Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() Additional content is available online at Elsevier eBooks for Practicing Clinicians
Additional content is available online at Elsevier eBooks for Practicing Clinicians
In 2018 the total cost of health care in the United States was approximately $3.6 trillion, 17.7% of the Gross Domestic Product, and more than 10% was spent on prescription drugs. Cardiovascular disease makes up the largest subcategory in this spending: in 2020 the American Heart Association estimated that the cost of care for cardiovascular disease in 2015 was $351.3 billion/year.
Not every patient responds to drug therapy in the same way; efficacy varies, and adverse drug reactions (ADRs) range from minor to potentially fatal. Multiple mechanisms can result in this variability, such as poor compliance, variable impact of diverse disease mechanisms on drug actions, drug interactions, and the increasingly well-recognized role of genomic variation. Indeed, ADRs across all therapeutic categories are estimated to be the fourth to sixth most common cause of death in the United States, costing over $30 billion annually and accounting directly for 3% to 6% of all hospital admissions. ,
The fundamental assumption underlying administration of any drug is that the real or expected benefit exceeds the anticipated risk. The benefits of drug therapy are initially defined in small clinical trials, perhaps involving several thousand patients, before a drug’s marketing and approval. Ultimately, the efficacy and safety profiles of any drug are determined after the compound has been marketed and used widely in hundreds of thousands of patients. Occasionally, unexpected drug actions detected during or after a development program can result in new indications: PDE5 inhibitors for pulmonary hypertension or SGLT-2 inhibitors for heart failure are examples.
When a drug is administered for the acute correction of a life-threatening condition, the benefits are often self-evident; insulin for diabetic ketoacidosis and nitroprusside for hypertensive encephalopathy are examples. However, extrapolation of such immediately obvious benefits to other clinical situations may not be warranted.
Randomized clinical trials (RCTs) have proven invaluable both to demonstrate the efficacy of drug therapy and to identify rare but serious ADRs. One of the first examples of an RCT identifying an unexpected serious ADR was the Cardiac Arrhythmia Suppression Trial (CAST), which tested the hypothesis that suppression of ventricular ectopic activity, a recognized risk factor for sudden death after myocardial infarction (MI), would reduce mortality; this notion was highly ingrained in cardiovascular practice in the 1970s and 1980s. In CAST, sodium channel–blocking antiarrhythmic drugs did suppress ventricular ectopic beats but also unexpectedly increased mortality threefold. The use of ectopic beat suppression as a surrogate marker did not produce the desired drug action—reduction in mortality—probably because the underlying pathophysiology was incompletely understood.
Similarly, drugs with positive inotropic activity augment cardiac output in patients with heart failure but also are associated with an increase in mortality, probably because of drug-induced arrhythmias. Nevertheless, clinical trials with these agents suggest symptom relief. Thus the prescriber and the patient may elect therapy with positive inotropic drugs to realize this benefit while recognizing the risk. This complex decision making is at the heart of the broad concept of personalized medicine, which incorporates into the care of an individual patient not only genomic (or other) markers of variable drug responses, but also factors such as patients’ understanding of their disease, presence of other diseases, willingness to tolerate minor or serious risks of treatment, and sociocultural factors which impact key health determinants such as exposure to pollution, ability to pay for care, and literacy and numeracy.
The risks of drug therapy may be a direct extension of the pharmacologic actions for which the drug is actually being prescribed. Hypoglycemia in a patient taking an antidiabetic agent and bleeding in a patient taking an anticoagulant are examples; sodium channel block in CAST is another. T cell activation by immune checkpoint inhibitors, with resultant myocarditis, may in this sense also be “on-target.”
In other cases, ADRs develop as a consequence of pharmacologic actions that were not appreciated during a drug’s initial development and use in patients. Examples include rhabdomyolysis occurring with 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitors (statins), angioedema developing during angiotensin-converting enzyme (ACE) inhibitor therapy, and torsades de pointes during treatment with noncardiovascular drugs such as methadone or hydroxychloroquine. Of importance, these rarer but serious effects generally become evident only after a drug has been marketed and extensively used. Even rare ADRs can alter the overall perception of risk versus benefit and can prompt removal of the drug from the market, particularly if alternate therapies thought to be safer are available or the benefits of drug therapy are modest or difficult to demonstrate. For example, withdrawal of the first insulin sensitizer, troglitazone, after recognition of hepatotoxicity was further spurred by the availability of other new drugs in this class.
