Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Primary amenorrhea is diagnosed if no menstrual function has occurred by age 15, or 5 years after initial breast development.
Menarche is delayed approximately 0.4 year for each year of premenarchal athletic training.
Gonadal failure is the most common cause of primary amenorrhea, accounting for almost 50% of patients with this disorder.
Individuals with gonadal failure should have a peripheral karyotype obtained to determine whether a Y chromosome is present. If it is present, or if there are signs of hyperandrogenism, the gonads should be removed to prevent the development of malignancy.
Amenorrhea with low estrogen levels is associated with decreased bone density.
If signs of pubertal progression (precocious puberty) are present in a girl, a workup is warranted by the age of 8 years.
The two primary concerns of parents of children with precocious puberty are the social stigma associated with the child being physically different from her peers and the diminished ultimate height caused by the premature closure of epiphyseal centers.
The exact cause of the majority of cases of GnRH-dependent (true or central) precocious puberty is unknown; however, approximately 30% of cases are secondary to central nervous system disease.
The most common cause of gonadotropin-releasing hormone–independent precocious puberty is a functioning ovarian tumor. Granulosa cell tumors are the most common type accounting for 60% of neoplasms.
Amenorrhea is defined as the absence of menstrual bleeding and may be primary (never occurring) or secondary (cessation sometime after initiation).
Primary amenorrhea is defined as the absence of menses in a woman who has never menstruated by the age of 15 years ( .) Another definition includes girls who have not menstruated within 5 years of breast development, if occurring by age 10. Breast development (thelarche) should occur by age 13 or otherwise requires evaluation as well. The incidence of primary amenorrhea is less than 0.1%. Secondary amenorrhea is defined as the absence of menses for an arbitrary period, usually longer than 6 to 12 months. The incidence of secondary amenorrhea of more than 6 months’ duration in a survey of a general population of Swedish women of reproductive age was found to be 0.7% but has been cited to be as high as 3% ( ). The incidence is significantly higher in women younger than 25 years and those with a history of menstrual irregularity.
