage, because the extent of coagulation necrosis is limited by the heat sink effect created by adjacent blood vessels. Flowing blood, in vessels of 3 mm or larger, carries away heat on a continuous basis, preventing the sustained elevation of high temperature in adjacent tissue. This can limit effectiveness, leading to possible residual tumor and increasing the chance of tumor recurrence. The use of multiple electrodes can mitigate this problem and help in increasing the ablation area.
There are three major manufacturers of monopolar RFA devices on the market. Generators manufactured by the three major RFA companies provide maximum outputs of 150–200 W, delivering high-frequency (450–500 kHz) alternating current via RF electrodes in the size range of 14–17 gauge.
The Boston Scientific RF system (Natick, MA), which includes the RF 3000 Generator that is an impedance-based direct feedback system designed to accurately monitor the extent of tissue desiccation, permits continued delivery of RF energy until desired ablation is achieved ( Figure 2-3 ). There are a range of LeVeen RFA array probes, which include the SuperSlim, standard, co-access systems in addition to the Soloist straight-tip needle, single-needle electrode ( Figure 2-4 ).

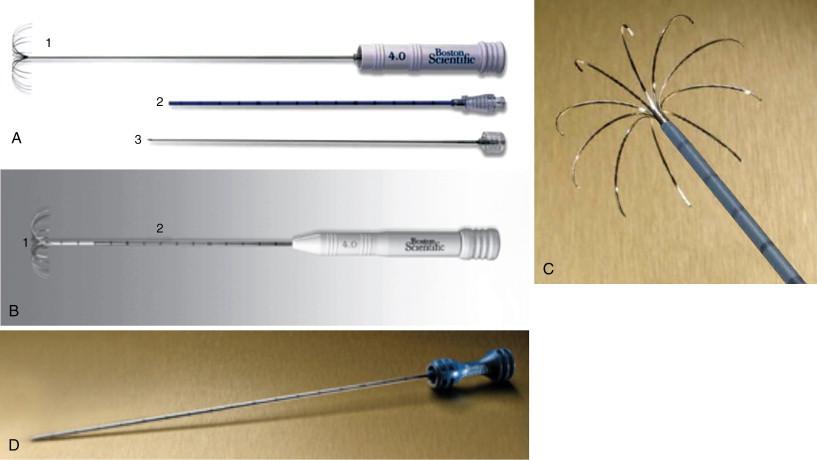
The SuperSlim RFA needle electrode (17-gauge) has size ranges from 2 to 3 cm and for each diameter size can be matched with cannula lengths of either 15 or 25 cm. The standard LeVeen needle electrode comes in diameters ranging from 2 cm (12 cm in length), 3 cm (12 cm in length), 3.5 cm (12 or 15 cm in length), 4 cm (15 cm in length), and 5 cm (15 cm in length). The needle electrodes for diameter sizes ranging from 2 to 3.5 cm, 4 cm, and 5 cm are 15, 14, and 13 gauge, respectively. The 15-gauge CoAccess electrode system has the ability to obtain a tissue biopsy by inserting an 18-gauge needle gun into the sheathed needle system before starting the ablation. The CoAccess probe has diameters ranging from 3, 3.5, and 4 cm with 15 cm length. The co-access RFA electrode shaft is not insulated and therefore cannot be used without the co-access sheath as this will cause skin burn ( Figure 2-5 ).