The recognition of multiple cyclooxygenase (COX) isoforms led to the development of specific COX-2 inhibitors to retain aspirin’s analgesic effects but reduce gastrointestinal side effects. However, one of these, rofecoxib, was withdrawn because of an apparent increase in cardiovascular mortality. The events surrounding the withdrawal of rofecoxib have important implications for drug development and utilization. First, specificity achieved by targeting a single molecular entity may not necessarily reduce ADRs; one possibility is that by inhibiting COX-2, the drug removes a vascular protective effect of prostacyclin. Second, drug side effects may include not only readily identifiable events such as rhabdomyolysis or torsades de pointes but also an increase—that may be difficult to detect—in events such as MI that are common in the general population.
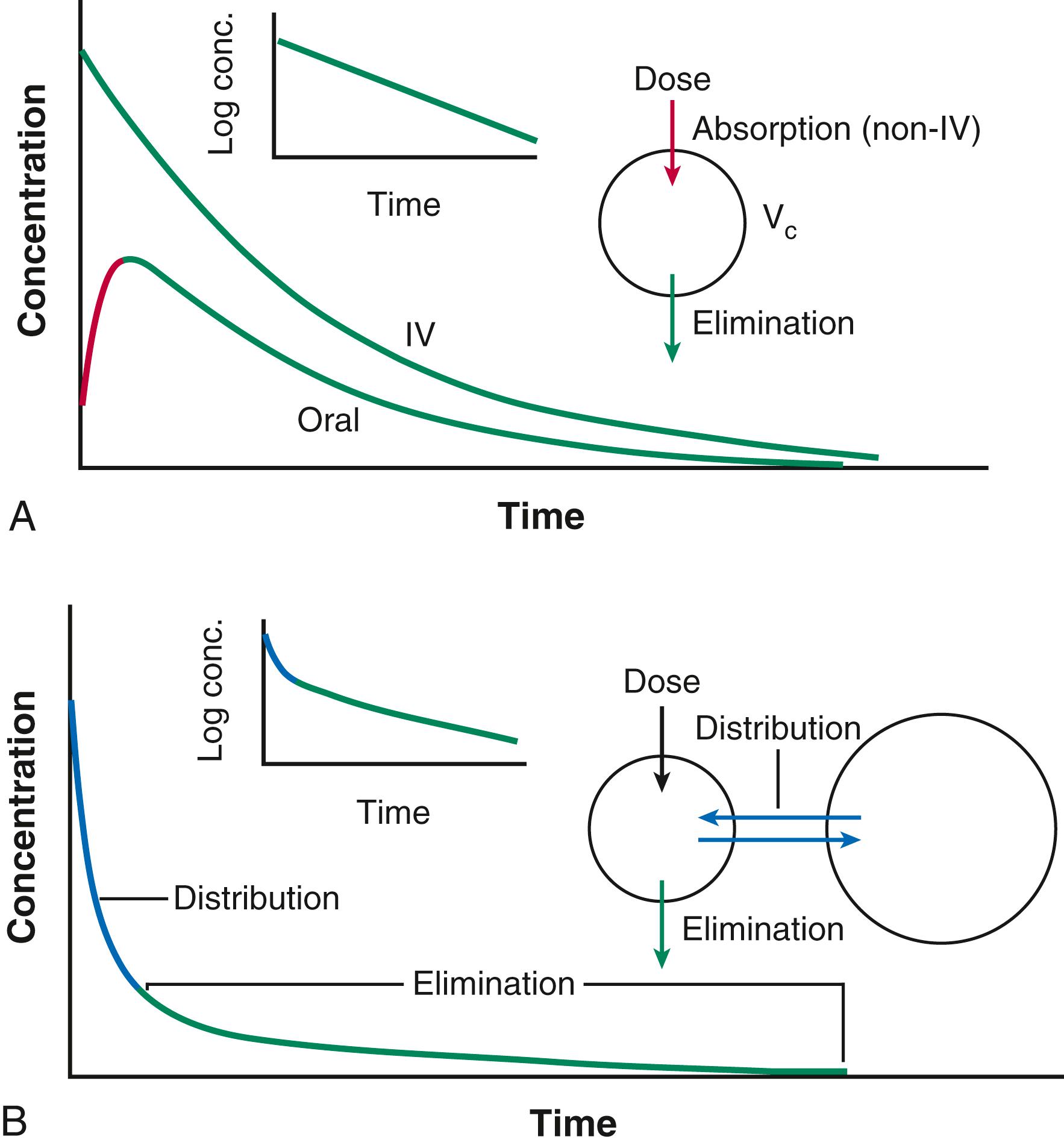
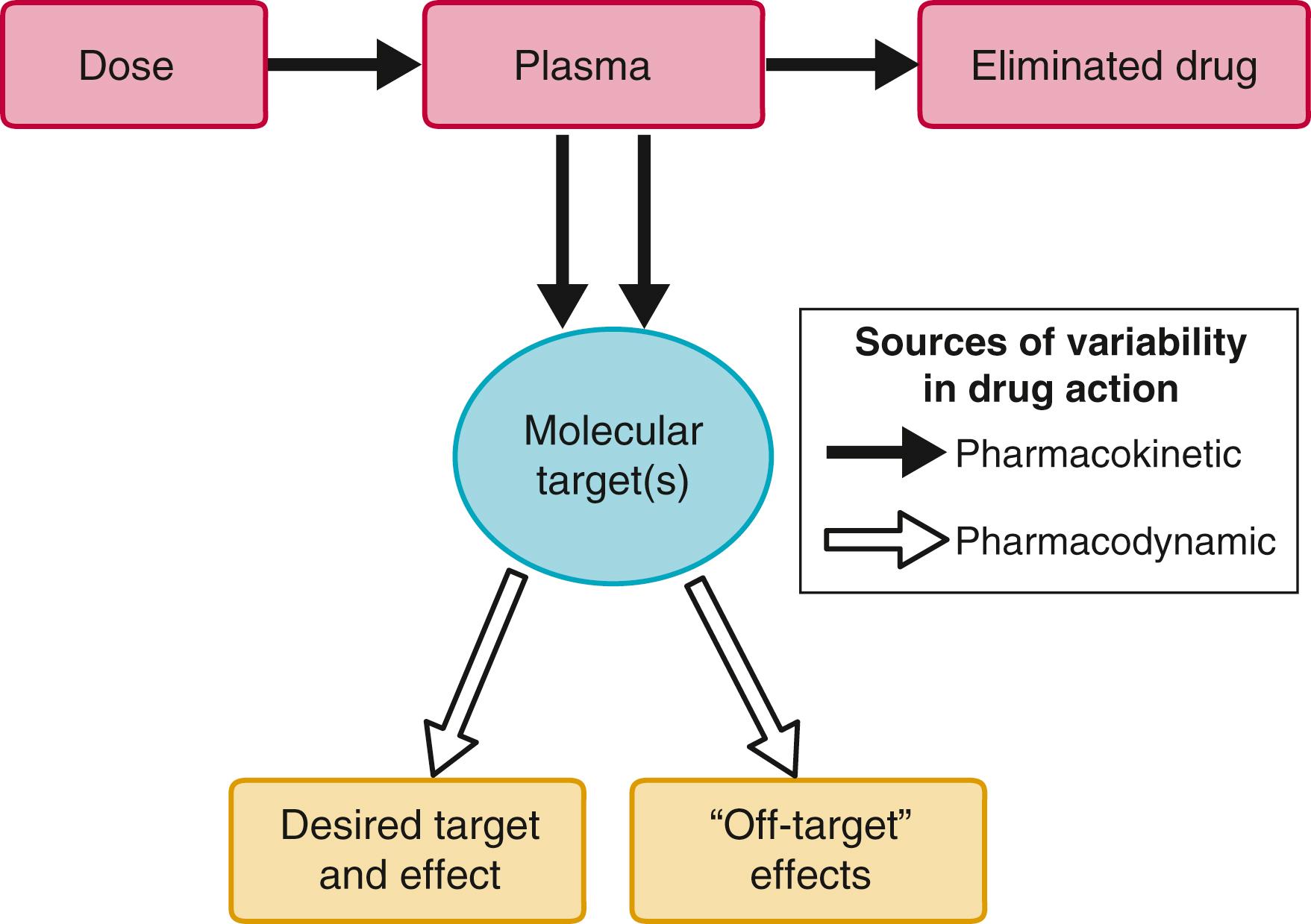
Two major processes determine how the interaction between a drug and its target molecule(s) can generate variable drug actions in a patient. The first, pharmacokinetics ( Fig. 9.1 ), describes drug delivery to and removal from the target molecule and includes the processes of absorption, distribution, metabolism, and excretion—collectively termed drug disposition. The second process, pharmacodynamics ( Fig 9.2 ), describes how the interaction between a drug and its molecular target(s) generates downstream molecular, cellular, whole-organ, and whole-body effects.
