Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
pediatric plastic surgery is performed in children of all ages, even in utero. However, the majority of children who undergo plastic surgical and reconstructive procedures are between 2 and 9 years of age, with a median age of 5 years. A wide spectrum of associated craniofacial abnormalities, underlying medical conditions, and surgical procedures characterizes this pediatric population. Consequently, a thorough preoperative assessment, consultation with medical and surgical teams, and anticipation and preparation for potential complications are of paramount importance to ensure a successful perioperative outcome. Many procedures are performed on the head and neck and require thoughtful coordination between the anesthesiologist and surgeon. A thorough understanding of the procedure allows for selection of the optimal anesthetic plan. The incidence of major morbidity and mortality has been reduced over the past 30 years from 16.5% and 1.6% to less than 0.1% and 0.1%, respectively, in children undergoing major craniofacial surgeries.
Cleft lip and palate are among the more common congenital malformations, occurring with an estimated incidence of approximately 1 in 600 births worldwide. More common in males than in females and more common in Asians and Latin Americans and least in Africans, this malformation likely results from both environmental and genetic causes. Parental occupation, in particular paternal farming, increases the risk of cleft lip or palate in the offspring, whereas the maternal occupation presents no additional risk. Folate metabolism disturbances and increased maternal homocysteine concentrations also may be contributory. Cleft lip with or without cleft palate has been linked to several loci on chromosomes 1, 2, 4, 6, 14, 17, 19, and 22, suggesting a genetic basis for some of these anomalies. Three genes have been associated with syndromic cleft lip and palate: T-box transcription factor-22, poliovirus receptor–like-1, and interferon regulatory factor-6 (IRF6) . Gene mutations have been identified in only a small fraction of nonsyndromic cleft lip and palate.
These disorders are associated with more than 400 syndromes; the more common syndromes are presented in Table 35.1 . Cleft lip and palate begin as a defect in palatal growth in the first trimester of pregnancy. Fetal magnetic resonance imaging (MRI) provides a greater degree of resolution of defects in the posterior palate and lateral extent of cleft with greater diagnostic accuracy than ultrasound. MRI also enables early detection of potential syndromic conditions by providing a complete study of the fetal head and biometric development of the facial bones.
| Pierre Robin sequence |
| Down syndrome |
| Klippel-Feil syndrome |
| Treacher Collins syndrome |
| Velocardiofacial syndrome |
| Fetal alcohol syndrome |
| Nager syndrome |
| Goldenhar syndrome |
Primary cleft lip repair is usually undertaken at approximately 2 to 3 months of age, whereas primary cleft palate repair occurs at 6 to 10 months. Surgery for lip or nose revision usually takes place in early childhood, and palatal revision and alveolar bone grafts occur at approximately 10 years of age. Rhinoplasty and maxillary osteotomy to complete the repair may take place at 17 to 20 years of age. Pharyngoplasty may be required for velopharyngeal incompetence secondary to anatomic or neurologic dysfunction to improve speech development and prevent nasal regurgitation during eating.
Surgical correction of a cleft lip defect is usually performed at 2 to 3 months of age to allow sufficient time for maturation and associated abnormalities to become apparent. Preoperative assessment may reveal abnormalities such as mandibular hypoplasia in Pierre Robin sequence (PRS) ( Fig. 35.1 and E-Fig. 35.1 ) or restricted neck movement as in Klippel-Feil syndrome ( E-Fig. 35.2 ). PRS is defined as the triad of micrognathia, glossoptosis (caudally displaced insertion of the tongue), and respiratory distress in the first 24 to 48 hours after birth. The presence of other anomalies might warrant additional clinical or laboratory investigations. Cleft lip repair usually involves minimal blood loss, so for children with hematocrit values greater than 30%, additional preoperative laboratory testing is unnecessary. A sample for type and screen is usually sufficient for infants with a hematocrit value less than 30%.
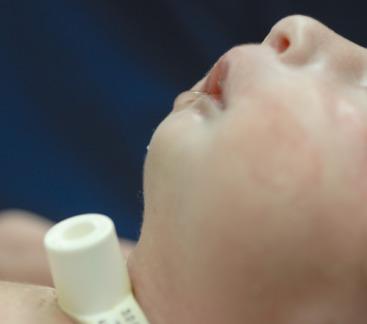
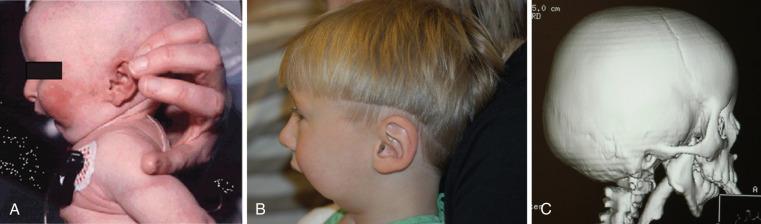
The frequency of difficult airways in children with cleft lip and palate varies from 2.9% to 23%. The incidence of difficult intubation in children with bilateral cleft is greater than that with unilateral cleft. Furthermore, micrognathia is an independent predictor of a difficult airway. The incidence of difficult direct laryngoscopy is approximately 50% in children with micrognathia but only about 4% in those without. In infants and young children, micrognathia may be subtle and not always easily detected. However, the presence of microtia, which is associated with a 42% incidence of difficult intubation with bilateral compared with 2% with unilateral microtia, should prompt a closer examination of the mandible for hypoplastic growth and raise the possibility of a hemifacial microsomia or Treacher Collins syndrome. Intubation difficulty (by direct laryngoscopy) with isolated micrognathia decreases with increasing age, with the greatest difficulty presenting in infants younger than 6 months. This has been attributed to rapid growth of the mandible, which catches up to the maxilla, thereby aligning the two bones, by 2 years of age in most cases. A careful review of previous anesthetic records may forewarn of a difficult airway. In a second study that reported the frequency of difficult intubations in young infants with cleft lip, cleft palate without PRS, cleft-lip-palate and cleft palate, and cleft palate with PRS was 0, 2.7%, 10%, and 23%. Difficult airways increased with early airway and feeding problems (P < 0.0001). In the isolated cleft palate, a wider cleft was associated with a significantly more difficult laryngoscopy.
