Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Maternofetal exchange across the placenta provides the solutes and water needed for fetal development and growth and enables the waste products of fetal metabolism to be transferred to the maternal circulation.
The placental exchange barrier consists of the syncytiotrophoblast epithelial cell layer, basement membrane and connective tissue, and the fetal capillary endothelium. All contribute to the barrier, but the syncytiotrophoblast is probably the most important locus of regulation of maternofetal exchange.
Driving forces for maternofetal exchange are, depending on the molecule in question, electrochemical gradients or hydrostatic gradients (or both) between maternal and fetal circulations.
The placenta is highly permeable to small lipophilic molecules such as oxygen. These therefore rapidly cross the exchange barrier with the rate of transfer being mainly dependent on uterine and umbilical blood flow.
The placenta has a low permeability to larger hydrophilic molecules. These therefore only diffuse slowly across the placenta with the rate of transfer being more dependent on the properties of the placental barrier rather than blood flow.
Transfer of a hydrophilic molecule is likely to require selective transporter proteins in the plasma membranes of the syncytiotrophoblast (channels or carriers) or, for larger molecules, vesicles enabling endocytosis at one membrane and exocytosis at the other.
Placental dysfunction includes abnormalities in maternofetal exchange and can lead to pathologies, including fetal growth restriction (FGR). Such dysfunction may be stratified into vascular defects with abnormal blood flow or nonvascular defects with abnormalities of the syncytiotrophoblast. Development of new treatments for FGR will need to target these different phenotypes of placental dysfunction.
This chapter summarises current understanding of the mechanisms of maternofetal exchange across the placenta. A full description of these mechanisms would combine details of the physiological processes involved with information about the identities, properties and genetic control of the relevant molecular species. However, there remains much to be learnt before such a comprehensive review is possible. Therefore we describe the key principles required for a full understanding of the maternofetal exchange of any solute and then provide examples of how these apply to selected substances. Finally, we consider the clinical relevance of maternofetal exchange in relation to fetal growth restriction. Additional detailed coverage of aspects of placental transfer which are beyond the scope of this chapter may be found elsewhere.
A variety of species have been used to study placental transport, but considerable care must be taken in extrapolating from these to the human because of the great diversity of placental morphology and function. Animal studies do provide an important foundation for understanding placental function, but here we focus on work on human placenta. A variety of in vitro and in vivo techniques have been used to study the human placenta, and these are considered in detail elsewhere. Complete characterisation of a transport mechanism should broadly include four sets of information: (i) the amount of substance transferred per unit time and per unit surface area, the flux, in both maternofetal and fetomaternal directions, the difference between the two giving the magnitude and direction of the net flux; (ii) the magnitude and factors controlling the driving force for the transfer of a substance (e.g., plasma concentrations along the length of the exchange surface and blood flow); (iii) the route of transfer, for example, whether across the plasma membranes and through the cytosol (transcellular route) or via extracellular water-filled channels (paracellular route); and (iv) the role and contribution of the placenta’s own metabolic processes. Historically, measurement of flux was the focus of attention, but more recently, the focus has shifted to the cellular and molecular aspects of transport with the use of in vitro and molecular techniques. However, there is an ongoing requirement for the physiological relevance of mechanistic components deduced from in vitro techniques to be reconciled with overall flux and accretion. It must also be borne in mind that transport by components of the placenta does not always lead to transfer across the placenta because some of the transport will satisfy the metabolic needs of the placenta itself.
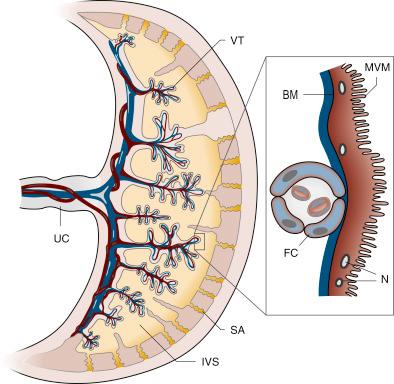
The human placenta is of the haemochorial type so that blood delivered into the intervillous space via the spiral arteries ( Fig. 8.1 ) directly bathes the syncytiotrophoblast lining of the villi (i.e., there is no endothelium separating blood from this epithelium). The syncytiotrophoblast is also unusual in that it a true multinucleated syncytium with no lateral intercellular spaces akin to those found in other epithelia (but see later discussion on paracellular routes) and is usually considered to be the main barrier to exchange. It has two plasma membranes: the microvillous, maternal-facing plasma membrane (MVM) and the fetal-facing basal plasma membrane (BM). Underlying the syncytiotrophoblast, there is an extracellular matrix (ECM) of connective tissue and finally the capillary endothelium bathed in fetal blood. Although ECM will not present a major barrier to most solutes, it is likely that, as in other epithelia, it will create a slow-moving pool of fluid (an ‘unstrirred layer’) that will affect the nature of electrochemical gradients across the syncytiotrophoblast. The fetal capillary endothelium has lateral intercellular spaces through which small molecules can diffuse. However, the diffusion of large proteins, known to cross the placenta, such as immunoglobulin G and alpha-fetoprotein, is restricted through these spaces so that the endothelium is a significant barrier to such molecules.