Genes encoding drug-metabolizing enzymes and drug transport molecules determine pharmacokinetics. Genes encoding drug targets and the molecules modulating the biology in which the drug-target interaction occurs (including those causing the disease being treated) determine pharmacodynamics. Pharmacogenetics describes the concept that individual variants in the genes controlling these processes contribute to variable drug actions. Pharmacogenomics is often used to describe the way in which variability across multiple genes, up to whole genomes, explains differences in drug response among individuals and populations. The following overview of broad principles of pharmacokinetics, pharmacodynamics, and pharmacogenomics is followed by more detailed discussion of the specific genes, their function, and important variants influencing cardiovascular drug responses.
Administration of an intravenous (IV) drug bolus results in maximal drug concentrations at the end of delivery of the bolus, followed by a decline in plasma drug concentrations over time ( Fig. 9.1A ), generally because of drug elimination. In the simplest case this decline occurs monoexponentially over time. A useful parameter to describe this decline is the half-life (t 1/2 ), the time in which 50% of the drug is eliminated; for example, after two half-lives, 75% of the drug has been eliminated, and after three half-lives, 87.5%. A monoexponential process can be considered almost complete in four or five half-lives. In some cases the decline of drug concentrations after administration of an IV bolus dose is multiexponential. The most common explanation is that the drug is not only eliminated (represented by terminal portion of time-concentration plot) but also undergoes more rapid distribution to peripheral tissues. Just as elimination may be usefully described by a half-life, distribution half-lives also can be derived from curves such as those shown in Figure 9.1B .
The plasma concentration measured immediately after a bolus dose can be used to derive a volume into which the drug is distributed. When the decline of plasma concentrations is multiexponential, multiple distribution compartments can be defined; these volumes of distribution can be useful in considering dose adjustments in cases of disease but rarely correspond exactly to any physical volume, such as plasma or total body water. With drugs that are highly tissue bound (e.g., some antidepressants), the volume of distribution can exceed total body volume by orders of magnitude.
Drugs are often administered by non-IV routes, such as oral, sublingual, transcutaneous, or intramuscular. Such routes of administration differ from the IV route in two ways (see Fig. 9.1A ). First, concentrations in plasma demonstrate a distinct rising phase as the drug slowly enters plasma. Second, the total amount of drug that actually enters the systemic circulation may be less than that achieved by the IV route. The relative amount of drug entering by any route, compared with the same dose administered intravenously, is termed bioavailability, calculated as the ratio of the area under the time-concentration curves, as shown in Figure 9.1A . Some drugs undergo extensive metabolism before entry into the systemic circulation, and as a result the amount of drug required to achieve a therapeutic effect is much greater (and often more variable) than that required for the same drug administered intravenously. Thus small doses of IV propranolol (5 mg) may achieve heart rate slowing equivalent to that observed with much larger oral doses (80 to 120 mg). Propranolol is actually well absorbed but undergoes extensive metabolism in the intestine and liver before entering the systemic circulation. Another example is amiodarone; its physicochemical characteristics make it only 30% to 50% bioavailable when administered orally. Thus an IV infusion of 0.5 mg/min (720 mg/day) is equivalent to 1.5 to 2 g/day orally.
Drug elimination occurs by metabolism followed by the excretion of metabolites and unmetabolized parent drug, generally by the biliary tract or kidneys. This process can be quantified as clearance, the volume that is cleared of drug in any given period. Clearance may be organ specific (e.g., renal clearance, hepatic clearance) or whole-body clearance. Drug metabolism is conventionally divided into phase I oxidation and phase II conjugation, both of which enhance water solubility and, consequently, biliary or renal elimination.
The most common enzyme systems mediating phase I drug metabolism are those of the cytochrome P-450 superfamily, termed CYPs. Multiple CYPs are expressed in human liver and other tissues. A major source of variability in drug action is variability in CYP expression and/or genetic variants that alter CYP activity. Table 9.1 lists CYPs and other proteins important for pharmacokinetics of cardiovascular drugs. Excretion of drugs or their metabolites into the urine or bile is accomplished by glomerular filtration or specific drug transport molecules, whose level of expression and genetic variation are only now being explored. One widely studied transporter is P-glycoprotein, the product of expression of the MDR1 (or ABCB1 ) gene. Originally identified as a factor mediating multiple drug resistance in patients with cancer, P-glycoprotein expression is now well recognized in normal enterocytes, hepatocytes, renal tubular cells, the endothelium of the capillaries forming the blood-brain barrier, and the testes. In each of these sites, P-glycoprotein expression is restricted to the apical aspect of polarized cells, where it acts to enhance drug efflux. In the intestine, P-glycoprotein pumps substrates back into the lumen, thereby limiting bioavailability. In the liver and kidney, it promotes drug excretion into bile or urine. In central nervous system capillary endothelium, P-glycoprotein–mediated efflux is an important mechanism limiting drug access to the brain. Transporters also play a role drug uptake into many cells. One example is OATP1B1, which is responsible for simvastatin uptake into hepatocytes; variants in SLCO1B1 , which encodes the transporter, have been associated with an increased risk for simvastatin-induced muscle toxicity.