Induction of anesthesia via face mask is usually uncomplicated in infants with cleft lip and palate. Laryngoscopy should be performed using a straight blade via a right paraglossal approach (blade inserted into the pharyngeal gutter with tongue displaced to the left), taking care to avoid dropping the blade into the cleft (see also Fig. 14.28A-C ). If the mandible is hypoplastic, external laryngeal manipulation may be required to bring the larynx into view. In some centers, the tongue is sutured to either the mandible or lower lip to preclude airway obstruction in infants with PRS in the postnatal period. In such instances, the tongue cannot be displaced to the left to expose the larynx. To facilitate laryngoscopy in such cases, the tongue is first released from the lower lip using ketamine sedation followed by direct laryngoscopy. Alternatively, selection of a primary airway management strategy other than direct laryngoscopy (e.g., video laryngoscopy, fiberoptic intubation through a supraglottic airway) circumvents this problem and may be a preferable approach with a greater success rate on the first attempt (see Chapter 14 ).
A variety of tracheal tubes can be used to secure the airway for cleft lip and palate surgery, although the ideal tracheal tube is perhaps the oral Ring-Adair-Elwyn (RAE) tube, which can be fixed centrally to the chin to facilitate optimal surgical access. It should be noted that preformed tracheal tubes vary in length from bend to tip for uncuffed and cuffed tubes. Seven brands of preformed tracheal tubes were compared for the same size inner diameter tubes; the distance from the bend to the tip varied by 0 to 1 cm for oral cuffed tubes but 0 to 4 cm for uncuffed oral tubes. Of greater concern was that the variability for preformed nasal tubes was even greater; cuffed nasal tubes varied by 0 to 5.5 cm and uncuffed varied from 2 to 9 cm (bend to tip). The risk of an endobronchial intubation when a cuffed oral preformed tube replaced an uncuffed tube of the same diameter was 0%–27%, whereas when a cuffed nasal preformed tube replaced an uncuffed tube of the same size, the risk of an endobronchial intubation increased to 50%–100%. Thus the risk for endobronchial intubation varies among manufacturers and is greater with nasal than oral preformed tracheal tubes.
Throat packs usually impinge on the surgical field and are not normally required for cleft palate repair. Ventilation is usually controlled for the duration of the procedure (~1–2 hours). Inhalational or intravenous (IV) anesthetics combined with a short-acting opioid such as fentanyl (1–2 µg/kg) can be used for maintenance of anesthesia. Bilateral infraorbital nerve blocks may be used to provide postoperative analgesia for cleft lip repairs (see Fig. 42.12A-C and Chapter 42 videos). Such blocks reduce the need for opioids and antiemetics, improve the ability to feed, and increase parental satisfaction. Additional evidence suggests that the incidence of emergence agitation is reduced with the use of infraorbital nerve blocks. A combination of infraorbital and external nasal nerve blocks for pain control after cleft lip repair is an alternative.
During cleft palate surgery, the pharyngeal space is reduced dramatically; postoperative nasal trumpets may be required (often placed by the surgeon) to maintain airway patency and permit suctioning the airway without damaging the palatal repair ( Fig. 35.2 and E-Fig. 35.3A and B ). At the end of surgery, the trachea is extubated after the upper airway reflexes have returned and the child is completely awake. These children are at particular risk for acute upper airway obstruction in the immediate postextubation period as a result of upper airway narrowing, edema, blood, and residual anesthetic effects. Accordingly, it is very important to extubate the trachea only when the child is completely awake. Intraoperative dexamethasone (0.5 mg/kg) may be administered to mitigate postoperative airway edema. Late postoperative edema and severe subcutaneous emphysema are additional complications. Upper respiratory tract infections are common in this age group. If airway infections are present, they should weigh heavily in favor of delaying surgery until they are resolved. Antibiotics may reduce the incidence of postoperative respiratory complications. Adverse airway events, including postoperative airway obstruction, oxyhemoglobin desaturation, bronchospasm, laryngospasm, reintubation, and unplanned postoperative intensive care unit (ICU) admission have been identified in as many as 23% of children undergoing cleft palate repair ; the presence of a craniofacial syndrome, history of preoperative airway problems, and both surgeon and anesthesiologist inexperience were significantly associated with airway complications.
