Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chemotherapy and radiation therapy both work primarily by disrupting nuclear deoxyribonucleic acid and inhibiting cellular division. They potentially kill all rapidly dividing cells. Targeted therapies are directed towards specific signaling pathways within cancer cells, to spare normal cells such as bone marrow.
Chronic radiation complications occur in 5-10% of patients who receive 50 centigray (cGy) or more of radiation therapy. The complications are caused by damage to small blood vessels (endarteritis), which in turn causes a decreased blood supply to relevant organs. This decreased vascularity results in progressive fibrosis and loss of organ parenchyma.
Toxicity from radiation therapy can be minimized by ensuring that the maximum dose of radiation is delivered to the cancer and the minimum dose to the surrounding normal tissues. This can be facilitated by the use of intensity modulated radiation therapy (IMRT), which relies on three-dimensional computed tomographic scanning to accurately plan the treatment and multileaf collimators to deliver radiation beams of variable intensity.
Hormonal therapy relies on the fact that some tumors contain receptors for estrogen and progesterone, and estrogen increases the growth of such tumors. Progestins or antiestrogens are often able to at least stabilize the growth rate of receptor positive tumors.
Nearly three-quarters of cancer patients experience significant pain that should be properly managed without delay. When it becomes clear that death is near, the goals should be to control symptoms, maintain dignity, and allow for time with loved ones.
Modern gynecologic cancer management requires a multidisciplinary approach and includes surgery, chemotherapy, radiation therapy, hormonal therapy, and targeted therapy. In this chapter, the principles of the nonsurgical modalities are discussed, together with the principles of pain management and end-of-life issues.
The characteristic feature of malignant tumor growth is its uncontrolled cellular proliferation, which requires replication of deoxyribonucleic acid (DNA). There are two distinct phases in the life cycle of all cells: mitosis (M phase), during which cellular division occurs, and interphase, the interval between successive mitoses.