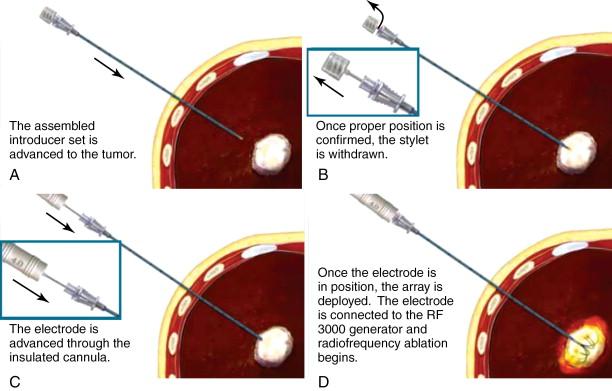
The LeVeen probe consists of multiple curved prongs that deploy from the central needle and project a short distance beyond the needle before directing recurving in the opposite direction. Fully deployed, this creates the appearance of an umbrella ( Figure 2-4 , C ). Each individual prong causes a separate area of coagulation necrosis that slowly increases in size by administering increasing amounts of RF energy in a stepwise fashion and creating a surrounding volume of necrosis ( Figure 2-6 ). Electrode probe tips provide impedance feedback. The resulting increase in tissue resistance to current flow—impedance—is measured in Ohms and is displayed as a series of illuminated bars that progress to Roll-Off ® Indication, an easy-to-read signal of the desired clinical endpoint. The end point of ablation with the LeVeen device is a dramatic increase in impedance, termed “roll-off” by Boston Scientific. The standard algorithm suggests a second “burn” starting at 70% of maximum Roll-Off power. At the end of the procedure, the probe tract can be ablated in order to reduce needle tract seeding.
There is also a straight-tip needle, the Soloist single-needle electrode (16.5 gauge), which is useful for treating small tumors and can avoid any significant collateral damage. The typical ablation zone with the Soloist needle is slightly oval in shape and measures ≈ 1 cm (diameter) × 1.5 cm (length).
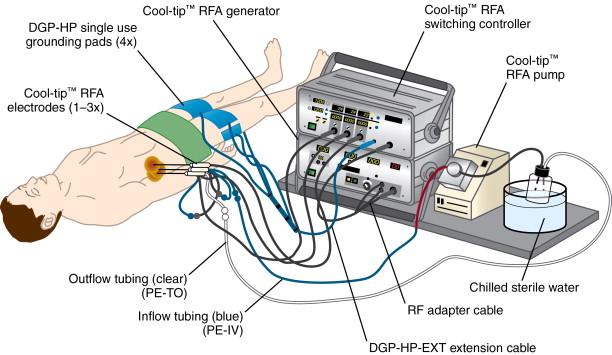
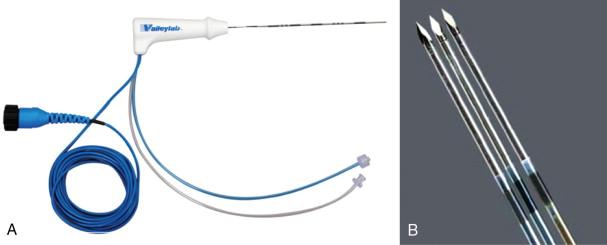
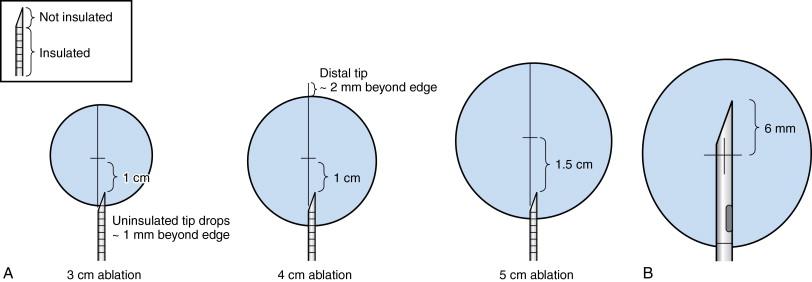
The Cool-tip Covidien RF system (Boulder, CO, formerly known as Tyco Healthcare Valleylab) works on the basis of internally cooled electrodes, where chilled saline is pumped through the chamber of the needle, in order to reduce charring, to increase tumor volume ablation and to shorten ablation time ( Figure 2-7 ). The internal electrode cooling mechanism keeps the temperature of the tissue around the electrode low, allowing the electrode to drive more energy into the tissue without the need to increase power. The Covidien RF Ablation Generator, a 200-W power source, connected to the Cool-tip electrode features a feedback algorithm that continuously monitors tissue impedance. The generator senses maximum energy deposition into the lesion and uses pulsing to control the energy output ( Figure 2-8 ). Impedance is adjusted to achieve the appropriate energy output for the electrode. Thermocouples at electrode tip also measure tissue temperature to help optimize energy deposition. Once the treatment cycle is complete, the electrode cooling can be stopped. The generator automatically shuts off at the end of the 12-minute ablation cycle. The Cool-tip electrode is a 17-gauge straight electrode with a beveled tip ( Figure 2-9 ). There are multiple electrode lengths (10, 15, 25 cm) with five different electrode exposure lengths (1, 2, 2.5, 3, 4 cm), allowing control for varying lesion sizes. The entire electrode can be imaged with computed tomography (CT) or ultrasound guidance during the procedure.