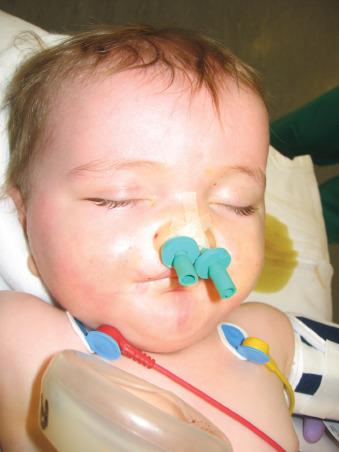
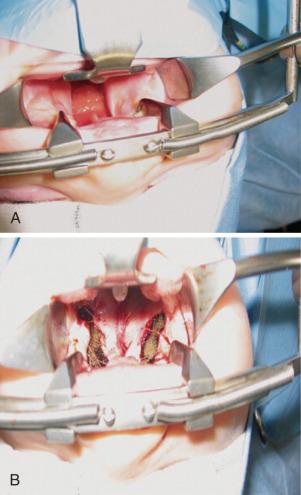
Arm restraints are used in many centers to prevent suture disruption. These children are monitored for signs of upper airway obstruction during the recovery period for approximately 48 hours. As soon as the child is awake, feeding with clear fluids is allowed. Postoperative pain is managed with a combination of opioids and acetaminophen. Rectal acetaminophen administered before surgery did not reduce postoperative opioid requirements in children undergoing cleft palate repair. In part this may be attributed to local infiltration at the surgical site and the slow and variable absorption of acetaminophen by the rectal route. However, IV acetaminophen has opioid-sparing effects in these children that may be useful in certain scenarios. Sphenopalatine and infraorbital nerve blocks (see Chapter 42 , Fig. 42.12 ) with a long-acting local anesthetic can be placed at the end of the procedure to prevent pain after cleft lip and palate repair. Palatal nerve block (nasopalatine, greater and lesser palatine) or a bilateral suprazygomatic maxillary nerve block reduce postoperative pain and favor early feeding.
Children who are scheduled for elective pharyngoplasty are usually school age, having undergone cleft palate repair at an earlier age. The primary objective of this procedure is to restore velopharyngeal competence for speech development, which can be achieved by a pharyngeal flap, sphincter pharyngoplasty, or palatal lengthening (Furlow double-opposing Z-plasty palatoplasty). The anesthetic goals and management are similar to those discussed for cleft palate repair.
Craniosynostosis, a congenital anomaly in which one or more cranial sutures close prematurely, occurs in approximately 1 in 2000 to 3000 births, affecting males more frequently than females. Embryologically, the cranial vault starts to ossify at 8 weeks after conception; fusion of the parietal and frontal bones is usually completed by 7 months after conception. Postnatally, the anterolateral fontanelle closes by 3 months, the posterior fontanelle by 3 to 6 months, the anterior fontanelle by 9 to 18 months, and the posterolateral fontanelle by 2 years. Premature osseous obliteration of a bony suture might result from the absence of osteoinhibitory signals from the suture. Craniosynostosis may be categorized as simple (or nonsyndromic) (60%-80% of cases), involving closure of one suture, or complex (or syndromic) (20%–30%), involving closure of two or more sutures and is often associated with a variety of clinical features and metabolic diseases ( Table 35.2 ).
| Nonsyndromic (primary) ~80%: Single suture closed, isolated finding |
| Syndromic ~20%: Two or more sutures closed, often with associated clinical findings. More than 150 syndromes have been described; the more common syndromes are as follows: |
| Crouzon |
| Apert |
| Pfeiffer |
| Saethre-Chotzen |
| Carpenter (acrocephalopolysyndactyly type II) |
| Muenke |
| Crouzonodermoskeletal |
| Shprintzen-Goldberg |
| Loeys-Dietz |
| Jackson-Weiss |
| Beare-Stevenson |
| Cole-Carpenter |
| Kleeblattschädel |
| Fibroblast growth factor receptor mutations 1 and 2 |
| Metabolic and other causes |
| Rickets |
| Bone metabolic disorders (hypophosphatasia) |
| Achondroplasia |
| Prematurity |
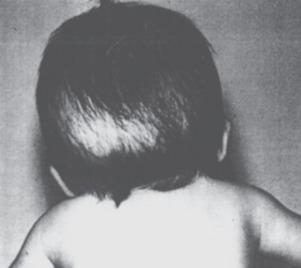
In the child with craniosynostosis, fusion of a cranial suture restricts normal bone growth perpendicular to the affected suture. Compensatory growth and expansion of the cranial vault occurs parallel to the affected suture, and there is a characteristic skull deformity associated with the different sutures involved ( Fig. 35.3 and E-Fig. 35.4 ). The frequency of single suture closures varies with the specific suture: sagittal (50%), coronal (20%), and metopic (10%). The coronal suture (especially bicoronal synostosis) is more commonly associated with syndromic craniosynostosis. Although approximately 80% of premature suture closures are isolated defects, the remaining 20% involve multiple suture closures associated with more than 400 syndromes that present with a myriad of clinical features (see Table 35.2 ); the more common syndromes that require craniofacial reconstruction are described later. Syndromic craniosynostoses are commonly associated with gene defects in the fibroblast growth factor receptor (FGFR), which is involved in bone and cartilage development.