Outside the United States, it is common to see women who have been categorized according to the World Health Organization (WHO) classification. WHO type I usually refers to women with low estrogen levels and low follicle-stimulating hormone (FSH) and normal prolactin (PRL) levels without central nervous system (CNS) lesions; type II refers to a normal estrogen status with normal FSH and PRL levels; WHO type III refers to low estrogen levels and a high FSH level, denoting ovarian failure.
Before the onset of menses, the normal female goes through a progressive series of morphologic changes produced by the pubertal increase in estrogen and androgen production. In 1969 Marshall and Tanner defined five stages of breast development and pubic hair development ( ; ) ( Fig. 36.1 ; Table 36.1 ). These changes sometimes are combined and called Tanner , or pubertal , stages 1 through 5 . The first sign of puberty is usually the appearance of breast budding, followed within a few months by the appearance of pubic hair.
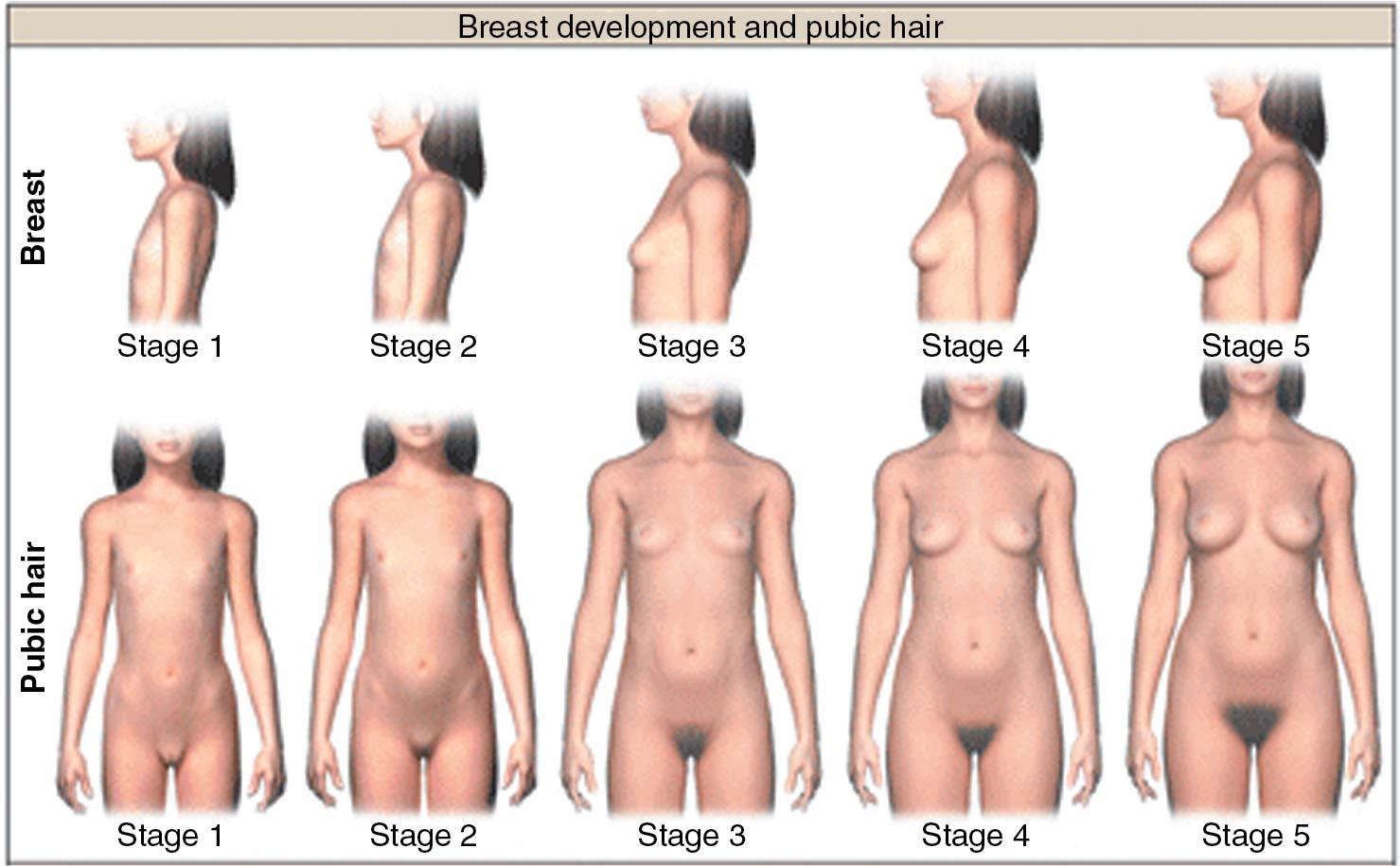
| Classification | Description |
|---|---|
| Breast Growth | |
| B1 | Prepubertal: elevation of papilla only |
| B2 | Breast budding |
| B3 | Enlargement of breasts with glandular tissue, without separation of breast contours |
| B4 | Secondary mound formed by areola |
| B5 | Single contour of breast and areola |
| P UBIC H AIR G ROWTH | |
| PH1 | Prepubertal—no pubic hair |
| PH2 | Labial hair present |
| PH3 | Labial hair spreads over mons pubis |
| PH4 | Slight lateral spread |
| PH5 | Further lateral spread to form inverse triangle and reach medial thighs |
