Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Posterior urethral valves (PUVs) are the most common cause of lower urinary tract obstruction (LUTO) in boys, with an incidence of 1 in 5000–8000 male births. Although the majority of boys with PUV are diagnosed antenatally, approximately one-third will be diagnosed during childhood or adolescence. Once diagnosed and surgically treated, PUV requires lifelong follow-up since it is the most common cause of LUTO leading to end-stage renal disease (ESRD) in children.
At 5–6 weeks gestational age (GA), the orifice of the mesonephric duct migrates from an anterolateral position in the cloaca to Müller’s tubercle on the posterior wall of the urogenital sinus, occurring simultaneously with division of the cloaca. Remnants of the mesonephric duct normally remain as small, distinct, paired lateral folds termed the inferior urethral crest and plicae colliculi. When the insertion of the mesonephric ducts into the cloaca is too anterior, normal migration of the ducts is impeded, and the ducts fuse anteriorly, resulting in abnormal ridges, which become the PUV. A smaller aperture between the leaflets causes more obstruction than those with a larger aperture and a less prominent anterior component.
Three distinct types of PUV have been described. Type I is an obstructing membrane that radiates distally and anteriorly from the verumontanum toward the membranous urethra, fusing in the midline. Approximately 95% of PUV are type I, in which the valves are thought to be a single membranous structure with the opening positioned posteriorly near the verumontanum. Type III appears as a membranous diaphragm with a central opening at the verumontanum. The obstructing tissue also has been termed a congenital obstructing posterior urethral membrane. It is thought that instrumentation with a urethral catheter might disrupt the posterior aspect of the membrane, resulting in the appearance of a type I valve. Type II valves are prominent longitudinal folds of hypertrophied smooth muscle that radiate cranially from the verumontanum to the posterolateral bladder neck, but these are nonobstructive and clinically insignificant.
About 10% of antenatally diagnosed obstructive uropathy is due to PUV and about two-thirds are diagnosed antenatally. It has been known for two decades that the GA at which hydronephrosis is detected influences prognosis. Typical antenatal ultrasound (US) findings include bilateral hydroureteronephrosis, a distended bladder, and a dilated prostatic urethra, called a “keyhole” sign. Discrete focal cysts in the renal parenchyma are diagnostic of renal dysplasia. Fetal megacystis is defined after the first trimester as a sagittal dimension (in mm) greater than the GA (in weeks). Amniotic fluid volume (AFV) is variable. Other diagnoses that may have antenatal US findings similar to those of PUV include prune-belly syndrome, urethral atresia or stenosis, and bilateral high-grade vesicoureteral reflux (VUR). US parameters alone are not able to predict postnatal renal function.
It had been thought that fetuses with LUTO and normal or slightly reduced amniotic fluid have a better prognosis. A very recent report by Johnson et al. for the North American Fetal Therapy Network (NAFTNet) centers evaluated the outcome and natural history of LUTO with normal AFV at midgestation. Of the 32 patients with LUTO and a normal AFV at 24 weeks, followed at 11 NAFTNet centers from August 2007 to May 2012, 18 (56%) had a final diagnosis of PUV. Normal AFV was defined as an amniotic fluid index (AFI) of ≥9 cm. For the entire cohort, the GA at delivery was 37.3 ± 2.8 weeks, and the mean creatinine level at discharge was 1.2 ± 0.8 mg/dL. Renal replacement therapy (RRT) was required in 32%, all of whom had an elevated creatinine and needed intervention early in the first year of life (median age at the start of RRT, 3.75 months; range, 0.5–11). Oligohydramnios and/or anhydramnios, the inability to spontaneously empty the urinary bladder on prenatal US, renal cortical cysts, the diagnosis of PUV, and a complicated, prolonged neonatal intensive care unit stay were findings associated with worsening renal function (serum creatinine level of ≥0.51 mg/dL) and the need for RRT. Multivariate analysis of these risk factors found that the development of oligohydramnios and/or anhydramnios and a younger GA at delivery were associated with worsening serum creatinine levels. Of note, younger GA at delivery was also predictive of the need for RRT. The presence of PUV and the development of oligohydramnios and/or anhydramnios were associated with the need for RRT on multivariate analysis, but did not reach statistical significance.