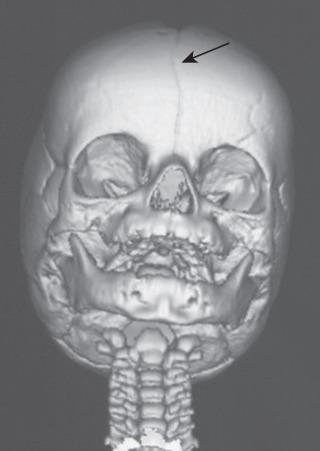
Apert syndrome occurs in less than 1 in 100,000 live births, usually as a sporadic mutation, although autosomal dominant inheritance patterns can occur with the FGFR2 gene on chromosome 10. This syndrome phenotypically manifests as cloverleaf skull (craniosynostosis), hypertelorism, proptosis, midface hypoplasia, and syndactyly (upper or lower extremity). Development is often complicated by increased intracranial pressure (ICP) and obstructive sleep apnea (OSA). Whether children with Apert syndrome develop normal intelligence quotients (IQs) is unclear; one study reported that 32% had IQs greater than 70. The timing of cranial surgery may affect the child's IQ; surgery in the first year of life was associated with an IQ greater than 70 in more than 50% of children in one study, whereas surgery after the first year of life was associated with an IQ greater than 70 in only 7%. Two other factors predicted improved IQ indexes: absence of a defect in the septum pellucidum and noninstitutional residence (i.e., family home residence). In contrast, more recent evidence failed to substantiate a salutary effect of early surgery on cognitive development. Indeed, cognitive development was related to the quality of the family environment and parental education and unrelated to brain malformation and the age of surgery.
Crouzon syndrome is phenotypically similar to Apert syndrome but has different ophthalmologic defects, specifically, optic atrophy occurring in up to 20%, and the absence of hand and foot defects (e.g., syndactyly). Fifty percent of Crouzon syndrome defects are sporadic mutations, and the remainder are familial with the same gene defect as Apert; FGFR2 on chromosome 10. Pfeiffer syndrome occurs in approximately 1 in 25,000 live births. Most cases are familial with an autosomal dominant inheritance pattern that has its origin in defects in the FGFR1 and FGFR2 genes on chromosome 10, although many remain sporadic. The phenotype of Pfeiffer syndrome is similar to that of Apert syndrome but includes broad thumb, large first toe, polydactyly, and may be associated with a cartilaginous sleeve trachea. Children with Pfeiffer syndrome have normal intelligence. Carpenter syndrome is associated with craniosynostosis, syndactyly, cardiac defects, and obesity. Cognitive impairment is common. Muenke syndrome is more common, occurring in 1 in 30,000 births. It results from a mutation in the FGFR3 gene. Affected patients have midface hypoplasia, ocular hypertelorism, strabismus, developmental delays, and intellectual disabilities. A relatively new but rare syndrome, Shprintzen-Goldberg, is characterized by craniosynostosis and a phenotype that resembles that of Marfan syndrome.
Indications for cranial vault reconstruction include increased ICP, severe exophthalmos, OSA, craniofacial deformity, and psychosocial reasons. If uncorrected, the deformed cranium may cause severe neurologic sequelae, including visual loss and developmental delay. Because rapid brain growth during infancy determines skull shape, surgical correction is undertaken within the first months of life to achieve the best cosmetic results.
Cranial vault reconstruction may involve the anterior or posterior aspect of the skull or both (total cranial vault reconstruction). Less invasive approaches to correct craniosynostosis are available and may be associated with reduced morbidity. Surgical correction may use an open approach where the synostotic suture is excised followed by barrel-stave osteotomies to release the adjacent cranium and allow for normalization of skull shape with subsequent brain growth ( Fig. 35.4 and E-Fig. 35.5 ). This technique is used in children younger than 6 months of age and is believed to be less invasive than total cranial vault reconstruction. Endoscopic strip craniectomy is increasingly being used in early infancy because of benefits including reduced transfusion requirements and reduced hospital stay, compared with the open procedures. The principal disadvantage of endoscopic techniques is the requirement of 4 to 6 months of wearing a helmet postoperatively to promote normalization of skull shape. Spring-assisted cranioplasty, a technique preferentially used in infants younger than 6 months, involves performing a midline osteotomy along the fused sagittal suture and placing springs across the osteotomy to increase the biparietal dimension. Spring-assisted cranioplasty may be associated with reduced intraoperative blood loss, reduced transfusion requirement, and shorter duration of hospital stay. In a single-center study of 100 children with spring-assisted craniosynostosis repair, no child was transfused and none was admitted to the ICU. The primary disadvantage of this approach is the need for a second surgery to remove the springs. A meta-analysis of the outcomes from calvarial remodeling, strip craniectomy, and spring-mediated cranioplasty in nonsyndromic sagittal synostosis demonstrated that the cephalic index (which is the ratio of the width of the outermost tables of the vault to the length of the outermost tables) with calvarial vault was superior to strip craniectomy and that the former approach required more surgical time, involved more blood loss, a greater duration of stay, and greater costs than the other two approaches (P < 0.0001).
