Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Three types of primary cutaneous CD30 + lymphoproliferative disorders (LPDs) are recognized in the World Health Organization classification: primary cutaneous anaplastic large cell lymphoma (C-ALCL), lymphomatoid papulosis (LyP), and borderline lesions. These entities represent a continuous spectrum of lesions that are not clearly demarcated by clinical appearance or histology in some instances. LyP occurs as multiple recurrent, often centrally necrotic, papulonodular lesions up to 2 cm in diameter and rarely with ulcers; the lesions regress spontaneously, usually in 4 to 6 weeks, occasionally leaving a hyperpigmented or hypopigmented scar. C-ALCL commonly presents as one to several tumors or thick plaques greater than 2 cm in diameter, often localized but occasionally multicentric. The tumors of primary C-ALCL frequently ulcerate. Partial or complete regression occurs at diagnosis or relapse in up to 42% of these tumors. On occasion, LyP and C-ALCL cannot be distinguished even by a clinicopathologic correlation. For these lesions, the term borderline lesion can be used. Borderline lesions are intermediate in size, clinical appearance, and histology and usually persist for several months if not treated ( Fig. 40-1 ; Table 40-1 ).
| LyP | C-ALCL | Borderline Lesions | |
|---|---|---|---|
| Clinical | Crops of papules with central necrosis; spontaneous regression mostly within a few weeks | One to several, often localized ulcerating nodules or tumors; occasional partial regression | Intermediate-size nodules (1-2 cm); tendency for slow regression |
| Histology |
|
|
|
| Immunophenotype | CD30 + , CD4 + , LCA + , TIA-1 + ; less frequently CD8 + | CD30 + , CD4 + , LCA + , TIA-1 + | CD30 + , CD4 + , LCA + , TIA-1 + |
| Genetics |
|
|
|
* This should be distinguished from the rare cases of primary cutaneous ALCL anaplastic lymphoma kinase (ALK)–positive primary, especially in children, that should not be considered part of the spectrum of CD30 + primary cutaneous lymphoproliferative disorders.
Primary cutaneous CD30 + T-cell LPDs present in the skin without extranodal manifestations at the time of diagnosis. These lesions must be distinguished from systemic ALCLs with secondary cutaneous manifestations as well as from progression of other cutaneous lymphomas, such as mycosis fungoides or Sézary syndrome. Secondary CD30 + cutaneous lesions generally have a poorer prognosis than primary cutaneous CD30 + LPDs.
The peak incidence of LyP is in the fifth decade, although children younger than 10 years and patients up to 80 years old can be affected. The male-to-female ratio for LyP patients from published series is 3 : 2. For primary C-ALCL, the male-to-female ratio is 2.5 : 1. Primary C-ALCL can occur in children; therefore, not all cases of C-ALCL in children should be considered secondary manifestations of systemic ALCL. Lack of anaplastic lymphoma kinase (ALK) expression favors primary C-ALCL (see later).
Our LyP registry data revealed an interesting bimodal distribution of patients at age of diagnosis. Most patients younger than 19 years were male, and most of those aged 19 or older at diagnosis were female. Two thirds of 35 patients who developed LyP in childhood (younger than 18 years) had atopy, which is significantly more than the expected prevalence (relative risk, 3.1; 95% confidence interval, 2.2-4.3). Fletcher and colleagues reported four cases of primary cutaneous CD30 + LPDs (one LyP, three C-ALCLs) in young adult patients with active atopic eczema since early childhood. These results from separate medical centers suggest an association between primary cutaneous CD30 + LPDs and atopy. Further studies are needed to determine whether there is a causal link between these two conditions.