Interphase is subdivided into three separate phases ( Figure 37-1 ). Immediately following mitosis is the G 1 phase, which is of variable duration and is characterized by a diploid content of DNA. DNA synthesis is absent, but ribonucleic acid (RNA) and protein synthesis occur. During the shorter S phase, the entire DNA content is duplicated. This is followed by the G 2 phase, which is characterized by a tetraploid DNA content and by continuing RNA and protein synthesis in preparation for cell division. When mitosis occurs, a duplicate set of chromosomal DNA is inherited by each daughter cell, thus restoring the diploid DNA content. Following mitosis, some cells leave the cycle temporarily or permanently and enter the G 0 or resting phase.
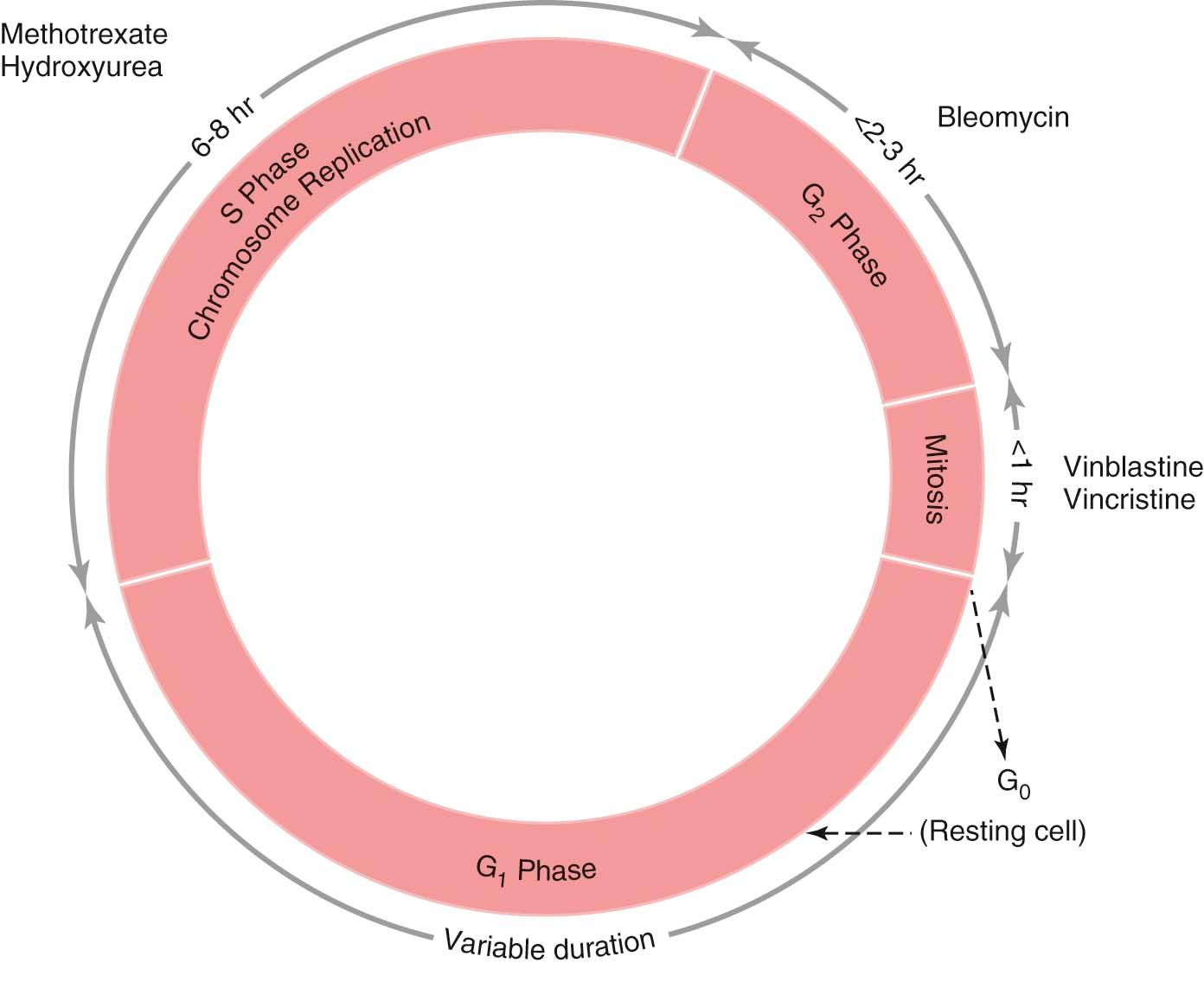
The growth fraction of the tumor is the proportion of actively dividing cells. The higher the growth fraction, the fewer the number of cells in the G 0 phase and the faster the tumor-doubling time.
Chemotherapeutic agents and radiation kill cells by first-order kinetics, which means that a constant proportion of cells is killed for a given dosage, regardless of the number of cells present. Both therapeutic modalities are most effective against actively dividing cells because cells in the resting (G 0 ) phase are better able to repair sublethal damage. Unfortunately, both therapeutic modalities also suppress rapidly dividing normal cells, such as those in the gastrointestinal mucosa, bone marrow, and hair follicles.
One of the major advances in medicine since the 1950s has been the successful treatment of certain disseminated malignancies, including choriocarcinoma and germ cell ovarian tumors, with chemotherapy.
Chemotherapeutic agents act primarily by disrupting nuclear DNA, and thus inhibiting cellular division. They may be subdivided into two categories according to their mode of action relative to the cell cycle:
Cell cycle–nonspecific agents, such as alkylating agents, cisplatin, and paclitaxel, which exert their damage at any phase of the cell cycle. They may damage resting as well as cycling cells, but the latter are much more sensitive.
Cell cycle–specific agents, which exert their lethal effects exclusively or primarily during one phase of the cell cycle. Examples include hydroxyurea and methotrexate, which act primarily during the S phase; bleomycin, which acts in the G 2 phase; and the vinca alkaloids, which act in the M phase.