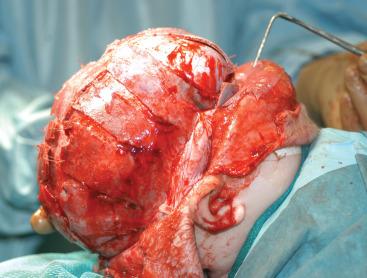
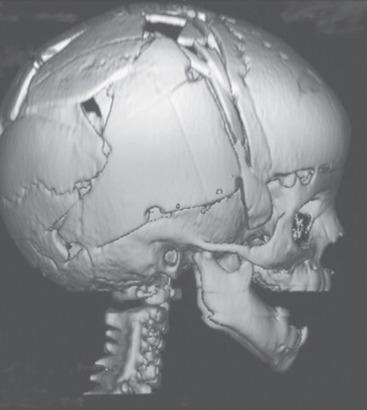
The application of cranial vault distractors to achieve distraction osteogenesis is a technique increasingly used in children with syndromic forms of craniosynostosis. This procedure involves a craniotomy (usually without reconstruction of the cut bone flap) combined with placement of distractors that are sequentially incrementally lengthened by turning an externalized screw beginning a few days postoperatively and continuing until the targeted cranial vault expansion is achieved. This technique has the advantage of shorter surgery and allows for greater expansion of the cranial vault than what can be achieved with a traditional cranial vault reconstruction. These children also need to return to the operating room for removal of the distraction hardware.
Preoperative assessment of children with craniosynostosis should focus on airway management, eye protection, and ICP. An important consideration in children with syndromic midfacial hypoplasia is its common association with OSA (50%–70% incidence) owing to associated narrowing of the nasopharyngeal space. Although some recommend preoperative adenotonsillectomy to treat OSA in children with syndromic craniosynostosis, neither airway dimensions nor propensity toward airway collapse are improved. Midfacial advancement may be required to resolve OSA, and even then, residual airway obstruction may persist. Preoperative endoscopy has been recommended to assess the severity of midfacial hypoplasia and whether OSA is likely to persist after midface advancement. Careful titration of opioids in the perioperative period is indicated if the child exhibits severe nocturnal desaturation (i.e., if the Sa o 2 nadir is <85%) (see Chapter 33 ); children with OSA who have severe nocturnal desaturation require half the dose of opioids that children without nocturnal desaturation require, so a normal dose of opioid is actually a relative overdose in this population. Upper airway obstruction may also occur postoperatively in children who received opioids as a direct effect of opioids on the hypoglossal nucleus. Preoperative laboratory assessment should include a complete blood cell count and a specimen for blood type, antibody screening, and crossmatching of blood. Many centers also routinely screen for coagulation abnormalities with a preoperative prothrombin time and the activated partial thromboplastin time.
Postoperative pain is generally not severe and is managed effectively with a combination of acetaminophen, nonsteroidal antiinflammatory drugs (NSAIDs), and IV opioids. Opioids remain the mainstay of pain management, but careful titration is indicated if OSA is present. Given the small incidence of craniosynostosis and the large variability in the management of these patients, multicenter trials are required to determine the optimal management strategy for these children.
Meticulous preoperative planning and evaluation of the airway are essential, particularly for children with known or possible OSA. Upper airway obstruction following induction of anesthesia in children with midface hypoplasia should be anticipated. This is usually readily managed with a jaw-thrust/subluxation technique or insertion of an oral airway. Occasionally, however, face mask ventilation may prove challenging owing to difficulty obtaining a good mask seal. External fixator devices on the face may also present challenges in managing the airway ( E-Fig. 35.6 ), and it is essential to craft the optimal plan and make necessary preparations should problems arise (e.g., emergent hardware removal, wire cutting). A laryngeal mask is a valuable rescue tool in these scenarios and should be readily available before anesthetic induction.
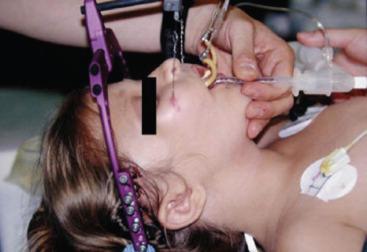
In the majority of children with craniosynostosis, the anatomy of the mandible and the temporomandibular joint is normal, as are upper airway dimensions, resulting in an easy direct laryngoscopy and tracheal intubation. Rarely, mandibular hypoplasia may complicate an otherwise straightforward laryngoscopy and tracheal intubation. Abnormal neck mobility also may pose additional challenges for laryngoscopy and tracheal intubation.
Crystalloid solutions are commonly administered for minimal to moderate surgical blood loss and fluid shifts during craniosynostosis surgeries. Although lactated Ringer's solution (or Hartmann's solution) is most commonly used in North America, some advocate using normal saline solution because it may be less likely to induce hyponatremia and an acid-base disturbance than lactated Ringer's solution. However, a recent study comparing the two solutions suggests that normal saline solution is more likely to induce (metabolic) acidosis than lactated Ringer's solution in infants undergoing craniosynostosis. Additionally, hyponatremia is usually mild, self-limited, and asymptomatic. The results of large adult cohort studies have demonstrated an association between hyperchloremic metabolic acidosis after normal saline and major complications, morbidity, mortality, and increased resource use. Isotonic balanced solutions may be the best choice for crystalloid management.