Thereafter the breasts enlarge, the external pelvic contour becomes rounder, and the most rapid rate of growth occurs (peak height velocity). These changes precede menarche. Thus breast budding is the earliest sign of puberty and menarche the latest. The mean ages of occurrence of these events in American women are shown in Table 36.2 and the mean intervals (with standard deviation [SD]) between the initiation of breast budding and other pubertal events are shown in Table 36.3 ( ). The mean interval between breast budding and menarche is 2.3 years, with an SD of approximately 1 year. Some individuals can progress from breast budding to menarche in 18 months, and others may take 5 years. As stated previously, if thelarche has not occurred by age 13, a diagnostic evaluation should be performed.
| Event | Mean Age ± SD (yr) |
|---|---|
| Initiation of breast development | 10.8 ± 1.10 |
| Appearance of pubic hair | 11.0 ± 1.21 |
| Menarche | 12.9 ± 1.20 |
| Interval | Mean Age ± SD (yr) |
|---|---|
| B2—peak height velocity | 1.0 ± 0.77 |
| B2—menarche | 2.3 ± 1.03 |
| B2-PH2 | 3.1 ± 1.04 |
| B2-B5 (average duration of puberty) | 4.5 ± 2.04 |
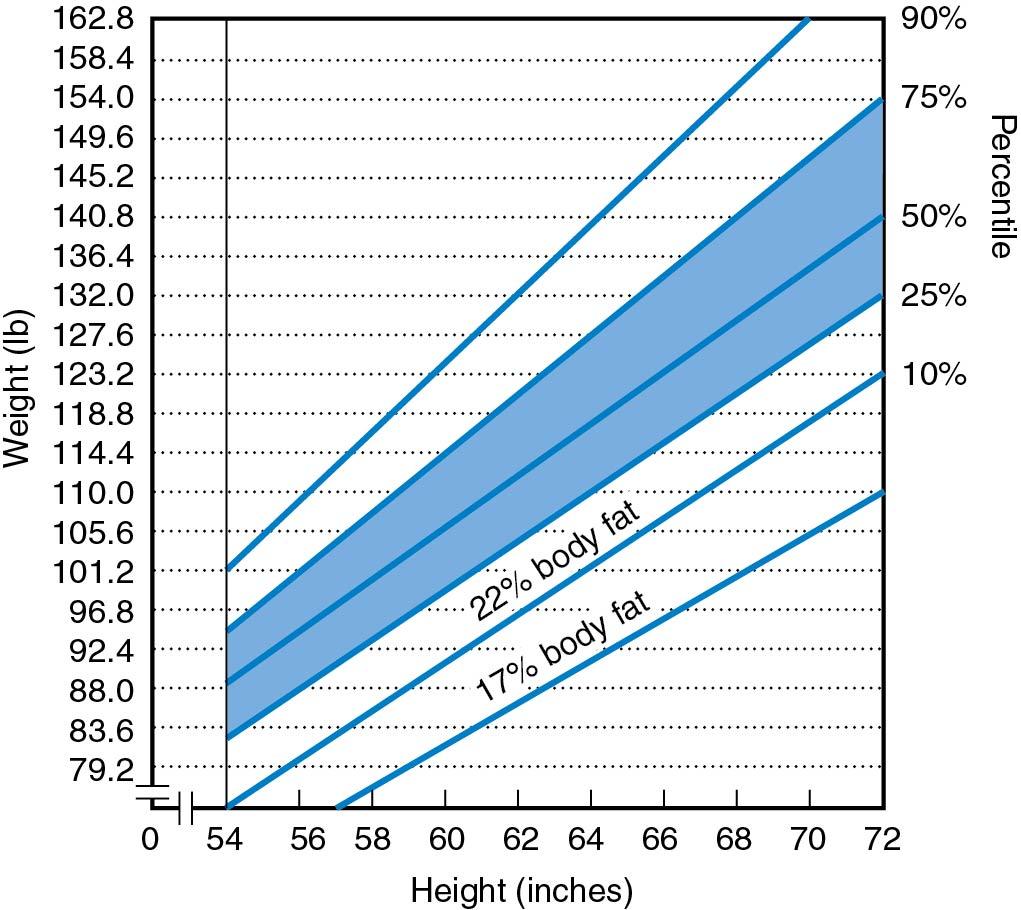
The mean time of onset of menarche was previously thought to occur when a critical body weight of approximately 48 kg (106 lb) was reached; however, it is now believed that body composition is more important than total body weight in determining the time of onset of puberty and menstruation. Thus the ratio of fat to both total body weight and lean body weight is probably the most relevant factor that determines the time of onset of puberty and menstruation. Individuals who are moderately obese, between 20% and 30% more than the ideal body weight, have an earlier onset of menarche than women who are not obese. Malnutrition, such as occurs with anorexia nervosa or starvation, is known to delay the onset of puberty.
One of the major links between body composition and the hypothalamic-pituitary-ovarian (HPO) axis, and thus menstrual cyclicity, is the adipocyte hormone leptin . Leptin is produced by adipocytes and correlates well with body weight. Leptin is also important for feedback involving gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) pulsatility and also binds to specific receptor sites on the ovary and endometrium. Leptin administration has been shown to affect LH pulsatile activity ( ) and to restore cyclicity in women with amenorrhea. Another hormone, a gastric peptide, ghrelin, interacts with leptin in this regard particularly when menstrual function is perturbed ( ).