The different types of mechanisms of exchange across the placenta are summarised in Fig 8.2 .
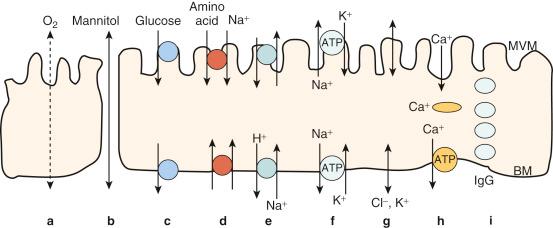
The placental exchange barrier limits solute transfer to varying degrees. Lipophilic substances (e.g., oxygen) that dissolve readily in the plasma membrane will rapidly diffuse across the barrier. On the other hand, for hydrophilic substances (e.g., sodium ions, amino acids), the placenta is a significant barrier to transfer. However, this barrier is not absolute because there are multiple pathways available for hydrophilic solutes to pass across the placenta, including pores, channels, carriers (including cotransporters and exchangers), pumps and vesicles. These can be matched to mechanisms such as filtration (pores), diffusion (pores and channels), facilitated diffusion and secondary active transport (carriers), primary active transport (pumps) and endocytosis and exocytosis (vesicles).
Pores allow for the movement of solute and solvent through a paracellular, extracellular water-filled pathway such that the transferred substances do not have to cross any plasma membranes to traverse a layer of tissue. Pores may allow transfer by diffusion of solute alone or, by bulk flow, of solute and solvent together. The extent of diffusion through pores can be altered by changes in electrochemical gradients and, in the case of bulk flow, by changes in plasma hydrostatic and osmotic pressures. There is clear physiological evidence, both in vivo and in vitro , that transfer through pores does occur in human placentas as well as in the placentas of several other species. Because the syncytiotrophoblast is a true syncytium as described earlier, the morphological correlates of the pores are unclear. However, there is evidence that naturally occurring areas of syncytial denudation, found in all normal placentas, could provide a large pore with contributions from other extracellular fluid-containing routes. This paracellular route of transfer is quantitatively of major importance for the transfer of small hydrophilic solutes such as calcium ions and chloride ions. Of course, the transcellular route through the syncytiotrophoblast, using channels and carriers, is likely to be qualitatively of greater importance, allowing fine tuning of net flux.
Channels are integral membrane proteins through which ions may diffuse down electrochemical gradients either into or out of cells. Although passive, the diffusion of solute through channels is selective, gated and saturable and may be functionally asymmetric. These properties allow cells to modify the extent of inward and outward solute movements caused by diffusion in response to homeostatic signals mediated by intracellular, autocrine, paracrine and endocrine agents and by effects at the genome. This probably allows a range of normal processes and a broad repertoire of reactions to abnormal processes.
Carriers are integral membrane proteins that selectively combine with a solute and can carry it from one side of the membrane to the other. The combining site is only exposed to one side of the membrane at a time. Channels and carriers show different behaviours. In general terms, if a solute is added to the far side (the trans side) of the membrane, then the combining site will be able to return to the near side (the cis side) more quickly than it would otherwise. The combining site will be on the near side more often and will remove solute more frequently. A carrier will thus carry more solute if it is ‘transstimulated’, but a channel will not respond in this way. Some carriers can transport more than one solute at a time. A cotransporter carries two solutes in the same direction; an exchanger swaps solutes. This allows cells to coordinate the movement of disparate solutes.