Surgery for craniosynostosis is associated with the potential for cardiac arrest as a result of sudden massive blood loss or underestimated blood loss. Although these procedures are extradural, significant bleeding from the scalp and cranium can occur, especially following inadvertent tears of dural venous sinuses. The blood loss can be so rapid during the surgery that the expression “trauma in progress” is applicable. The risks of massive blood loss and the need for invasive monitoring are primarily determined by the type of surgery. In children undergoing endoscopic strip craniectomy, weight less than 5 kg, those undergoing sagittal endoscopic craniectomy, those with syndromic craniosynostosis, and earlier date of surgery are associated with blood transfusion. Some centers advocate commencing blood transfusions at the time of skin incision (particularly in infants) to prevent hemodynamic instability and the need for a rapid transfusion, but this must be tempered by whether the surgery is open or endoscopic (see later discussion).
To manage the large volume and rapidity of the blood loss, it is essential to establish large-bore peripheral venous access. Central venous access (see also Figs. 49.3 and 49.4 ), usually via the internal jugular vein, is most commonly reserved for children in whom obtaining adequate peripheral venous access proves difficult but overall has been used in a minority of cases for rapid transfusion. Strategies that are important to preclude hyperkalemic cardiac arrest include infusing blood stored less than 7 days old (see Wake Up Safe; http://www.wakeupsafe.org/Hyperkalemia_statement.pdf?201501300915 ; accessed January 7, 2015) and (most importantly) avoiding hypovolemia. If only older blood is available, it should be warmed and administered slowly through a peripheral IV line to reduce the risk that hyperkalemia is present when the blood reaches the right atrium. Estimation of ongoing blood loss can be difficult because of the use of large volumes of irrigation fluid and difficulty quantifying blood loss onto surgical drapes and gowns. Invasive arterial blood pressure monitoring and serial blood gas sampling are indicated in this type of surgery (see Fig. 49.11A-D ). A urinary catheter should be inserted to monitor urine output.
Several blood conservation strategies have been proposed for this type of surgery, including preoperative recombinant human erythropoietin, acute normovolemic hemodilution, antifibrinolytics, and induced hypotension (see Chapter 12 for a more detailed discussion). Of primary importance is meticulous surgical technique and attention to hemostasis. Bleeding from the scalp incision may be reduced by infiltration with a dilute (1 : 400,000) epinephrine-containing solution. The use of the reverse Trendelenburg position may help to decrease venous pressure and blood loss from osteotomy sites but may increase the risk of venous air embolism (with a reported frequency of 5%–80%; see later discussion). For this reason, the horizontal position is preferred.
Blood-conserving dual therapy with recombinant human erythropoietin (to optimize preoperative hematocrit) and use of a cell saver has reduced transfusion in children undergoing craniosynostosis repair. Administration of preoperative recombinant human erythropoietin, in combination with elemental iron (4 mg/kg per day orally to a maximum of 200 mg/day for 6 weeks) increases the preoperative hematocrit value and may decrease the need for autologous blood transfusion. If iron stores are at all compromised, iron therapy combined with oral vitamin C (to increase gastrointestinal absorption) should begin 3 weeks before erythropoietin therapy. Currently this technique is rarely used, likely because of high costs as well as the black box warning applied to synthetic erythropoietin drugs related to increased likelihood of major complications in adult populations receiving these drugs.
Little evidence exists to suggest that autologous blood donation decreases perioperative morbidity in craniosynostosis surgery. While infants as young as 3 months have predonated, this technique is of questionable value and should not be pursued in this population. Instead, simpler and more cost-effective techniques should be used such as meticulous surgical attention to hemostasis and administration of antifibrinolytics.
Acute normovolemic hemodilution is a labor-intensive technique in which blood is collected from the child after induction of anesthesia but immediately before surgery and replaced with an appropriate volume of crystalloid or colloid, such as 5% albumin. This technique has been used in combination with other techniques, especially preoperative erythropoietin to reduce transfusion in craniosynostosis surgery. The maximum potential blood savings with this technique is modest at best. Despite some reports of efficacy, owing to the labor-intensive nature of the technique and modest blood savings, this technique is rarely used in craniofacial surgery.
The coagulation profile and clotting factors after fresh frozen plasma (FFP) or 5% albumin during craniofacial surgery have been compared in a nonrandomized study in infants younger than 12 months of age. The increases in activated partial thromboplastin time and decreases in the plasma concentration of factors XI and XIII and antithrombin III were less after intraoperative FFP than after 5% albumin. Fibrinogen concentrations remained stable in the FFP-treated group but decreased in the albumin-treated group. A hemostatic resuscitation strategy, similar to that used in massive hemorrhage from trauma, has been applied in pediatric craniofacial surgery. With this approach, blood loss is replaced using red blood cells and FFP in a 1 : 1 ratio. The central tenet of this approach is to prevent dilutional coagulopathy (see also Chapter 12 ). At one center where blood loss frequently exceeds a blood volume, FFP and packed red blood cells from the same blood donors were used for blood loss replacement; this technique essentially eliminated coagulopathy, resulted in fewer perioperative blood donor exposures, and is most likely to be useful where blood loss is expected to approach or exceed the circulating blood volume. Recombinant factor VIIa has been used successfully for intractable hemorrhage during cranial vault reconstruction in an infant, although this was an extreme circumstance in an isolated case.