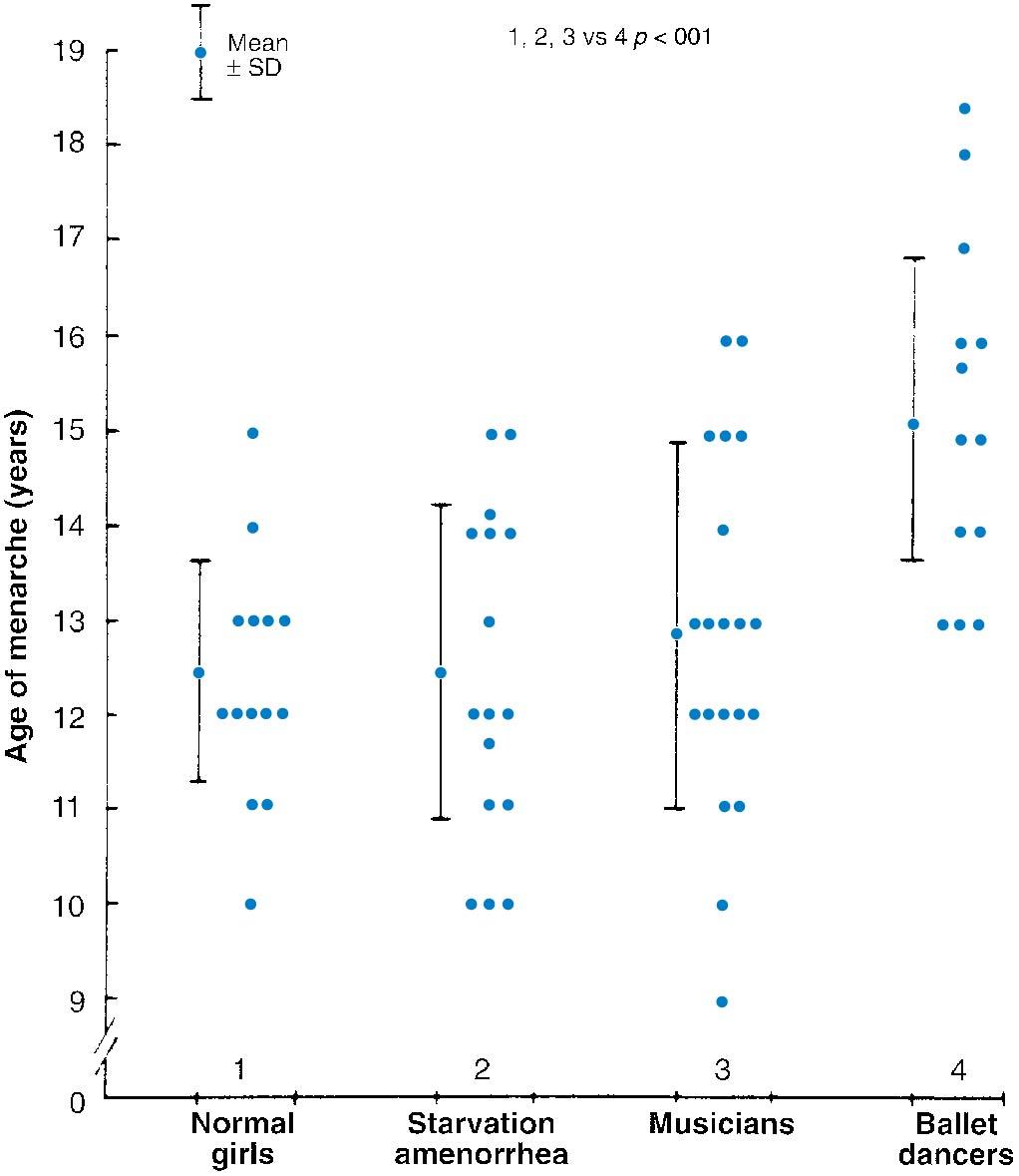
Body weight and body fat content have been shown to be important for menstruation; a fatness nomogram is depicted in Fig. 36.2 ( ). Well-nourished individuals with prepubertal strenuous exercise programs resulting in less total body fat have also been shown to have a delayed onset of puberty. Warren and colleagues have reported that ballet dancers, swimmers, and runners have menarche delayed to approximately age 15 if they began exercising strenuously before menarche ( ) ( Fig. 36.3 ). It is greater in those athletic activities requiring lower body weight and where success is more subjective (ballet, gymnastics) compared with swimming. It was also determined that stress per se is not the cause of the delayed menarche in these exercising girls, because girls of the same age with stressful musical careers did not have a delayed onset of menarche ( ). Young women with strenuous exercise programs have sufficient estrogen to produce some breast development and thus do not need extensive endocrinologic evaluation if concern arises about the lack of onset of menses. Frisch and coworkers have reported that for girls engaged in premenarchal athletic training, menarche is delayed 0.4 year for each year of training. Individuals who exercise strenuously should be counseled that they will usually have a delayed onset of menses, but it is not a health problem. They should be told that they will most likely have regular ovulatory cycles when they stop exercising or become older.
The metabolic features of amenorrheic athletes, who are considered to be in a state of negative energy balance, are fairly characteristic. These include elevated serum FSH and insulin-like growth factor-binding protein 1 (IGFBP-1) and lowered insulin-like growth factor (IGF) levels.
Emotional stress can lead to inhibition of the GnRH axis. The mechanism involves an increased secretion of corticotropin-releasing hormone (CRH), releasing adrenocorticotropic hormone (ACTH), opioid peptides such as beta-endorphin, and cortisol. CRH itself is known to inhibit GnRH.