A remarkable association between LyP and other lymphomas occurs in 10% to up to 62% of patients. The most common lymphomas are mycosis fungoides, Hodgkin's lymphoma, and ALCL. A case-control study revealed that of 57 LyP patients, 3 had Hodgkin's lymphoma, 3 had non-Hodgkin's lymphoma, 10 had mycosis fungoides, and 4 had non-lymphoid malignant neoplasms (1 brain tumor, 2 lung cancers, 1 breast cancer). In addition, 4 patients had received radiation therapy 8 to 40 years before the onset of LyP. None of 67 age- and gender-matched controls had any history of radiation or lymphoid or non-lymphoid malignant disease. A Dutch study of 118 LyP patients found that 23 (19%) had lymphomas (11 mycosis fungoides, 10 C-ALCL, 2 Hodgkin's lymphoma). In addition to lymphomas, prospective follow-up of our 57 LyP case-control patients revealed a high frequency of non-lymphoid malignant neoplasms (10 of 57; 18%). The relative risk for development of lymphoid and non-lymphoid malignant neoplasms in this cohort of patients during 8.5 years of follow-up was 13 (95% confidence interval, 2.2-44) and 3.1 (95% confidence interval, 1.206-6.47), respectively. The factors that predispose these patients to development of malignant neoplasms are unknown. Possible risk factors based on small case series include age at onset or years at risk for LyP, male gender, histologic subtype, and expression of fascin by CD30 + cells in LyP. For example, Dutch investigators found that patients with type C LyP have an increased risk for development of malignant lymphoma, whereas none of seven patients with pure type B lesions had or developed a malignant lymphoma. Clinical manifestations of mycosis fungoides or Hodgkin's lymphoma can occur before, after, or simultaneously with LyP. In nearly all cases, LyP lesions precede the development of C-ALCL.
The etiology of LyP and C-ALCL is unknown. A viral origin was initially suspected but has not been confirmed. Human T-lymphotropic virus 1, herpesviruses (6, 7, and 8), and Epstein-Barr virus (EBV) could not be detected in primary skin lesions or in cell lines derived from CD30 + cutaneous lymphomas.
CD30 expression is a hallmark of LyP and C-ALCL. CD30 is a “late” activation antigen maximally expressed 72 hours after lymphocyte activation in vitro. Engagement of CD30 by its natural ligand CD30L (CD156) can lead to sustained proliferation, cell cycle arrest, or apoptosis, depending on the target cell, its state of differentiation, and environmental costimulatory signals. CD30 cross-linking of ALCL cell lines clonally derived from LyP causes upregulation of nuclear factor-κB (NF-κB) and ERK/MAP kinases, promoting cell survival and proliferation. CD30 activation also enhances the expression of FLICE-like inhibitory protein, which protects lymphocytes from apoptosis induced by Fas/CD95. Our studies suggest that the level of CD30 transcription is genetically determined, rendering some individuals more or less susceptible to CD30 + LPDs, including primary cutaneous LPDs. This might explain LyP patients' increased risk for development of Hodgkin's lymphoma and ALCL. Because LyP patients have a significantly increased risk of both lymphoid and non-lymphoid malignant neoplasms, an as yet undefined genetic defect that is not limited to lymphoid cells is suspected.
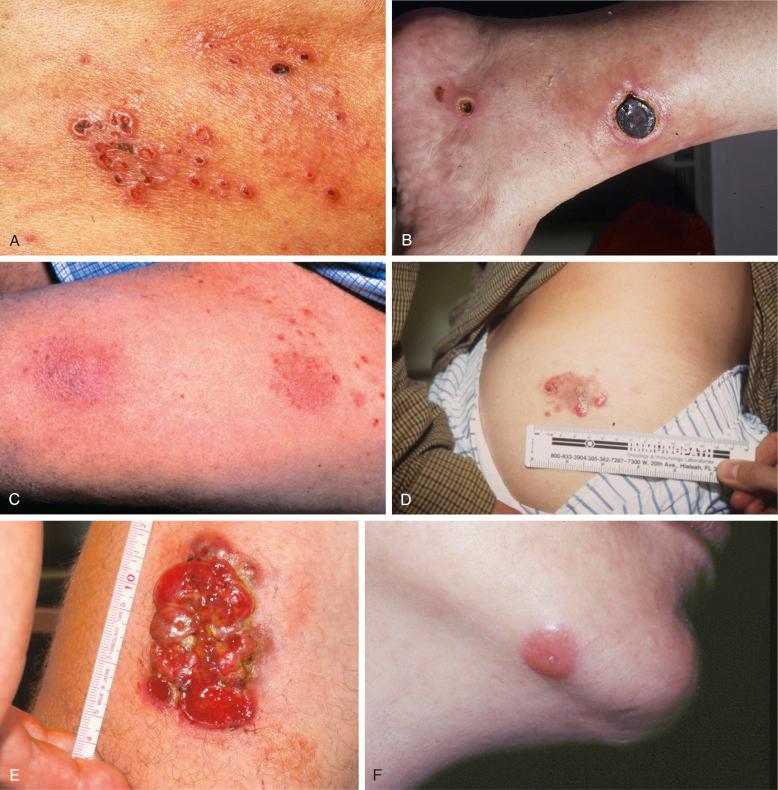
LyP lesions appear as small self-healing papules, often with a necrotic center ( Fig. 40-1, A ). On occasion, larger eschar-type lesions result from angioinvasive LyP ( Fig. 40-1, B ).