Chemotherapeutic agents are selected on the basis of previous experience with particular agents for a given tumor, particularly after randomized controlled clinical trials. The drugs are usually given systemically so that the tumor can be treated regardless of its anatomic location. To increase the local concentration, certain drugs may occasionally be administered topically, by intraarterial infusion, or by intrathecal or intracavitary instillation (e.g., intraperitoneal therapy for ovarian cancer).
Chemotherapy is generally not administered if the neutrophil count is less than 1500/mm 3 or if the platelet count is less than 100,000/mm 3 . Nadir blood counts are obtained 7 to 14 days after treatment, and subsequent doses may need to be reduced if there is significant myelosuppression or if the patient develops febrile neutropenia. Dosage reduction may also be necessary because of toxicity to other organs, such as the gastrointestinal tract, liver, or kidneys.
Resistance to chemotherapeutic agents may be temporary or permanent. Temporary resistance is mainly related to the poor vascularity of bulky tumors, which results in poor tissue concentrations of the drugs and an increasing proportion of cells in the relatively resistant G 0 phase of the cell cycle. Permanent resistance mainly results from spontaneous mutation to phenotypic resistance and occurs most commonly in bulky tumors. Permanent resistance may also be acquired by frequent exposure to chemotherapeutic agents.
The common agents used in the management of gynecologic malignancies may be classified as shown in Table 37-1 . This table also contains a summary of the main indications and side effects of these drugs.
| Drug | Main Indications | Side Effects | Precautions |
|---|---|---|---|
| Alkylating Agents | |||
| Chlorambucil | Ovarian carcinoma | Bone marrow depression | |
| Melphalan | Ovarian and tubal carcinoma | Bone marrow depression, leukemia | Avoid prolonged courses (more than 12 cycles) to avoid leukemia |
| Cyclophosphamide | Ovarian carcinoma, germ cell tumors, squamous carcinomas, sarcomas | Bone marrow depression, nausea and vomiting, alopecia, hemorrhagic cystitis, sterility | Maintain adequate fluid intake to avoid cystitis |
| Antimetabolites | |||
| Methotrexate | Gestational trophoblastic disease | Bone marrow depression, nausea and vomiting, stomatitis, alopecia, liver and renal failure, dermatitis | Ensure normal kidney and liver function |
| 5-fluorouracil | Vaginal intraepithelial neoplasia (topical application) | Pain and ulceration | |
| Gemcitabine | Ovarian carcinoma | Bone marrow depression, flu-like illness, skin rash | IV infusion |
| Antibiotics | |||
| Actinomycin-D | Gestational trophoblastic disease | Bone marrow depression, nausea and vomiting, diarrhea, stomatitis, alopecia, dermatitis, local tissue necrosis | Administer through running intravenous infusion to avoid extravasation |
| Doxorubicin | Ovarian carcinoma, recurrent endometrial carcinoma, sarcoma | Bone marrow depression, nausea and vomiting, cardiomyopathy, cardiac arrhythmias, alopecia, local tissue necrosis | Administer through running intravenous infusion; do not exceed total dose of 550 mg/m 2 to avoid cardiac toxicity; avoid if significant heart disease is present |
| Liposomal doxorubicin | Ovarian cancer | Hand-foot syndrome; less cardiotoxic than doxorubicin | IV infusion |
| Bleomycin | Germ cell tumors, squamous carcinomas | Pneumonitis and pulmonary fibrosis, alopecia, stomatitis, cutaneous reactions | Do not exceed total dose of 400 U; monitor pulmonary function with carbon monoxide diffusion capacity |
| Plant Alkaloids | |||
| Vinblastine | Germ cell tumors, sarcomas | Bone marrow depression, nausea and vomiting, stomatitis, diarrhea, local tissue necrosis | Administer through running intravenous infusion |
| Vincristine | Germ cell tumors, sarcomas | Neurotoxicity, constipation, alopecia, local tissue necrosis; bone marrow depression less marked | Administer through running IV infusion; prophylactic cathartics may be helpful |