In contrast, oligohydramnios observed in the second trimester suggests significant obstructive uropathy and portends a poor prognosis. Fetal obstructive uropathy is known to affect lung development. Metanephric urine production begins at 12 weeks, but the fetal contribution to amniotic fluid begins after 18 weeks. Normal AFV is essential to bronchial branching in the lung, especially during the critical canalicular phase between 16 and 28 weeks. Therefore, oligohydramnios is associated with pulmonary hypoplasia and an arrest of normal lung development resulting in an abnormally low number or size of bronchopulmonary segments or alveoli.
Matsell et al. assessed prenatal findings as predictors of long-term renal outcomes in boys with PUV. In their series, 21% (17/82) of the boys developed ESRD. They found that younger GA, oligohydramnios, renal cortical cysts, and the combination of oligohydramnios, cortical cysts, and echogenic kidneys were associated with ESRD. The combination of all of these factors was an independent predictor of poor long-term renal function.
In the fetus with suspected PUV and normal amniotic fluid volume, serial fetal sonograms are necessary to monitor the status of the hydronephrosis and AFV. If oligohydramnios develops, bladder drainage may help restore the amniotic fluid and allow normal pulmonary development. Before any intervention, a karyotype should be obtained to confirm the male gender and to detect chromosomal abnormalities, which occur in about 12%. Fetal renal function can be assessed with serial urinary electrolytes and β 2 -microglobulin levels. Normally, fetal urine is hypotonic (favorable prognosis), with a sodium <100 mEq/L, chloride <90 mEq/L, osmolality <200 mEq/L, and β 2 -microglobulin levels <6 mg/L between 18 and 30 weeks GA. Elevated fetal urine electrolytes and β 2 -microglobulin levels are an indication of irreversible renal dysfunction. Sequential bladder aspiration every 48–72 hours should be performed because the initial urine sample may be stagnant and fresh urine more accurately reflects the function of the fetal kidneys.
Previous observations have suggested that if the fetal urine is hypotonic, and oligohydramnios is present, then fetal intervention to restore the AFV should be considered, with the goal of preventing pulmonary hypoplasia. This procedure has been performed in the first trimester although the majority of fetuses are diagnosed and treated in the second trimester . The problem with fetal intervention in managing LUTO is that not only is the diagnosis not definitive but also the severity of the obstruction is often variable, and the effect of the obstruction is dependent upon the GA at onset and whether the resulting renal dysplasia or cystic dysplasia is unilateral or bilateral. In theory, though, diverting the urine into the amniotic fluid with a percutaneously placed vesicoamniotic shunt (VAS) should decompress the urinary tract, but it does not allow the bladder to cycle. Therefore, when counseling expectant parents, they need to understand that their newborn may have limited renal function or ESRD, even if the drainage procedure is successful. In addition, VAS can have complications in up to 45% including herniation of the omentum or bowel through the fetal abdominal wall. Also, these shunts can become obstructed or displaced, necessitating additional procedures that increase morbidity to the mother and fetus with a 5% procedure-related fetal loss. In a recent review by Kurtz et al., shunt dislodgement was found to be the most common technical complication of fetal intervention for LUTO, occurring in about 50%, and appeared to be related to the Harrison Fetal Bladder Stent, which was found to have more complications than the Rocket KCH Fetal Bladder Catheter.
In an early review of 14 fetuses with proven PUV and favorable fetal urinary electrolytes undergoing VAS at a mean GA of 22.5 weeks, six deaths occurred before term delivery. Of the surviving eight neonates, three had ESRD, and the other five had an elevated serum creatinine. In a more optimistic study of 20 boys with LUTO managed by VAS, the overall 1-year survival was 91%. In this study, the mean birth weight was 2574 g, 40% had acceptable renal function, 20% had mild renal insufficiency, and 30% required dialysis. Of this group, seven had PUV and seven had prune-belly syndrome that did not need fetal intervention.
This lack of evidence regarding fetal drainage was the impetus for the study on Percutaneous Shunting in Lower Urinary Tract Obstruction (PLUTO) trial, which was an international, randomized controlled trial comparing the effect of VAS versus no intervention. This study evaluated prenatal and perinatal mortality, renal function, and other variables. The study closed early due to low recruitment. Of the 16 women randomly assigned to VAS (with 1 intrauterine death and 3 pregnancy terminations) and the 15 women randomly assigned to conservative management (1 intrauterine death and 2 pregnancy terminations), each group had 12 live births. Of the 16 pregnancies in the VAS group, 8 survived 28 days while 4 of the 15 pregnancies conservatively managed survived 28 days. Pulmonary hypoplasia was the cause of death in the 12 who died in both treatment arms. Only 2 children, both in the VAS group, survived to 2 years with normal renal function. Six fetuses had 7 complications in the VAS group including spontaneous rupture of membranes and shunt blockage and dislodgement that led to fetal demise in 4. These data suggest improved perinatal survival with intervention, but there was no difference in the incidence of ESRD.