Patients may experience itching and, less often, fever or other systemic symptoms. LyP lesions often appear in clusters and recur in the same region of the body. In a few patients, continual eruptions of papulonodules histologically typical of LyP occur in a well-circumscribed area, equivalent to limited plaque mycosis fungoides ( Fig. 40-1, C ), which is also referred to as persistent agminated LyP. The extremities, trunk, and particularly the buttocks are most commonly affected. Lesions occur infrequently on the face, palms, soles, and anogenital areas and only rarely on mucous membranes. These clinical observations raise the possibility that cytokines or chemokines released from epidermal keratinocytes or Langerhans cells may contribute to the development of LyP.
LyP lesions occur in crops, often with long lesion-free intervals. Many patients experience the development of new lesions while others are regressing, and lesions may be continuous. LyP lesions often recur in the original site. In some women, LyP lesions appear to be modulated by the menstrual cycle or to develop during pregnancy. LyP lesions can coalesce to form one or more large lesions indistinguishable from C-ALCL ( Fig. 40-1, D ). In other patients, one or a few lesions grow progressively to form a primary C-ALCL without prior LyP lesions ( Fig. 40-1, E ). Large lesions often ulcerate centrally and show some degree of spontaneous regression, even after 2 to 3 months. In borderline lesions, the distinction between C-ALCL and LyP cannot be established ( Fig. 40-1, F ); however, in most patients, follow-up clarifies the lesion type.
Regional lymphadenopathy can develop and likely represents the local spread of tumor cells ( Fig. 40-2 ). The prognosis does not appear to be affected by regional lymphadenopathy. Regional lymph node enlargement can also represent dermatopathic lymphadenopathy.
A new TNM classification system for patients with C-ALCL has been proposed by the Dutch Cutaneous Lymphoma Group. Patients with leg involvement had significantly worse 5-year disease-specific survival (76% versus 96%).
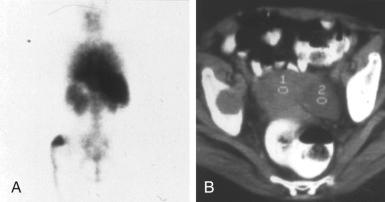
The development of systemic symptoms of fatigue, fever, weight loss, night sweats, or bone pain should raise the possibility of systemic lymphoma complicating LyP. In these individuals, more extensive staging with imaging of the chest and abdomen should be done. Abdominal or intrathoracic lymphadenopathy should be regarded as highly suspicious for malignant lymphoma. Bone lesions have been observed in several LyP patients who developed systemic CD30 + ALCL ( Fig. 40-3 ).
