Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Molecular imaging is defined as the in vivo measurement of biological processes at the cellular and molecular levels . The technique visualizes pathophysiologic processes noninvasively in real time, with the potential for serial monitoring, and provides information regarding specific molecular alterations underlying the disease status of individual subjects. By complementing conventional “anatomical or physiological” imaging, molecular imaging enables early detection of disease, staging of disease, and quantitative assessment of therapeutic response. Molecular imaging also contributes to the understanding of stroke pathophysiology in living animals and humans. This chapter describes the principles and methods of molecular imaging techniques, while providing a translational perspective in stroke.
Various modalities of molecular imaging, such as optical imaging, computed tomography (CT), magnetic resonance imaging (MRI), and radionuclide imaging (single photon emission tomography, positron emission tomography, i.e., PET), have their own specific advantages and disadvantages . Optical imaging has a high sensitivity and high spatial resolution, but it has poor depth penetration. CT has the advantage of short scan times, but disadvantages include the exposure to radiation. MRI has an excellent soft-tissue contrast and a high spatial resolution, and also allows for physiological and biochemical imaging. Unlike fluorescence imaging with femtomolar or picomolar detection limits, MRI and CT suffer from relatively poor sensitivity. Radionuclide imaging is inherently molecular, and has the potential for true signal quantification as well as a high sensitivity. Micro-PET, micro-MRI, and micro-CT are increasingly popular tools dedicated to molecular imaging of small animals; they provide higher spatial resolution, compared with clinical scanners .
For CT-based molecular imaging, gold nanoparticles (AuNPs) are frequently used. For MRI, superparamagnetic iron oxide (SPIO) and ultrasmall SPIO (USPIO) nanoparticles are widely used. Optical imaging largely depends on fluorescent proteins, fluorochromes, or quantum dots. Multimodal imaging probes report the signal through more than one imaging modality, thereby complementing each other in terms of spatiotemporal resolution, sensitivity, or depth penetration. Many studies have shown that MRI combined with ex vivo reflectance fluorescence imaging or in vivo fluorescence tomography is very useful for small-animal research. Dual modality magnetic resonance (MR)–PET scanners have begun to show promise as a powerful platform for molecular imaging in animals and humans .
Targeted imaging probes are synthesized through attachment to the nanoparticle of a ligand directed against a known target on the cell surface or modification of the nanoparticle surface with small molecules to modulate its uptake. High-throughput screening of phage and chemical libraries are useful for the identification of peptides specific for targets, leading to the discovery of novel probes . Activatable imaging probes are silent at baseline and turned on after responding to specific biomolecular or environmental changes in real time, thereby amplifying output signal while reducing background noise .
Some atherosclerotic plaques are prone to rupture and thromboembolism, whereas others are clinically silent. Thus there is an unmet clinical need for tools to localize rupture-prone vulnerable plaques, so that risk-altering treatments can be offered to prevent stroke. These high-risk plaques are not well identified by current imaging techniques that measure stenosis and depict anatomic features, such as: large lipid-rich necrotic core, thin fibrous cap, intraplaque hemorrhage or thrombi, neovascularization, and microcalcification. As a molecular hallmark of vulnerable atherosclerotic plaques, researchers have focused on inflammatory activity by intraplaque monocytes/macrophages that secrete cathepsins and matrix metalloproteinases (MMPs), disorganizing the extracellular matrix and thus rendering the plaque susceptible to rupture .
USPIO nanoparticles, which consist of ferromagnetic iron oxide particles with an overall size of 30 nm, are taken up by monocytes/macrophages that infiltrate atherosclerotic plaques . Kooi et al. showed that these nanoparticles could produce a T 2 ∗ -weighted MR susceptibility effect, visualizing carotid inflammation in humans after intravenous infusion (2.6 mg/kg suspended in normal saline) over 30 min .
18 F-fluorodeoxy glucose (FDG) is well known to accumulate in macrophage-rich areas of plaques. Carotid arteries harboring inflammatory atheromas exhibit a higher FDG PET signal than unaffected vessels, suggesting that the nuclear molecular imaging tool has the potential to detect vulnerable plaques .
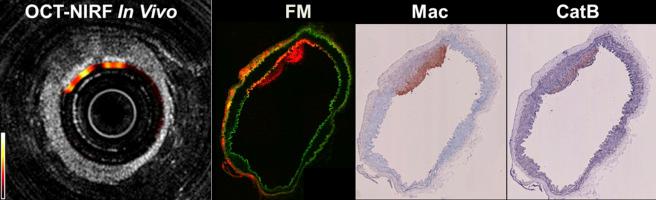
Unlike “always-on” probes, such as targeted fluorescent probes relying on affinity ligands, protease-sensing fluorescent imaging agents developed by the Weissleder group are “optically silent at injection” because of intramolecular autoquenching between closely spaced fluorochromes. After protease-mediated cleavage of the substrate sequence (e.g., GGPRQITAG for MMP-2/9), fluorochromes are spatially separated, dequenched, and become brightly fluorescent. Animal studies using cathepsin-B or MMP-2/9 activatable probes have shown that in vivo and ex vivo fluorescent imaging could visualize the protease activity by monocyte/macrophages in the aortic or carotid atheromata of ApoE knockout mice or in human carotid endarterectomy specimens .
The clinical use of optical imaging is limited due to poor tissue penetration capability . The attenuation of light by tissue is lowest in the near-infrared (NIR, 700–900 nm) region, with less photon absorption by blood hemoglobin, lipid, and water. Thus NIR fluorescent (NIRF) molecular imaging allows light to penetrate several centimeters into the body and offers substantially reduced tissue autofluorescence. Furthermore, this technology can be combined with a noninvasive optical tomography system or a fluorescence-sensing catheter-based system ( Fig. 70.1 ), as shown by Jaffer’s group .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here