A recent meta-analysis evaluated the effectiveness of VAS in 112 fetuses with LUTO versus 134 fetus treated conservatively. More than 400 abstracts were retrieved, but only 9 studies were eligible for inclusion. Although the study design had heterogeneity, the authors report improvement in perinatal survival in the VAS group, but no difference in survival at 6–12 months or at year 2 between the treatment groups. There was no effect on renal function between fetuses undergoing VAS versus conservative management.
Since its advent in the late 1990s, fetal cystoscopy has been shown to improve the accuracy of the prenatal US diagnosis by elucidating the urethral phenotype of the fetus. A recent retrospective cohort study of 48 fetuses with normal karyotype who underwent cystoscopy for sonographic findings of LUTO after 18 weeks of gestation at three tertiary referral centers with the intent of diagnosis and treatment in cases of PUV. PUV were found in 30 fetuses, urethral atresia in 13, and urethral stenosis in 5. There were no survivors in the urethral atresia group. One fetus with urethral stenosis underwent urethral stenting and survived with normal renal function at 2 years of life. Of the 48 fetuses, 17 underwent termination of pregnancy, 2 cases had intrauterine fetal demise after fetal intervention, and 11 died during the neonatal period. The cause of the LUTO was determined in the 18 survivors and in 17 by autopsy. The diagnosis at the time of cystoscopy was accurate in 32/35 (91.4%). One case of suspected valves had hypoperistalsis–microcolon–megacystis syndrome, and another with valves also had distal urethral stenosis. Of the 18 survivors at 1 year, 14 had normal renal function. Sixteen survivors had reached the age of 2 by the end of the study, and 12/16 (75%) had normal renal function.
Percutaneous endoscopic ablation is currently being performed in a few centers in the United States A meta-analysis in 2011 showed a perinatal survival advantage using endoscopic ablation compared with observation, but there was no significant improvement in survival with ablation when compared with VAS. Martinez et al. reported fetal cystoscopy in 20 fetuses (mean GA of 18.1 weeks) using diode laser ablation of PUV. The median operative time was 24 minutes. Access to the urethra was obtained in all but one, and decompression of the bladder was achieved in 80%. Termination of pregnancy was elected in 45% (9), and 11 delivered at a mean of 37 weeks. Of the 11 who were alive at 15–110 months, 73% had normal renal function and 27% had ESRD and were awaiting renal transplantation.
Ruano et al., in a recent retrospective case-control analysis from two centers, divided 111 fetuses into three groups: vesicoamniotic shunting (16), fetal cystoscopy/valve ablation (34), and no intervention (61). In neonates with a postnatal diagnosis of PUV (57), fetal cystoscopy improved both the 6-month survival rate and renal function, which trended toward normal while VAS affected only the 6-month survival. Fetal cystoscopy with valve ablation permits the fetal bladder to cycle and replenish amniotic fluid in a physiologic pattern. In addition, the amnio-infusion needed for VAS makes the procedure more difficult and puts the fetus at increased risk of premature delivery, despite VAS being less challenging and less invasive than valve ablation.
Complications of fetal cystoscopic laser fulguration of PUV were recently reported from three large centers. Of the 40 fetal cystoscopies, laser fulguration was performed in 23 with a survival rate of 60.9% (14/23) and normal renal function in 85.7%. Urethrocutaneous and urethrorectal thermal fistulas occurred in 17% (4/23) and were associated with the type, energy, and power settings of the laser .