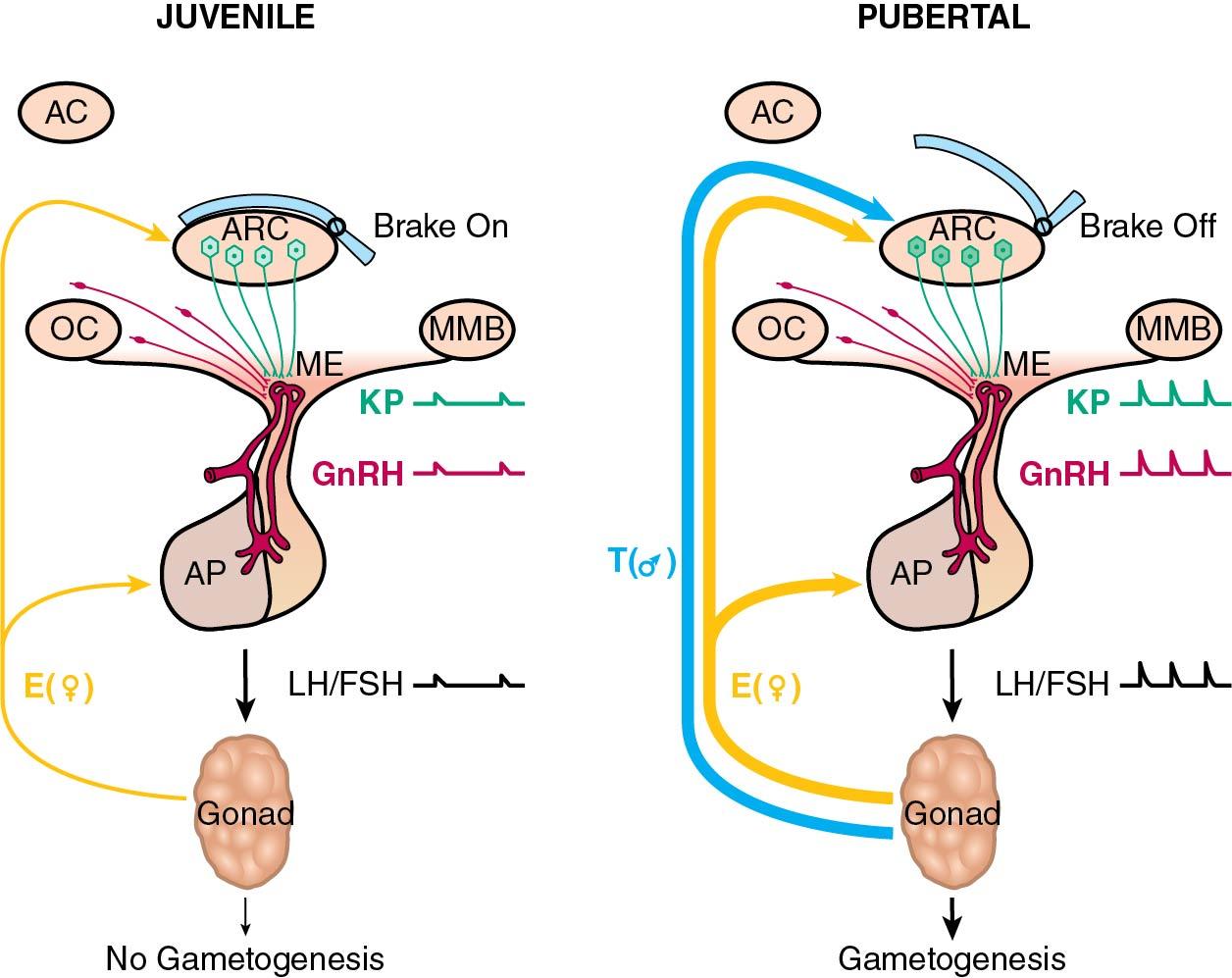
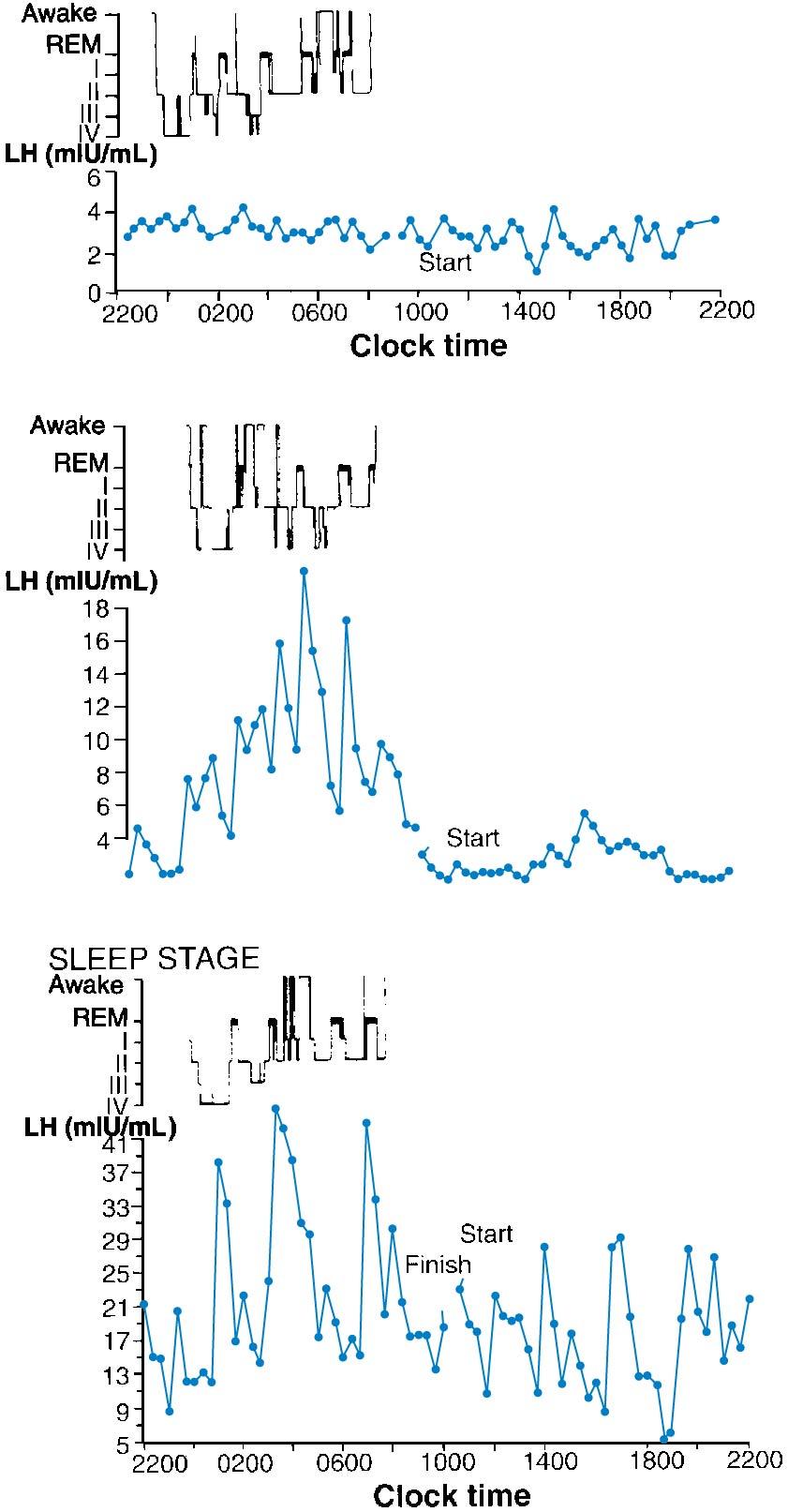
Before puberty, circulating levels of LH and FSH are low, with an FSH/LH ratio greater than 1. The CNS-hypothalamic axis is extremely sensitive to the negative feedback effects of low levels of circulating estrogen. As the critical weight or body composition is approached, the CNS-hypothalamic axis becomes less sensitive to the negative effect of estrogen and GnRH is secreted in greater amounts, causing an increase in LH and, to a lesser extent, FSH levels. This release from the prepubertal “brake” on GnRH secretion is depicted in Fig. 36.4 , which also illustrates the integral role of neuropeptides such as kisspeptin ( ). The initial endocrinologic change associated with the onset of puberty is the occurrence of episodic pulses of LH during sleep ( ) ( Fig. 36.5 ). These pulses are absent before the onset of puberty. After menarche, the episodic secretions of LH occur during sleep and while awake. The last endocrinologic event of puberty is activation of the positive gonadotropin response to increasing levels of estradiol (E 2 ), which results in the midcycle gonadotropic surge and ovulation.
Although numerous classifications have been used for the various causes of primary amenorrhea, it has been found useful to group causes on the basis of whether secondary sexual characteristics (breasts) and female internal genitalia (uterus) are present or absent ( Box 36.1 ). Thus the findings on a physical examination can alert the clinician to possible causes and indicate which laboratory tests should be performed. In a series of 62 individuals reported by Mashchak, the largest subgroup with primary amenorrhea (29; 47%) were individuals in whom breasts were absent but where a uterus was present; the second largest subgroup (22; 35%) had both breasts and a uterus; lack of a uterus together with breast development accounted for the third largest category (9; 14.5%); and individuals without breasts or a uterus were the least common (2; <1%) ( ). This breakdown of the various accompanying conditions of primary amenorrhea reflects the referral pattern to the center. In one study, a “physiologic delay” occurring in 14% and polycystic ovary syndrome (PCOS) in 7% ( ).