C-ALCL usually presents as solitary or grouped tumors or thick plaques and infrequently as multicentric tumors. Extensive staging procedures are generally not warranted for asymptomatic patients with LyP or clinically localized primary C-ALCL. Bone marrow examination is not recommended owing to the low frequency of involvement. A Sézary preparation or flow cytometry of the peripheral blood is not indicated in patients with uncomplicated primary cutaneous CD30 + LPDs. A chest radiograph is recommended to exclude asymptomatic mediastinal lymphadenopathy, which can be a presenting feature of Hodgkin's lymphoma. Importantly, LyP lesions do not generally show a concordant response to therapy for Hodgkin's lymphoma. A summary of recommendations for the management of patients with primary cutaneous CD30 + LPDs has been compiled by the Dutch Cutaneous Lymphoma Group. More recent consensus recommendations for management and treatment of primary cutaneous CD30 + LPDs have been reported in detail by the European Organization for Research and Treatment of Cancer, the International Society for Cutaneous Lymphomas, and the United States Cutaneous Lymphoma Consortium.
The development of persistent patches, plaques, or scaly erythematous lesions and hair loss or onychodystrophy can indicate the presence of mycosis fungoides complicating LyP (see Fig. 40-1, C ). This would necessitate the use of a systemic approach to therapy (e.g., psoralen with ultraviolet A [PUVA] or ultraviolet B, topical nitrogen mustard or carmustine, bexarotene, total skin electron beam therapy, extracorporeal photoimmunotherapy), depending on the stage of disease.
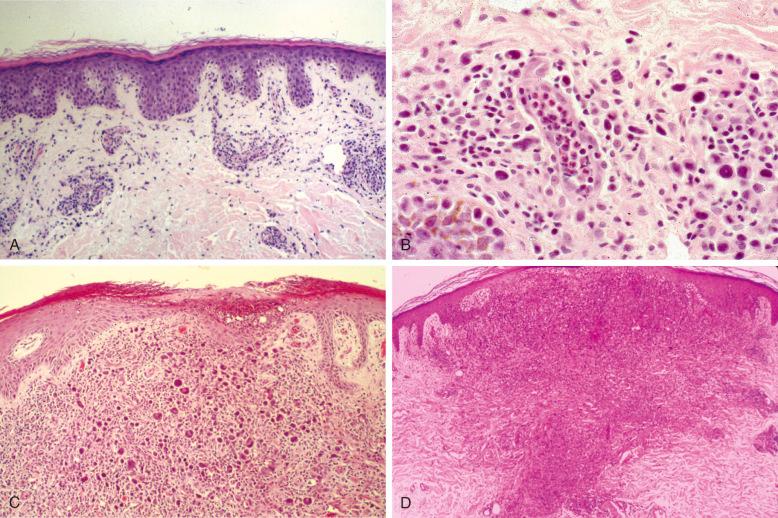
LyP lesions vary in appearance, depending on their stage of development at the time of biopsy ( Fig. 40-4 ). Early lesions reveal mainly perivascular and superficial dermal accumulations of atypical lymphoid cells surrounded by variable numbers of inflammatory cells ( Fig. 40-4, A ). Neutrophils within the lumens of blood vessels are a nearly constant feature of LyP ( Fig. 40-4, B ). Surrounding the large atypical cells are variable numbers of neutrophils, eosinophils, histiocytes, and small lymphocytes. Few too many neutrophils often percolate through the epidermis, accounting for the pustular appearance of LyP lesions ( Fig. 40-4, C ). Plasma cells generally are not prominent. Fully developed or late lesions are often wedge shaped, sometimes extending into the deep dermis with little or no involvement of the subcutis in most cases ( Fig.40-4, D ). Hair follicles and sweat glands may be infiltrated by atypical cells. Other unusual histopathologic patterns associated with LyP include follicular mucinosis, syringosquamous metaplasia, angiocentric/angiodestructive, and bandlike rather than wedge-shaped distribution of lymphoid cells. The atypical cells often concentrate around and can be found within the lumens of blood vessels.