Ruano et al. also recently reported a single center experience using a standardized prenatal protocol for fetal intervention in LUTO which considers a combination of prenatal parameters correlated with GA as well as in utero disease progression. They performed a retrospective cohort study of fetal intervention with VAS in 25 consecutive patients with LUTO (between 16 and 34 weeks). Strict criteria defined a classification for LUTO based on fetal urinary biomarker for renal injury, amniotic fluid volumes, and imaging studies to assess renal dysplasia or cortical cysts. Intervention was considered depending on bladder capacity and rate of bladder refilling after vesicocentesis. Fetal intervention was offered for severe LUTO, oligohydramnios/anhydramnios, and normal renal function parameters. Based on their observations of fetal renal function and renal function outcomes, the authors propose a classification of LUTO based on disease severity with recommendations for intervention based on three stages of obstruction. Stage I included low-risk fetuses with normal amniotic fluid, “normal renal function” by urine biochemical parameters, and no renal cysts/dysplasia who required no intervention. Stage II included high-risk fetuses with finding suggestive of preserved renal function. In this group, oligohydramnios after 18 weeks GA, echogenic kidneys but no cortical cysts or dysplasia, and normal urinary biochemistries were thought to benefit from intervention with VAS to prevent pulmonary hypoplasia and severe renal impairment. Stage III had oligohydramnios, echogenic kidneys with or without renal cortical cysts/dysplasia, and poor urine chemistries. Of the 25 fetuses with LUTO, 14/16 meeting stage II criteria underwent VAS at a mean GA at diagnosis of 18.5 + 4.2 weeks. Outcomes included survival and renal function status at 6 months of age. Normal renal function was defined at <0.5 mg/dL at 6 months of age. For stage I, all patients (2/2) at 6 months survived and had normal renal function. For stage II undergoing intervention, the survival rate was 85.7% (12/14) and 57.1% (8/14) had normal renal function. One fetus died 1 week after shunt placement, and the other fetus died postnatally at 27 weeks GA from factors related to the prematurity. The overall survival in the entire stage II group was 75% (12/16) with ESRD developing in 33% by 6 months requiring dialysis. Stage III had signs of abnormal renal function, and none of the seven patients in this group were offered intervention. One pregnancy was terminated, one fetus survived