| Etoposide | Germ cell tumors | Bone marrow depression; nausea and vomiting | Administer slowly intravenously |
| Paclitaxel | Ovarian carcinoma, breast carcinoma | Myelosuppression, alopecia, allergic reactions, cardiac arrhythmias | Intravenously as a 3-24 hr infusion |
| Docetaxel | Ovarian and breast cancer | Myelosuppression, alopecia, dermatologic reactions | Intravenous infusion, IV dexamethasone to reduce fluid retention |
| Other Drugs | |||
| Cisplatin | Ovarian carcinoma, germ cell tumors, squamous carcinomas | Renal toxicity, ototoxicity, neurotoxicity, severe nausea and vomiting, bone marrow depression less marked, hypokalemia, hypomagnesemia | Administer intravenous fluids to maintain urinary output of 100 mL/hr during infusion; discontinue if creatinine clearance <35 mL/hr |
| Carboplatin | Ovarian carcinoma, germ cell tumors | Bone marrow depression, less gastrointestinal toxicity, less renal toxicity, less neurotoxicity | Suitable for outpatient therapy because no need for high urinary output |
| Topotecan | Ovarian cancer | Bone marrow depression | IV for 5 days every 3 weeks |
The cytotoxicity of alkylating agents results from their ability to cause alkylation to DNA, resulting in cross-linkage between DNA strands and prevention of DNA replication. There is cross resistance among the various alkylating agents.
Antimetabolites are compounds that closely resemble normal intermediaries, for which they may substitute in biochemical reactions, and thereby produce a metabolic block; for example, methotrexate competitively inhibits the enzyme dihydrofolate reductase, thus preventing the conversion of dihydrofolate to tetrahydrofolate. The latter is required for the methylation reaction necessary for the synthesis of purine and pyrimidine subunits of nucleic acid.
Antibiotics are naturally occurring antitumor agents elaborated by certain species of Streptomyces. They have no single, clearly defined mechanism of action, but many agents in this group intercalate between strands of the DNA double helix, thereby inhibiting both DNA and RNA synthesis and causing oxygen-dependent strand breaks.
The most common plant alkaloids are the vinca alkaloids, which are derived from the periwinkle plant. These include vincristine and vinblastine. They are spindle toxins that interfere with cellular microtubules and cause metaphase arrest.
Other plant alkaloids include the epipodophyllotoxins such as etoposide (VP16), which are extracts from the mandrake plant, and paclitaxel (Taxol), an extract from the bark of the Pacific yew tree. Docetaxel (Taxotere) is the first semisynthetic analogue of paclitaxel. Etoposide appears to act by causing single-strand DNA breaks. Paclitaxel binds preferentially to microtubules, and results in their polymerization and stabilization.
Cisplatin, one of the more important drugs in gynecologic oncology, causes inhibition of DNA synthesis by forming interstrand and intrastrand linkages. Carboplatin is an analogue of cisplatin with a similar mechanism of action and efficacy, but with less gastrointestinal and renal toxicity.
Radiation may be defined as the propagation of energy through space or matter.
There are two main types of radiation: electromagnetic and particulate.
Examples of electromagnetic radiation include the following:
Visible light
Infrared light
Ultraviolet light
X-rays (photons)
Gamma rays (photons)
X-rays and gamma rays are identical electromagnetic radiations, differing only in their mode of production. X-rays are produced by bombardment of an anode by a high-speed electron beam; gamma rays result from the decay of radioactive isotopes, such as cobalt 60 ( 60 Co).
X-rays and gamma rays (photons) are differentiated from electromagnetic radiation of longer wavelength by their greater energy, which allows them to penetrate tissues and cause ionization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here