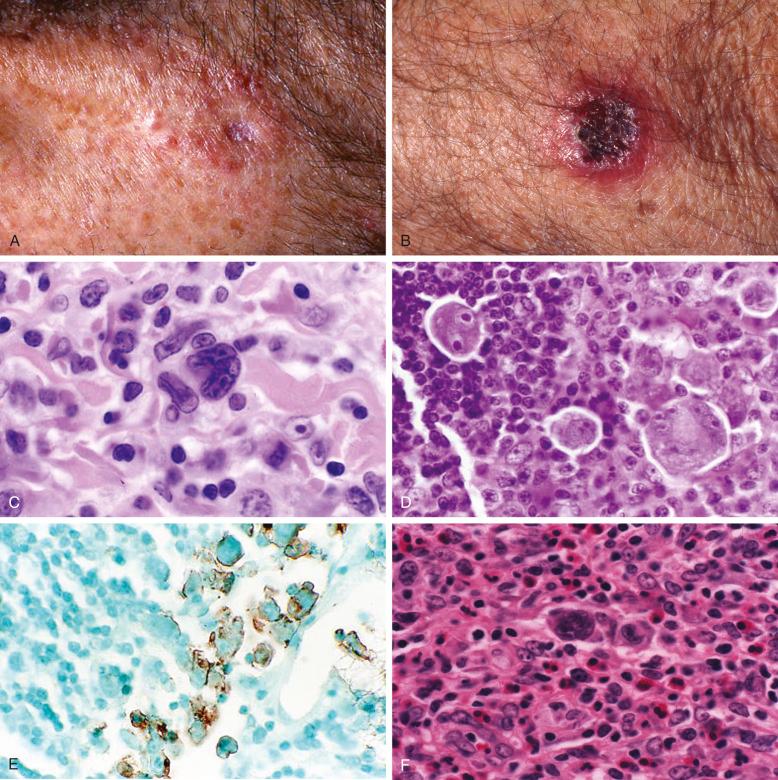
LyP comprises six main histologic types, with some overlapping features ( Table 40-2 ; Fig. 40-5 ). Importantly, none of the histologic types has an impact on disease course or prognosis. Type A may resemble Hodgkin's lymphoma because of the presence of large Reed-Sternberg–like cells with prominent, often eosinophilic nucleoli ( Fig. 40-5, A ). These cells are surrounded by variable numbers of inflammatory cells ( Fig. 40-5, B ). In some lesions, the atypical cells resemble immunoblasts with amphophilic to basophilic cytoplasm, slightly eccentric nuclei, and conspicuous but usually not huge nucleoli. In some cases, LyP type A cells have a plasmacytoid appearance not previously described ( Fig. 40-5, C ). This may be a discriminating feature from pityriasis lichenoides. When large atypical cells are confluent or occur in sheets confined to the dermis, with relatively few inflammatory cells, the lesion is classified as LyP type C ( Fig. 40-5, D ). Type B lesions resemble mycosis fungoides with epidermotropism of small to medium-sized atypical lymphocytes ( Fig. 40-5, E ). The predominant cell is a mononuclear cell with nuclear irregularities, sometimes cerebriform, without prominent nucleoli. Mitoses are infrequent. Epidermotropism is often present. Neutrophils and other inflammatory cells are not abundant. There is some controversy about whether LyP type B lesions represent a papular variant of mycosis fungoides. In contrast to LyP lesions, the papular lesions in mycosis fungoides do not show spontaneous regression. It is not uncommon to find LyP lesions that contain a spectrum of cerebriform cells to larger immunoblasts or Reed-Sternberg–like cells with abundant inflammatory cells, which makes distinction from inflammatory skin disorders challenging in those cases. These lesions can be referred to as type A/B to indicate a hybrid or mixed histology. Different histologic LyP types can be observed in lesions of the same patient at the same time point.