Antifibrinolytic therapy may decrease blood loss during craniosynostosis repair in children. Tranexamic acid reduces blood loss and transfusions in pediatric craniofacial surgery ; the recommended dosing regimen is a loading dose of 10 mg/kg followed by a continuous infusion of 5 mg/kg per hour. The incidence of adverse events, including seizures and thromboembolic events, in children treated with or without antifibrinolytics during craniofacial reconstructive surgery was similar.
Another antifibrinolytic, ε-aminocaproic acid (EACA), is effective in reducing bleeding in cardiac and spinal procedures. In observational studies, EACA has been associated with reduced blood loss and transfusion in craniosynostosis surgery; these findings have yet to be confirmed in a controlled study. The dosing for infants undergoing craniofacial surgery is a loading dose of 100 mg/kg followed by 40 mg/kg per hour. A recent single-center prospective study of 120 infants undergoing craniosynostosis repair used thromboelastography and the platelet fibrinogen product to guide the administration of blood products. Using multivariate analysis and receiver operating curves to assess four parameters: K-time >2 : 1, MA <55 mm, α-angle <62 degrees, and the platelet-fibrinogen product <343, infants with all four predictors had a 92% probability of a blood loss of 60 mL/kg or more, whereas those with none of these parameters had an approximately 10% probability.
Induced hypotension, defined as a 10% to 20% reduction in the mean arterial blood pressure, may decrease intraoperative surgical blood loss and operating time, although studies demonstrating its effectiveness and safety during craniosynostosis surgery are lacking. The lower limits of safe blood pressure reduction in infants are unknown. A variety of pharmacologic agents have been used to induce hypotension, including inhalational agents, vasodilators, β-blockers, and remifentanil. Invasive arterial pressure monitoring is essential whenever hypotensive anesthesia is used. Induced hypotension should be used with great caution in the presence of increased ICP because of the risk of compromising cerebral perfusion pressure (i.e., the difference between mean arterial pressure [MAP] and either ICP or central venous pressure, whichever is greater). The risks of inadequate cerebral and end-organ oxygen delivery during hypotension are magnified by coexistent anemia. It is considered prudent to maintain normovolemia and normocapnia when induced hypotension is used (see Chapter 12 ). Many practitioners have determined that the potential benefits (reduction of blood loss, shorter surgery) of this technique are outweighed by the risks (cerebral ischemia and irreversible brain injury, blindness, end-organ damage). Rather than an approach of induced hypotension, some use a strategy of deliberate normotension, where the anesthetic is tailored to avoid blood pressures greater than baseline.
Whether used alone or in combination, the preceding techniques seldom obviate the need for any blood transfusions during craniofacial surgery. In a retrospective review of 60 children who underwent craniofacial surgery at a single institution, half of the children required fewer transfusions and had a reduced length of stay that were attributed to the preoperative use of iron and erythropoietin, the use of a blood-recycling device intraoperatively, and a reduced minimum hemoglobin concentration for transfusion (<7 mg/dL) compared with a placebo group. Given the potential for acute large-volume blood loss IV access with large-bore catheters remains essential and at least 2 units of packed red blood cells (PRBCs) should be crossmatched and available in the operating room at all times. IV fluids should be administered via a fluid warmer to prevent hypothermia. Maintaining normothermia preserves coagulation function and theoretically reduces bleeding and transfusion-related complications. Based on adult data, coagulopathy from dilution of soluble clotting factors on average develops when 142% of the circulating blood volume has been lost and replaced with PRBCs and crystalloid, and with thrombocytopenia developing after an average of 2.3 blood volumes were lost (see also Chapter 12 ). Estimating the blood loss based on the number of blood volumes of blood products administered, physical estimates of blood loss, and an assessment of hemostasis in the surgical field are useful empiric and clinical indicators for the need for hemostatic blood product administration. Serial determinations of the international normalized ratio prothrombin time (INR), partial thromboplastin time (PTT), platelet count, and fibrinogen concentration and the use of thromboelastography help to identify coagulopathy and guide hemostatic blood product administration.
Endoscopic repair of craniosynostosis has become a rapidly growing surgical approach to reduce bleeding and decrease morbidity. Independent risk factors for bleeding during endoscopic strip craniectomy include low body weight (<5 kg), sagittal suture surgery (related to proximity to the sagittal venous sinus), syndromic craniosynostosis, and earlier date of surgery.