requiring dialysis, and the remainder died immediately after birth from respiratory distress due to severe pulmonary hypoplasia. This study suggests that intervention with VAS in this select group of fetuses with LUTO and favorable renal parameters improves postnatal outcomes. The authors attribute their improved survival of 85.7% versus approximately 65% in prior reports to VAS placement within 2 days of diagnosis and close monitoring to detect shunt migration or obstruction with immediate replacement.
Despite advances in technology, fetal intervention has not significantly impacted on the development of ESRD. However, perinatal intervention has reduced perinatal mortality by ameliorating oligohydramnios at the critical time of lung development between 16 and 28 weeks of gestation.
Neonates with PUV not diagnosed prenatally can present with symptoms of delayed voiding or a reduced urinary stream. Also, respiratory distress secondary to pulmonary hypoplasia may be the primary manifestation of PUV. Other postnatal signs and symptoms include an abdominal mass (49%), failure to thrive (10%), lethargy, poor feeding, urosepsis (8%), and urinary ascites (7%). On physical examination in the newborn, a palpable walnut-sized bladder secondary to the hypertrophic detrusor muscle is found. Urinary ascites can result in significant abdominal distention. Older boys can have persistent diurnal incontinence or abdominal distention.
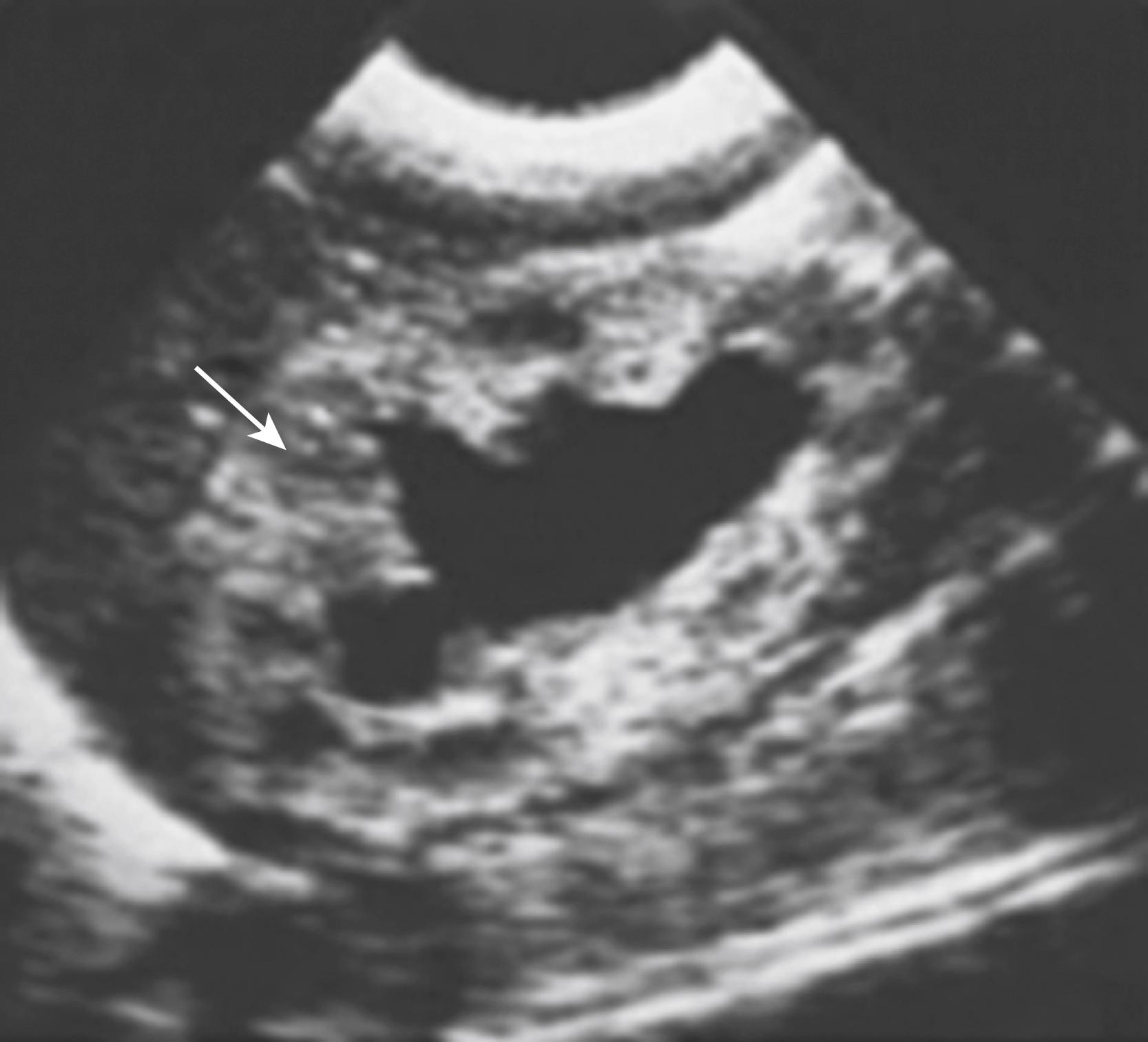
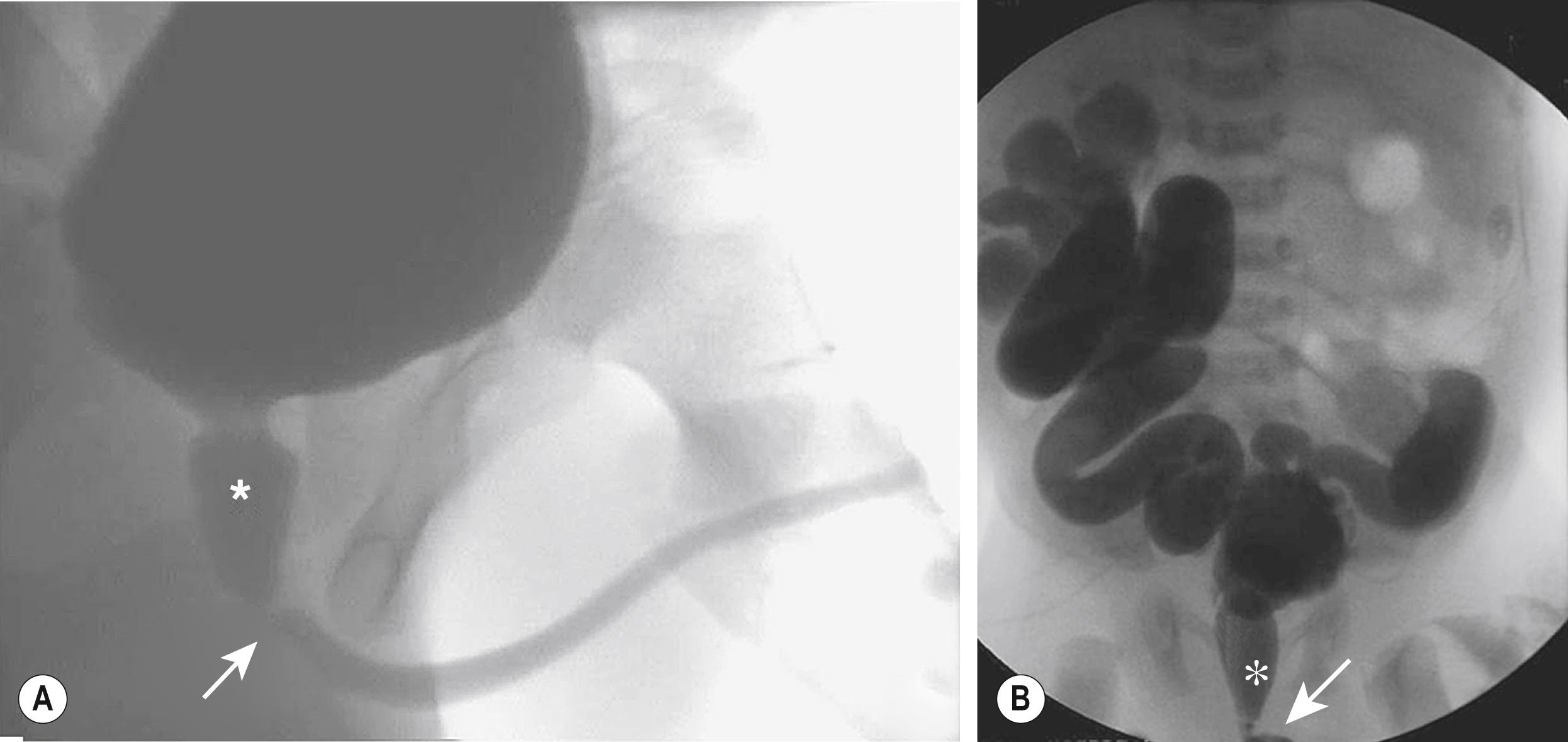
Significant bilateral hydroureteronephrosis and a thick-walled, distended bladder are seen on US. Corticomedullary differentiation is a favorable prognostic sign regarding renal function ( Fig. 57.1 ). Conversely, echogenic kidneys or subcortical cysts and the loss of corticomedullary differentiation are unfavorable signs. Suprapubic or perineal US may demonstrate a dilated prostatic urethra, which is pathognomonic for PUV. The voiding cystourethrogram (VCUG) is the only radiographic study that definitively establishes the diagnosis of PUV ( Fig. 57.2 ). The valves appear as a defined lucency in the distal prostatic urethra. The posterior urethra is dilated and elongated. The bladder is trabeculated with cellules and diverticuli, and bladder neck hypertrophy is seen. Unilateral VUR is present in 25% and bilateral VUR in 25% of infants with PUV.