| Type A | Type B | Type C | Type D | Type E | |
|---|---|---|---|---|---|
| Growth pattern | Dermal, wedge-shaped infiltrate | Epidermotropic | Dermal, nodular, or wedge shaped |
|
Angioinvasive |
| Cytology | Immunoblasts, sometimes Reed-Sternberg–like cells | Cerebriform cells | Immunoblasts, sometimes a spectrum of cerebriform cells and immunoblasts | Small to medium-sized pleomorphic cells | Medium-sized pleomorphic cells |
| Inflammatory cells | Numerous | Infrequent | Few to moderate | Few to moderate | Few to moderate |
| Mitoses | Frequent | Infrequent | Frequent | Infrequent | Infrequent |
| Clinical regression | 4-6 weeks | 8 weeks | Slow and incomplete | 4-8 weeks | Up to 4 months |
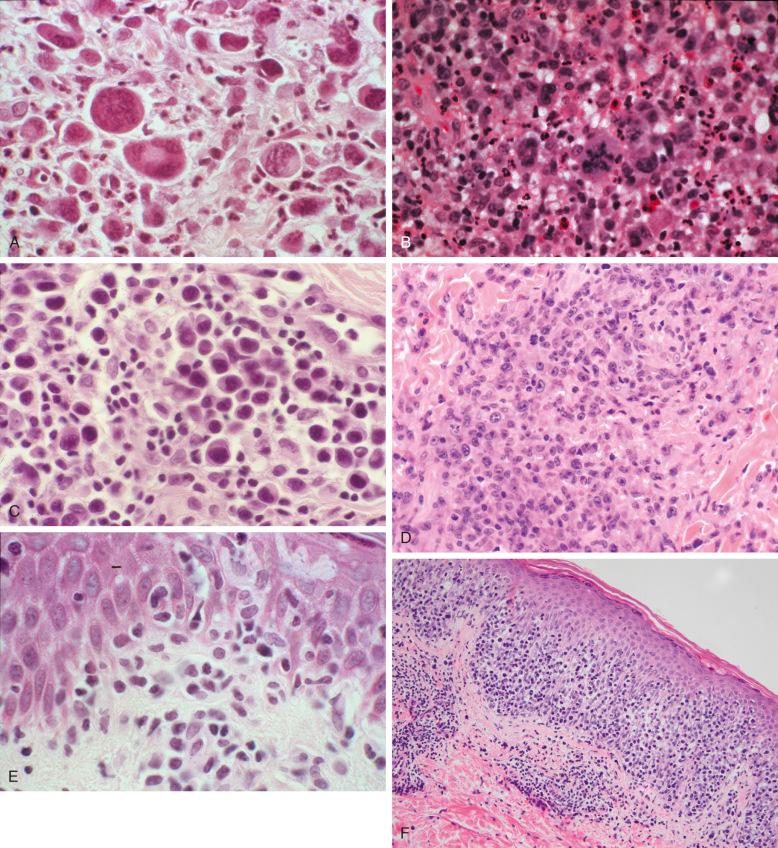
Additional histologic types have recently been identified, such as LyP type D and type E and genetic variant with 6p25.3 rearrangement. LyP type D is characterized by an epidermotropic infiltrate of CD30 + atypical lymphocytes, which express CD8, in contrast to LyP type B, which displays a CD4 + phenotype ( Fig. 40-5, F ). Because of the prominent epidermotropism and in particular because of the immunophenotype, LyP type D is histologically prone to be misinterpreted as cutaneous epidermotropic aggressive CD8 + cytotoxic T-cell lymphoma. LyP type E is characterized by angioinvasive infiltrates of mostly medium-sized CD30 + and often CD8 + atypical lymphocytes, resulting in necrosis and eschar-like ulcers ( Figs. 40-1, B , and 40-5, G ). It simulates other angiocentric and angiodestructive lymphomas, such as extranodal NK/T-cell lymphoma, nasal type, or cutaneous gamma delta T-cell lymphoma. The atypical lymphocytes in LyP type E infiltrate the vessel walls but do not accumulate in the lumina of the vessels like in the intralymphatic variant of ALCL. LyP with 6p25.3 rearrangement is distinctive for a biphasic epidermal epidermotropic component of small to medium-sized atypical lymphocytes and a dermal nodular component composed of larger cells, which may infiltrate the skin adnexae ( Fig. 40-5, H ).
C-ALCL occurs as an extensive dermal infiltrate, usually sparing the epidermis, and is composed almost entirely of large anaplastic cells ( Fig. 40-6, A ). The deep part of the lesion usually extends into the subcutis ( Fig. 40-6, B ). Inflammatory cells are less frequent than in LyP; they are often nearly absent or confined to the lesion's periphery. An exception is the neutrophil-rich variant of C-ALCL. A rare intravascular variant of C-ALCL contains intralymphatic accumulation of CD30 + T-cells and exhibits an excellent prognosis.