Gonadal failure
45,X (Turner syndrome)
46,X, abnormal X (e.g., short- or long-arm deletion)
Mosaicism (e.g., X/XX, X/XX,XXX)
46,XX or 46,XY pure gonadal dysgenesis
17α-hydroxylase deficiency with 46,XX
Hypothalamic failure secondary to inadequate GnRH release
Insufficient GnRH secretion because of neurotransmitter defect
Inadequate GnRH synthesis (Kallmann syndrome)
Congenital anatomic defect in central nervous system
CNS neoplasm (craniopharyngioma)
Pituitary failure
Isolated gonadotrophin insufficiency (thalassemia major, retinitis pigmentosa)
Pituitary neoplasia (chromophobe adenoma)
Mumps, encephalitis
Newborn kernicterus
Prepubertal hypothyroidism
Androgen resistance (testicular feminization)
Congenital absence of uterus (uterovaginal agenesis)
17,20-desmolase deficiency
Agonadism
17α-hydroxylase deficiency with 46,XY karyotype
Hypothalamic cause
Pituitary cause
Ovarian cause
Uterine cause
It would seem logical, because breast development is a biomarker of ovarian estrogen production, that individuals with no breast development and a uterus present have no estrogen production. This is either the result of a primary ovarian disorder or an abnormality of the CNS hypothalamic–pituitary axis, which provides the normal signal to the ovary. The phenotype of individuals with either of these causes of low estrogen status is similar.
Failure of gonadal development is the most common cause of primary amenorrhea , occurring in almost 50% of those with this symptom. Gonadal failure is most commonly caused by a chromosomal disorder or deletion of all or part of an X chromosome, but it is sometimes caused by another chromosomal genetic defect and, rarely, defective CYP-17 leading to 17α-hydroxylase deficiency. The chromosomal disorders are usually caused by a random meiotic or mitotic abnormality (e.g., nondisjunction, anaphase lag) and thus are not inherited; however, if gonadal development is absent in the presence of a 46,XX (called pure gonadal dysgenesis ), a gene disorder may be present, because it has been reported to occur in siblings. Reindollar reported that all individuals with gonadal failure and an X chromosome abnormality were shorter than 63 inches in height ( ). Approximately one-third also had major cardiovascular or renal anomalies.
Deletion of the entire X chromosome (as occurs in Turner syndrome) or of the short arm (p) of the X chromosome results in short stature. Deletions of only the long arm (q) usually do not affect height. In place of the ovary a band of fibrous tissue called a gonadal streak is present ( ) ( Fig. 36.6 ). When ovarian follicles are absent, synthesis of ovarian steroids and inhibin does not occur. Breast development does not occur because of the low circulating E 2 levels. Because the negative hypothalamic-pituitary action of estrogen and inhibin is not present, gonadotropin levels are markedly elevated, with FSH levels being higher than LH. Estrogen is not necessary for müllerian duct development or wolffian duct regression, so the internal and external genitalia are phenotypically female.
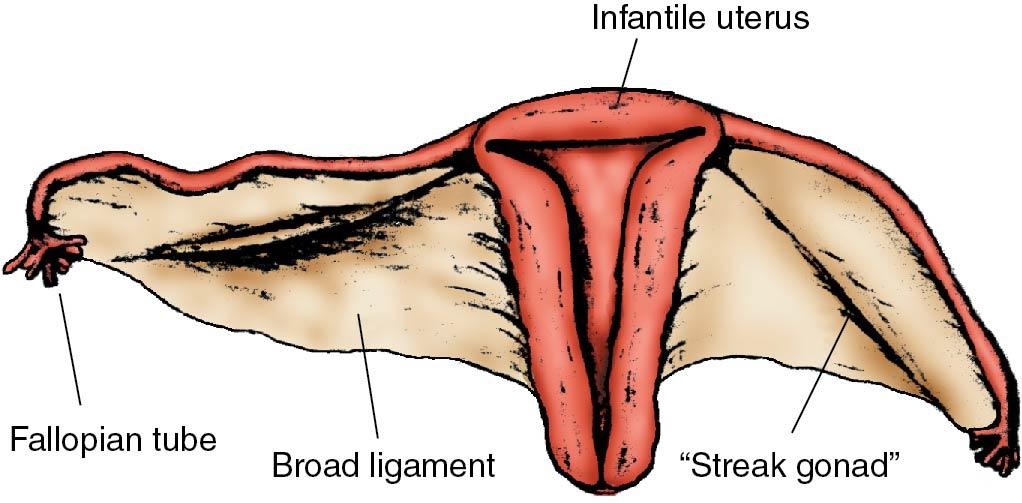
An occasional individual with mosaicism, an abnormal X chromosome, pure gonadal dysgenesis (46,XX), or even Turner syndrome (45,X) may have a few follicles that develop under endogenous gonadotropin stimulation early in puberty and may synthesize enough estrogen to induce breast development and a few episodes of uterine bleeding, resulting early in premature ovarian failure , usually before age 25. Rarely, ovulation and pregnancy can occur.
Goldenberg reported that all individuals with primary amenorrhea and plasma FSH levels higher than 40 mIU/mL have no functioning ovarian follicles in the gonadal tissue. Thus in women with primary amenorrhea, the diagnosis of gonadal failure can be established if the FSH levels are consistently elevated, without requiring ovarian tissue evaluation.