Renal nuclear scintigraphy with a technetium-99m-labeled dimercaptosuccinic acid ( 99m Tc-DMSA) is performed if imaging studies show thin or abnormal parenchyma in either kidney on US and/or high-grade VUR. The study should be delayed until 6–8 weeks of age to allow maturation of renal function. This study is effective in establishing baseline differential renal function. However, if renal function is poor, visualization of the kidneys will be suboptimal. An alternative to renal scintigraphy is dynamic contrast-enhanced magnetic resonance urography (MRU). This study provides high-resolution renal images and assessment of differential renal function, but requires an anesthetic.
The initial treatment of neonates may focus not only on bladder decompression but also on any degree of respiratory distress that may require immediate pulmonary resuscitation with endotracheal intubation and positive-pressure ventilation. If urinary ascites is present, paracentesis may be necessary to correct the fluid and electrolyte imbalance and/or improve ventilation. The bladder can be decompressed with a 5 or 8 French feeding tube. The catheter can be difficult to pass due to significant dilation of the prostatic urethra and hypertrophy of the bladder neck as well as the valvular obstruction. The catheter often tends to coil in the prostatic urethra, and a Coudé tip catheter may be helpful. US can confirm placement of the catheter within the bladder. Insertion of a urinary catheter is discouraged because the inflated balloon can obstruct the ureteral orifices when the thick-walled bladder is decompressed, and can cause bladder spasms, which further obstruct the intramural ureters. Penna and colleagues have described the use of a 6 French 12 cm Double-J stent for decompression of the bladder postnatally. They found that a 5 French feeding tube and a 6 French Foley catheter drain more slowly than the Double-J due to their diminished cross-sectional luminal area.
Amoxicillin or cephalexin prophylaxis should be started. Electrolytes, blood urea nitrogen (BUN), creatinine, and fluid status are monitored carefully. The serum creatinine concentration at birth reflects maternal renal function and should gradually decrease to 0.3–0.4 mg/dL when there is favorable renal function. However, when there is compromised renal function, the creatinine will remain the same or increase, even after bladder decompression. Metabolic acidosis and hyperkalemia can occur if renal function is impaired. Consultation with a pediatric nephrologist is invaluable because metabolic abnormalities due to renal insufficiency and ESRD lead to somatic growth abnormalities requiring long-term monitoring.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here