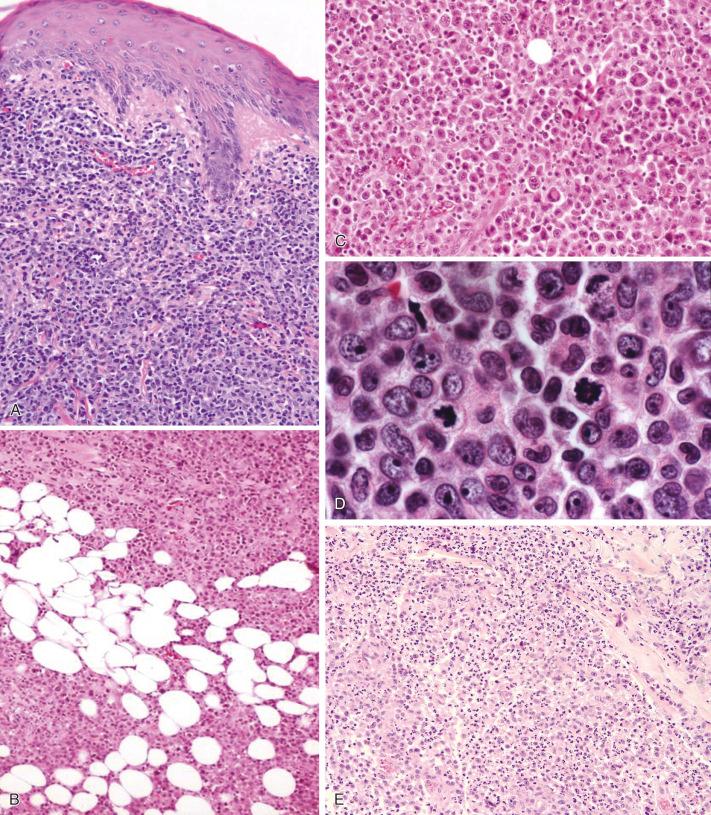
Mitoses are common among the large atypical cells in LyP and in C-ALCL ( Fig. 40-6, C ). Several studies indicate a high ratio of apoptotic cells to dividing cells in LyP. A significantly higher apoptotic index is found in LyP (12.5%) than in CD30 + large T-cell lymphoma (3.1%). The high rate of apoptosis in LyP can be attributed in part to the low expression of BCL2 and the high expression of the proapoptotic protein BAX. The proportion of CD30 + cells expressing death receptor apoptosis pathway mediators FADD and cleaved caspase 3 is significantly higher in primary cutaneous CD30 + LPDs than in systemic ALCL. C-ALCL cells are resistant to apoptosis induced by death ligands tumor necrosis factor-α, CD25, and TRAIL as a result of CD30-mediated overexpression of c-FLIP. CD30 ligation leads to NF-κB–mediated c-FLIP upregulation in C-ALCL cells, which in turn confers enhanced resistance to CD95-mediated apoptosis. In regressing LyP lesions, binding of CD30 and its ligand was detected by immunohistochemistry.
Borderline lesions contain an extensive infiltrate or sheets of atypical cells with focal extension into the subcutis, making them difficult to distinguish from LyP type C or C-ALCL.
It is still unclear whether involvement of lymph nodes by LyP exists at all or whether it should be regarded as nodal ALCL or Hodgkin's lymphoma arising as a second lymphoid neoplasm in the context of LyP. In primary C-ALCL, spread to local regional lymph nodes occurs in less than 10% of the patients and is not linked to an impaired prognosis. On histologic evaluation, nodal involvement by cutaneous CD30 + T-cell lymphoma is characterized by cohesive infiltrates of large CD30 + pleomorphic or anaplastic tumor cells, but rare cases may mimic classical Hodgkin's lymphoma.