Turner syndrome occurs in approximately 1 per 2000 to 3000 live births but is much more common in abortuses . In addition to primary amenorrhea and absent breast development, these individuals have other somatic abnormalities, the most prevalent being short stature (<60 inches in height), webbing of the neck, a short fourth metacarpal, and cubitus valgus. Cardiac abnormality, renal abnormalities, and hypothyroidism are also more prevalent.
A wide variety of chromosomal mosaics are associated with primary amenorrhea and normal female external genitalia, the most common being X/XX. In addition, individuals with X/XXX and X/XX/XXX mosaicism have primary amenorrhea. These individuals are generally taller and have fewer anatomic abnormalities than individuals with a 45,X karyotype. In addition, some of them may have a few gonadal follicles and approximately 20% have sufficient estrogen production to menstruate. Occasionally, ovulation may occur, as stated earlier. Isolated phenotypic features of Turner syndrome (without gonadal failure) may also occur in males and is known as Noonan syndrome.
Although individuals with this disorder have a 46,XX karyotype, part of one X chromosome is structurally abnormal. If there is deletion of the long arm of the X chromosome (Xq), normal height has been reported to occur, but, in Reindollar’s series, these individuals were all relatively short ( ). They have no somatic abnormalities; however, if there is deletion of the short arm of the X chromosome (Xp), the individual will be short. A similar phenotype occurs in those with isochromosome of the long arm of the X chromosome. Other X chromosome abnormalities include a ring X and minute fragmentation of the X chromosome.
As noted, this abnormality may have a familial/genetic association and has been reported in siblings. Abnormalities in genes involved in gonadal development are expected to be involved. These individuals have normal stature and phenotype, absence of secondary sexual characteristics, and primary amenorrhea. Some of these women have a few ovarian follicles, develop breasts, and may even menstruate spontaneously for a few years.
46,XY gonadal dysgenesis is the result of an abnormal testis in utero. There can be incomplete forms with some degree of testicular tissue, but in this context the “pure” form as a dysgenetic streak as in other forms of ovarian dysgenesis and previously has been referred to as Swyer syndrome.
If a Y chromosome is present (as in 46,XY gonadal dysgenesis) or is found as part of a mosaic karyotype, with or without any clinical signs of androgenization, gonadectomy should be performed.
A rare gonadal cause of primary amenorrhea without breast development and normal female internal genitalia is deficiency of the enzyme 17α-hydroxylase (P450 CYP-17) in an individual with a 46,XX karyotype (it can also occur in genetic males 46,XY), who may present in a similar fashion. Only a few such individuals have been described in the literature, but it is important for the clinician to be aware of this entity because, in contrast to those described earlier, these individuals have hypernatremia and hypokalemia. Because of decreased cortisol, ACTH levels are elevated. The mineralocorticoid levels are also elevated, because 17α-hydroxylase is not necessary for the conversion of progesterone to deoxycortisol or corticosterone. Thus there is excessive sodium retention and potassium excretion, leading to hypertension and hypokalemia. Serum progesterone levels are also elevated because progesterone is not converted to cortisol. In addition to sex steroid replacement, these individuals need cortisol administration. They usually have cystic ovaries and viable oocytes. Pregnancies have been documented after in vitro fertilization–embryo transfer (IVF-ET), despite low levels of endogenous sex steroids.
Hyperandrogenism occurs in approximately 10% of women with gonadal dysgenesis. Most have a Y chromosome or fragment of a Y chromosome, but some may only have a DNA fragment that contains the testes-determining gene (probably SRY ) without a full Y chromosome. Those with hypergonadotropic hypogonadism and a female phenotype who have any clinical manifestation of hyperandrogenism, such as hirsutism, should have a gonadectomy , even if a Y chromosome is not present, because gonadal neoplasms are common.
With CNS-hypothalamic-pituitary disorders, the low estrogen levels are caused by an abnormal or absent signal to the ovary, resulting in very low circulating gonadotropin levels. The cause of low gonadotropin production may be morphologic or endocrinologic.
Any anatomic lesion of the hypothalamus or pituitary can cause low gonadotropin production. These lesions can be congenital (e.g., stenosis of aqueduct, absence of sellar floor) or acquired (tumors). Many of these lesions, particularly pituitary adenomas, result in elevated PRL levels (see Chapter 37 ); however, non–PRL-secreting pituitary tumors ( chromophobe adenomas ) and craniopharyngiomas may not be associated with hyperprolactinemia and can rarely be the cause of primary amenorrhea with low gonadotropin levels. Thus all individuals with primary amenorrhea and low gonadotropin levels, with or without an elevated PRL level, should have computed tomography (CT) scanning or magnetic resonance imaging (MRI) of the hypothalamic-pituitary region to rule out the presence of a lesion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here