Hyponatremia and cerebral salt-wasting syndrome are associated with craniosynostosis repair. Both intraoperative and postoperative hyponatremia have been described, with the latter occurring in approximately 30% of children. In a retrospective review of a cleft palate and craniofacial database, postoperative hyponatremia was associated with preoperative increased ICP, blood loss, and female gender with normal preoperative ICP. The average reduction in sodium concentration was more pronounced in children who received hyponatremic (hypotonic) (5% dextrose and 0.2% or 0.5% NaCl) compared with normonatremic (isotonic) postoperative IV fluids. The perioperative use of balanced salt solutions is recommended to prevent hyponatremia (see also Chapter 9 ).
Early surgery for craniosynostosis is often indicated to prevent increases in ICP. One-third of children with craniofacial dysostosis syndrome and 15% to 20% of children with single-suture craniosynostosis have increased ICP (>15 mm Hg). Approximately 40% to 50% of children with syndromic craniosynostosis have associated hydrocephalus, although differentiation from nonprogressive ventriculomegaly may be difficult. Timing of surgery may affect neurocognitive development and intelligence because these are adversely affected by sustained increased ICP. Associated OSA resulting in hypoxemia and hypercapnia may lead to an increase in cerebral blood volume and thereby exacerbate intracranial hypertension. Untreated intracranial hypertension may lead to optic atrophy and visual impairment. As a consequence, when increased ICP has been identified either preoperatively or postoperatively, placement of a ventriculoperitoneal shunt should be considered ; this occurs more commonly in Crouzon and Pfeiffer syndromes.
For children who present with signs of intracranial hypertension, it is important to follow basic principles of neuroanesthesia to prevent further increases in ICP and decreases in cerebral perfusion pressure (see also Chapter 26 ). It may be prudent to use protective measures to attenuate the hypertensive response to laryngoscopy and intubation, including the administration of a short-acting opioid, a β-blocker, or topical local anesthesia to the upper airway. Intraoperatively, the anesthesiologist faces numerous challenges to control ICP. Strategies to control ICP include mild to moderate hyperventilation (end-tidal carbon dioxide [ etco 2 ] of 30 to 35 mm Hg), especially when signs of herniation are evident; avoidance of hypervolemia; and, where indicated, appropriate use of hypertonic (3%) saline solution, mannitol, furosemide, and dexamethasone to reduce ICP, reduce brain volume, and facilitate brain retraction. Although cranial vault reconstruction increases intracranial volume and reduces ICP, children remain at risk of increased ICP after surgery and require close ophthalmologic and clinical follow-up, even after a cosmetically successful cranial expansion.
Venous air embolism (VAE) may occur during any operative procedure in which the operative site is above the level of the heart and noncollapsible veins are exposed to atmospheric pressure. The incidence of VAE in children undergoing craniectomy for craniosynostosis repair has been reported to be as great as 83%, although hemodynamically significant VAE is rare. The incidence associated with endoscopic craniectomy may be as small as 2%. Significant hypovolemia resulting from surgical blood loss can lead to a decrease in both systemic and central venous pressures and the development of a pressure gradient between the right atrium and the surgical site. This gradient increases the potential to entrain air via open dural sinuses or bony venous sinusoids. If the entrained volume of air is sufficiently large, right ventricular outflow obstruction may ensue, causing acute right-sided heart failure and cardiovascular collapse. Smaller volumes of air may cause a reduction in cardiac output, hypotension, and myocardial or cerebral ischemia. Transesophageal echocardiography (documenting the presence of air in the right ventricular outflow tract), precordial Doppler ultrasonography (continuous windmill murmur), end-tidal carbon dioxide (precipitous decrease in carbon dioxide tension), and nitrogen monitoring (sudden increase in nitrogen concentration in the exhaled breath) have been used to identify VAE with different sensitivities, well before cardiovascular collapse occurs (see Figs. 26.6 and 26.7 ). Applying bone wax to the open edges of cut bone, reducing the degree of or avoiding the reverse Trendelenburg position, maintaining positive-pressure ventilation with 5 cm of PEEP, and ensuring normovolemia help to prevent VAE. Fluid resuscitation, vasopressors, and aspiration of air from the right side of the heart may prevent episodes of VAE from progressing to cardiovascular collapse.
As with all surgeries that last several hours, preventing the complications associated with prolonged anesthesia is paramount. Nerve palsies, pressure ulcers of the skin, ophthalmic complications, hypothermia, and acidosis may occur. Careful positioning of the extremities, use of an egg-crate–type mattress or foam padding, and avoiding pressure to the eyes, particularly when the surgical procedure requires the prone position, will prevent the majority of these adverse outcomes. In children with proptosis, such as Crouzon syndrome, it may be necessary to suture the eyelids closed after applying lubrication to prevent corneal abrasions. Anterior ischemic optic neuropathy that can cause transient or permanent postoperative blindness is a rare complication that occurs in the absence of external pressure to the eyes.
Hypothermia is another major concern and is a largely preventable complication when the appropriate steps are taken. Factors that predispose to hypothermia include the large surface area exposed during surgery and the potential infusion of large volumes of relatively cold IV fluids. Effective measures to prevent hypothermia include warming the operating room, the use of forced-air warmers and radiant warming lamps, insulating the child, and warming devices for blood and IV fluids. Preventing hypothermia and limiting blood loss and transfusion requirements are key factors in preventing the development of perioperative metabolic disturbance ; even mild hypothermia has been associated with increased blood loss and transfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here