Pumps carry solutes against concentration gradients. This is called ‘active transport’ because energy (as adenosine triphosphate (ATP)) is used up in the process. A good example is the active extrusion of sodium by cells in exchange for potassium on the Na + /K + -ATPase (sodium pump). This is primary active transport. Carriers can harness the gradients generated by pumps by linking solute movements to sodium movements. This is secondary active transport, which allows cells to move solutes against concentration gradients and thus to control their surroundings more subtly than if diffusion gradients were the only forces present.
Vesicles are formed on one side of an epithelium such as the syncytiotrophoblast by invagination of the plasma membrane and, on the opposite side of the cell, fuse with the plasma membrane and open onto the extracellular space. Solute and water may be taken up into the forming vesicle by simple entrapment (‘fluid-phase’ endocytosis), or solute may be taken up specifically by binding to receptors on the surface of the area of membrane about to vesiculate. Vesicles may move around the cytoplasm randomly by Brownian motion or may be directed by the cytoskeleton.
Most placental exchange is driven by diffusion or modifications of this process. Factors affecting diffusion will thus affect the magnitude of net flux. The rate of diffusion is determined by the concentration gradient across the barrier and the permeability of the barrier and its components. Different substances have different concentration gradients. Permeability varies among solutes according to their size, shape and lipophilicity (permeability to hydrophobic molecules being much greater than that of hydrophilic ones as mentioned earlier). The permeability of the placenta to hydrophilic solutes increases towards term in various animals, although this has not been studied in humans.
A potential difference across the exchange barrier will affect the transfer of charged solutes. Potential differences between mother and fetus have been measured in some species, but it is unclear whether these are generated by the placenta. In humans, a potential difference has been measured in vitro both across the MVM and across the entire exchange barrier of isolated mature intermediate villi derived from term placentas (magnitude ∼4 mV; fetal side, negative.) In vivo , a small maternofetal potential difference was measured in women in the third trimester of similar magnitude to that found in vitro . However, at term, there is reported to be no significant maternofetal potential difference. This question of the magnitude and polarity of potential difference across the placental exchange barrier is difficult to address experimentally in humans but is of fundamental importance in understanding the driving forces for ions and other charged solutes.
The pattern and magnitude of blood flow affects exchange. A hydrophobic substance (e.g., oxygen) crosses the membrane so quickly that it has effectively gone from the maternal side of the placenta as soon as it arrives; the rate-limiting steps of transfer will be the rate at which it arrives and the rate at which it is taken away. The transfer of such substances is said to be ‘flow limited’; if placental blood flow is deranged, oxygen delivery, for example, will be impaired. Furthermore, the pattern of blood flow will affect the efficiency of exchange. If the blood flows are in opposite directions (countercurrent flows), the exchange will be more efficient than if they are in the same direction (concurrent flows). The human placenta is thought to have an intermediate arrangement in efficiency called ‘multivillous pool flow’. A hydrophilic substance, on the other hand (e.g., an amino acid) will have much lower permeability across the placenta, transfer is slow and its concentration in the maternal circulation hardly changes across the exchange barrier. The transfer of such substances is therefore relatively unaffected by blood flow, and their transfer is said to be ‘membrane’ or ‘diffusion’ limited. It is important to understand the distinction between flow- and membrane-limited diffusion when considering the phenotypes of placental dysfunction as related to FGR (see final section of this chapter).
The placenta is a metabolically active organ, which significantly affects the traffic of solutes such as oxygen, amino acids and carbohydrates. Control of placental metabolism, as well as of transport, by hormonal, genetic or intrinsic means, by the mother or fetus, is likely to be of considerable importance.
Finally, it should be noted that although the placenta shares many characteristics with other tissues (e.g., sodium pumps and intracellular signalling apparatus), we can identify features which are less prominent in other organs, and these need to be mentioned as a background to any discussion of function or pathology. First, the supply of some substances is vastly in excess of fetal accretion, but other fluxes are more obviously related to fetal requirements. Second, transfer represents diverse phenomena; some substances (e.g., glucose) are transferred by one or two mechanisms only, and alterations in these mechanisms are relatively easy to detect. Other substances (e.g., sodium) are transferred by many specific mechanisms, none of which is dominant, and alterations in these systems are more difficult to detect. Water flux, which is greater than for any other molecule, appears to be particularly complex. There is evidence in rats that net water flux may be the balance of osmotic flow in the maternofetal direction following active transport of ions and bulk flow in the fetomaternal direction down a hydrostatic pressure gradient. Alteration in water transfer may thus reflect a wide range of changes and is therefore not simple to understand and will not be easy to manipulate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here