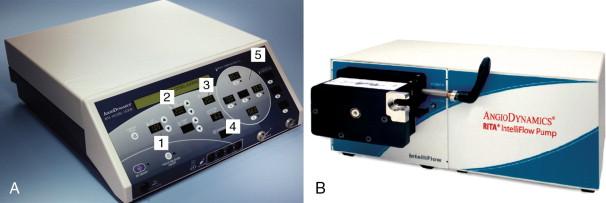
The AngioDynamics system (Latham, NY, formerly known as RITA Medical Systems) has a 1500X RF generator that is equipped with computer-driven protocols that automatically adjust wattage to maintain optimal temperatures during the ablation. The generator, with 250 W of power, combined with the IntelliFlow saline peristaltic infusion pump, is thought to allow for faster and larger ablations by reducing the charring effect ( Figure 2-10 ).
The AngioDynamics StarBurst ® family of probes consists of an attachable or preattached interface cable and an insulated primary trocar with needles and temperature sensors at the distal end. The temperature sensors determine the average temperature.
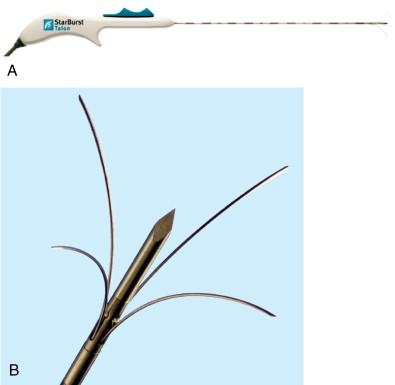
The AngioDynamics system has several electrodes, with the 14-gauge StarBurst XL as the basic probe capable of ablations from 3 to 5 cm ( Figure 2-11 ). The probe contains nine deployable curved prongs oriented forward from the main needle shaft. The maximum diameter is 5 cm in a configuration of a Christmas tree when fully deployed. The tips of the AngioDynamics system hooks have thermocouples that monitor real-time temperature at the treatment volume margin. Real-time temperature readings are displayed on the generator console. Flexible electrodes are also available (StarBurst SemiFlex) ( Figure 2-12 ) with the ability to bend up to 90 degrees in all directions, allowing for easy entry and navigation into the CT gantry. Similar to other systems, electrodes with perfusion capabilities are also offered, the StarBurst Xli Enhanced ( Figure 2-13 ) and StarBurst Talon electrodes ( Figure 2-14 ), whereby saline or hypertonic saline is infused into the ablation tissue, to augment tissue electrical conductivity with the aim of creating an ablation volume diameter up to 7 cm. The probe is placed at the proximal portion of the lesion, allowing the tine tips to be deployed forward ( Figure 2-15 , A ). The one exception is the StarBurst Talon probe, which uses side deployment of the tines, and therefore requires insertion to the distal aspect of the lesion ( Figure 2-15 , B ). The StarBurst Talon RFA electrode with side-deployment tines may be used in lesions adjacent to critical structures. AngioDynamics also manufactures a linear deployment “UniBlate” 17-gauge probe, which provides linear, scalable ablations from 1 to 3 cm in length and 1 to 2.5 cm in diameter. The UniBlate probe has a very low profile for CT gantry compatibility ( Figure 2-16 ).