The usual immunophenotype of the large atypical cells in LyP is that of activated helper T lymphocytes expressing CD4 and lymphocyte activation antigens such as CD30, CD25, CD71, and HLA-DR. Other T-cell antigens (e.g., CD3, CD2, CD5, CD7) are often not expressed, resulting in an aberrant T-cell phenotype ( Fig. 40-7 ) characteristic of CD30 + T-cell lymphoproliferations. A natural killer cell phenotype for the large atypical cells was noted in 10% to nearly 50% of cases in one study but in none of 18 cases in another study. Expression of CD8 by CD30-positive atypical lymphocytes can be seen in all histologic LyP types except for type B. It is by definition expressed in all cases of LyP type D and is commonly found in LyP type E (70% of the cases), and it is also commonly seen in LyP arising in childhood. Expression of IRF4/MUM1 has been observed in LyP and C-ALCL and is not disease specific. Moreover, expression of IRF4/MUM1 does not correlate with underlying IRF4 translocation detected by break-apart fluorescence in situ hybridization probes. Recently, aberrant expression of PAX5 by CD30-positive tumor cells was observed in three cases, but further studies are needed to confirm this observation.
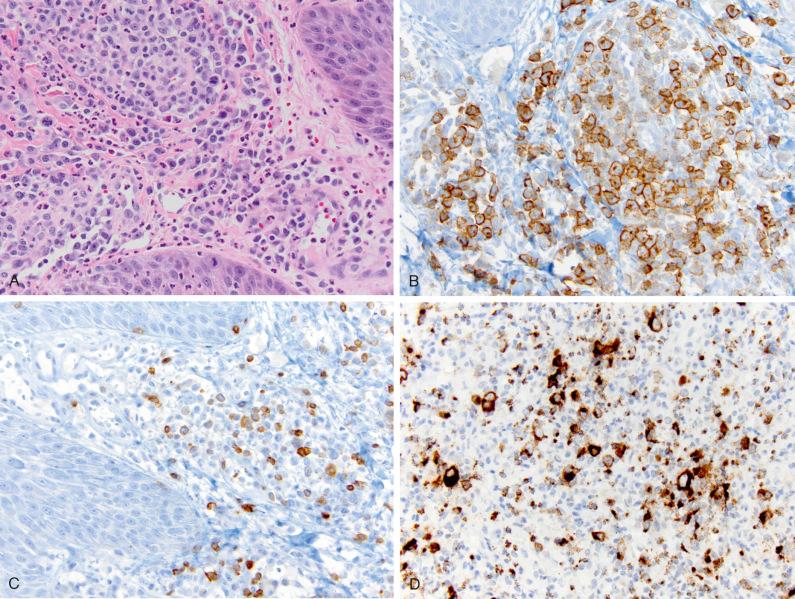
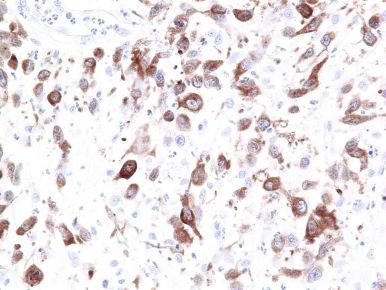
In most cases, the atypical cells express cytotoxic proteins, including T-cell intracellular antigen-1, granzyme B, and perforin ( Fig. 40-7 ). ALK is negative, leukocyte common antigen (LCA; CD45) is characteristically expressed, and CD15 is absent in most LyP cases. EBV (EBER, LMP-1) is not found in LyP and C-ALCL. This profile helps distinguish LyP and ALCL from Hodgkin's lymphoma. C-ALCL also displays an activated T-cell phenotype, with frequent expression of cytotoxic molecules. In C-ALCL, CD30 must be expressed by at least 75% of large cells. Epithelial membrane antigen (EMA) and ALK staining is usually absent in C-ALCL, in contrast to systemic ALCL. Rare cases of C-ALCL are associated with a cytoplasmic variant of ALK ( Fig. 40-8 ). Homeobox gene HOXC5 is often expressed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here