| Protein | Substrates |
|---|---|
| Cytochrome P-450s (CYPs) | |
| CYP3A4, CYP3A5 ∗ | Erythromycin, clarithromycin; quinidine, mexiletine; many benzodiazepines; cyclosporine, tacrolimus; many antiretrovirals HMG-CoA reductase inhibitors: atorvastatin, simvastatin, lovastatin; not pravastatin Many calcium channel blockers; apixaban, rivaroxaban |
| CYP2D6 ∗ | Some beta blockers: propranolol, timolol, metoprolol, carvedilol Propafenone; desipramine and other tricyclics; codeine † ; tamoxifen † ; dextromethorphan |
| CYP2C9 ∗ | Warfarin, phenytoin, tolbutamide, losartan, † rosuvastatin |
| CYP2C19 ∗ | Omeprazole, clopidogrel † |
| Other Drug-Metabolizing Enzymes | |
| N -acetyltransferase ∗ | Procainamide, hydralazine, isoniazid |
| Thiopurine methyltransferase ∗ | 6-Mercaptopurine, azathioprine |
| Pseudocholinesterase ∗ | Succinylcholine |
| Serine esterase 1 (CES1) | Clopidogrel, dabigatran |
| Uridine diphosphate-glucuronosyltransferase ∗ | Irinotecan, † atazanavir |
| Drug Transporters | |
| P-glycoprotein | Digoxin, dabigatran |
| SLCO1B1 ∗ | Simvastatin, atorvastatin; methotrexate; troglitazone; bosentan |
Drugs can exert variable effects, even in the absence of pharmacokinetic variability. This can arise as a function of variability in the molecular targets with which drugs interact to achieve their beneficial and adverse effects, as well as variability in the broader biologic context within which the drug-target interaction takes place (see Fig. 9.2 ). Variability in the number or function of a drug’s target molecules can arise because of genetic factors (see later) or because disease alters the number of target molecules or their state (e.g., changes in extent of phosphorylation). Simple examples of variability in the biologic context are high dietary salt, which can inhibit the antihypertensive action of beta blockers, and hypokalemia, which increases the risk for drug-induced QT prolongation. In addition, disease itself can modulate drug response. For example, the effect of lytic therapy in a patient with no clots is manifestly different from that in a patient with an acute coronary syndrome, or the vasodilating effects of nitrates, beneficial in patients with coronary disease with angina, can be catastrophic in patients with aortic stenosis. These examples highlight the requirement for precision in diagnosis to avoid situations in which risk outweighs potential benefit. One hope is that emerging genomic or other molecular approaches can add to this precision.
The targets with which drugs interact to produce beneficial effects may or may not be the same as those with which drugs interact to produce ADRs. Drug targets may be in the circulation, at the cell surface, or within cells. Many drugs widely used in cardiovascular therapeutics (e.g., digoxin, amiodarone, aspirin) were developed when the technology to identify specific molecular targets was not available. Some drugs (e.g., amiodarone) have many drug targets. In other cases, however, even older drugs are found to have rather specific molecular targets. The actions of digitalis glycosides are mediated primarily by the inhibition of sodium/potassium–adenosine triphosphatase (Na + ,K + -ATPase). Aspirin permanently acetylates a specific serine residue on the COX enzyme, an effect that is thought to mediate its analgesic effects and its gastrointestinal toxicity. Most newer drugs have been developed to interact with a specific drug target identified in the course of basic mechanistic studies; examples of such targets are HMG-CoA reductase, ACE, G protein–coupled receptors (GPCRs; e.g., alpha, beta, angiotensin II, histamine), and platelet P2Y12 receptors.
An emerging approach is to use modern genetic techniques to identify loss-of-function DNA variants that are tolerated throughout life and associated with a desired phenotype, such as greatly reduced MI risk. Inhibitors of the corresponding gene products are thus predicted to exert a beneficial effect and lack serious on-target ADRs. PCSK9 inhibitors are an excellent example (see Chapter 27 ), and other potential drug targets are now being identified using this approach. , Furthermore, an emerging understanding of the way in which genetic variation produces mendelian diseases such as cystic fibrosis is leading to new, mechanism-based therapies. Cardiovascular diseases such as hypertrophic cardiomyopathy appear ripe for such development (see Chapter 54 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here