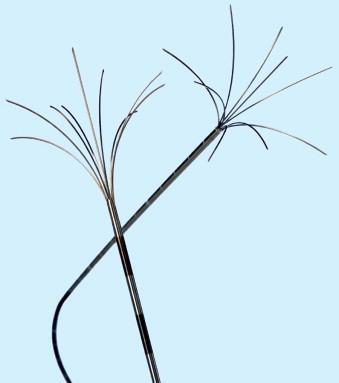



Bipolar systems do not require attaching the patient to a grounding pad, thus minimizing risks of skin burns. The applied RF current is limited to between the two poles within the ablative electrode. The InCircle (RFA Medical, Fremont, CA) bipolar probes are placed on each side of the tumor without penetrating the lesion. Bipolar systems have only recently been available in the United States; hence, experience is currently limited.
Image-guided percutaneous cryoablation is most commonly used for the treatment of small renal cancers, prostate cancer, soft tissue, and bone tumors. Renal cell carcinoma is the tumor most commonly treated with cryoablation. In addition, percutaneous cryoablation is used for the palliation of painful bone lesions and to a lesser extent for the treatment of liver and chest neoplasms, especially pleural-based lesions. More recently, cryoablation has been used for salvaging prostate applications and lung and breast tumors.
Cryoablation reduces the temperature of the target lesion to lethal levels (−20°C to −40°C). Additionally, cycling between cooling and thawing results in disruption of cell membranes, leading to a variety of cytotoxic effects. Cytotoxicity results from extracellular ice formation (producing dehydration, protein denaturation, lipid peroxidation, and increased free radicals), apoptosis (programmed cell death and mitochondrial damage), vascular stasis (creating hypoxia and lack of nutrients), and intracellular ice formation (shearing the cellular membrane caused by freezing and thawing).
The low thermal energy can be transferred from one tissue volume to another by conduction or convection. The direct physical contact and the molecular kinetic energy exchanges are seen in the conduction. However, flowing blood surrounding ablated tissue can transfer away the low thermal energy as seen in convection. Conduction constitutes stable energy transfer dependent on the temperature difference between ablated tissue and the cryoablation probe, whereas convection depends on the flow rate of the surrounding blood adjacent to the volume of interest. Therefore, large-diameter arteries will limit the extent of cryoablation.
The iceball created by cryoablation is visible under CT, ultrasonography, or magnetic resonance guidance, allowing visual monitoring throughout the procedure in most organs. The iceball margin demarcates a zone cooled to 0°C, which is not a lethal temperature. The zone starting 5 mm inside this 0°C isotherm zone will usually harbor the tissue at −20°C, which is considered a lethal temperature. A cryoprobe tip should be placed beyond the distal margin of the tumor to provide 5–10 mm tumor margin as the ablation zone does not extend more than 2 mm past the probe tip.
Argon or helium are the two expanding gases used in commercial cryoablation systems. The inversion temperature is the critical temperature below which a gas that is expanded will experience a temperature decrease, and above which it will experience a temperature increase. According to the Joule-Thomson law, an expanding gas cools if its inversion temperature is greater than the ambient temperature and warms if its inversion temperature is lower than the ambient temperature. Therefore, argon creates the cooling when expanding and circulating in the cryoprobe. The inversion temperature of helium is lower than room temperature; therefore, helium warms when it expands and circulates in the dual-chambered cryoprobe. Separate gas tanks are required to supply argon and helium to the probe via pressure regulators. Argon or helium, once expanding, cools or warms and exchanges thermal energy with the adjacent tissues before circulating back into the dual-chambered cryoprobe.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here