Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Q18.1 What are the most important differences between small molecule drugs (such as apremilast and tofacitinib) and biologics? (Pg. 200)
Q18.2 What is the role of phosphodiesterase 4 in inflammatory diseases? (Pg. 200 )
Q18.3 What drugs inhibit phosphodiesterase 4 and for what diseases are these drugs being used for? (Pgs. 200, 201, 202)
Q18.4 What can be expected of apremilast in terms of efficacy for psoriasis and psoriatic arthritis, based on clinical trial results? (Pg. 201x2 )
Q18.5 What is the most common adverse effect experienced by patients on apremilast? (Pgs. 201, 202)
Q18.6 What is the role of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway in inflammatory dermatoses? (Pg. 203)
Q18.7 What drugs inhibit the JAK/STAT pathway and for what diseases are these drugs used? (Pg. 203x6)
Q18.8 What are the Boxed Warnings in the prescribing information for tofacitinib? (Pgs. 204, 205x2)
Q18.9 What laboratory monitoring is recommended for patients taking tofacitinib? (Pg. 206x3)
Q18.10 What other drugs targeting the JAK/STAT pathway are currently in development? (Pg. 207x3)
Adverse events
American College of Rheumatology
Absolute neutrophil count
Adenosine triphosphate
Behçet disease
Body surface area
Cyclic adenosine monophosphate
Complete blood count
Disease-modifying antirheumatic drugs
Eczema Area and Severity Index
US Food and Drug Administration
Gastrointestinal
Hepatitis B virus
Interferon-gamma
Interleukin
Investigators Static Global Assessment
Janus kinase
Major adverse cardiovascular events
Nail Psoriasis Severity Index
Nonsteroidal anti-inflammatory drug(s)
Psoriasis Area and Severity Index
Phosphodiesterase 4
Physicians Global Assessment
Protein kinase A
Palmoplantar Psoriasis Physician Global Assessment
Severity of Alopecia Tool
Synovitis, acne, pustulosis, hyperostosis, osteitis
Scalp Physicians Global Assessment
Signal transducer activator transcription
Tumor necrosis factor-alpha
Since 2000, we have witnessed a large number of approved immunomodulatory therapies for inflammatory diseases. Many of these are biologic medications: injectable or infusable large, high molecular weight proteins. However, a variety of small molecule drugs administered either topically or orally have also been recently developed. New targets of small molecule drugs include phosphodiesterase 4 (PDE4) and the Janus kinase (JAK) family of nonreceptor tyrosine kinases. These approaches show promise in treating various inflammatory cutaneous conditions, including atopic dermatitis (AD), psoriasis, alopecia areata, and vitiligo.
Q18.1 Small molecule drugs are not new; in fact, they make up over 90% of drugs currently prescribed. Their small size allows them to be readily absorbed into the bloodstream after oral or topical administration and subsequently reach many different tissues in the body. At the target cell, unlike larger biologic drugs, these molecules can also easily penetrate the cell membrane to interact with intracellular proteins.
Q18.2 Cyclic adenosine monophosphate (cAMP) was the first identified second messenger of extracellular ligand signals and is a key regulator in an array of cellular actions necessary for the function of nearly every system in the body, with the immune system as no exception. cAMP is synthesized from adenosine triphosphate (ATP) by adenylate cyclase, an enzyme anchored intracellularly on the plasma membrane. Once synthesized, cAMP can activate a multitude of signaling molecules that mediate downstream activities. Specifically, in immune cells, high levels of cAMP activate the protein kinase A (PKA) pathway, which results in the suppressed expression of proinflammatory cytokines , including tumor necrosis factor-alpha (TNF-α), interleukin-17 (IL-17) and interferon-gamma (IFN-γ) and, concomitantly, the production of antiinflammatory mediators, such as interleukin-10 (IL-10). These effects are inhibited by PDE4, the predominant cAMP-degrading enzyme in inflammatory cells. Effects of PDE4 and the mechanism of action of apremilast are depicted in Fig. 18.1 .
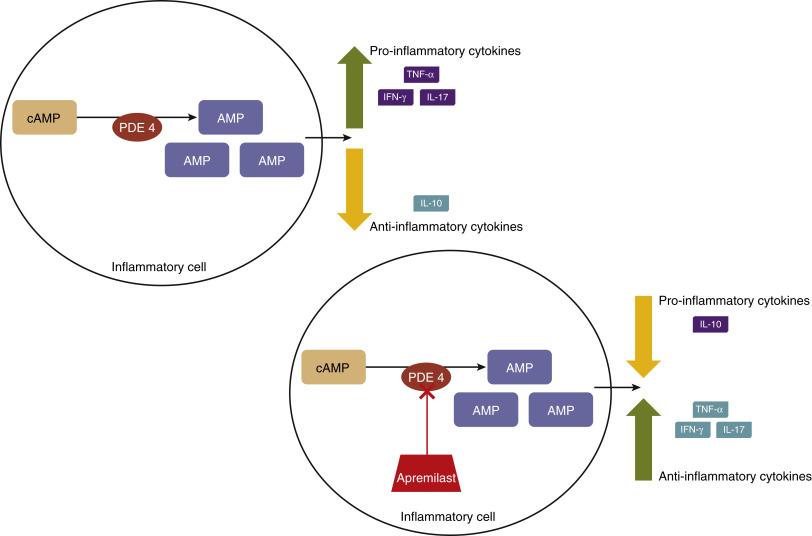
Q18.3 There are several PDE4 inhibitors approved for the treatment of multiple diseases, including depression, chronic obstructive pulmonary disease, psoriasis and AD. When PDE4 is inhibited, cAMP cannot be degraded. Therefore high levels of cAMP are permitted to exert downstream anti-inflammatory effects, via the PKA pathway.
Q18.3 Apremilast was approved by the US Food and Drug Administration (FDA) initially in March 2014 for treatment of active psoriatic arthritis. Several months later in September 2014, its approved indications expanded to include patients with moderate to severe plaque psoriasis for whom phototherapy or other systemic therapy is appropriate. Subset analyses within its phase 3 registrational studies demonstrate efficacy in specifically treating scalp and nail psoriasis, as well as palmoplantar psoriasis associated with moderate-to-severe plaque psoriasis affecting large areas of the rest of the body.
Apremilast is available as 30 mg tablets and as 10 mg, 20 mg, and 30 mg tablets in a titration pack used at the beginning of therapy and that might reduce gastrointestinal (GI) adverse effects (AE). Patients can take apremilast with or without food, but should not alter the tablet in any way including crushing, splitting, or chewing. Patients with creatinine clearance of less than 30 mL/min should only take apremilast 30 mg daily. No dose adjustment is necessary for geriatric patients or those with hepatic impairment. No laboratory monitoring is required for patients on apremilast therapy.
Q18.4 In patients with moderate to severe plaque psoriasis, Psoriasis Area and Severity Index (PASI)-75 was achieved in 28.8% to 39.8% of patients receiving apremilast compared with 5.3% to 11% in patients receiving placebo after 16 weeks of therapy. Patients, taking apremilast in general, have reported improvements in quality of life measures when compared with patients in matched placebo groups.
After 16 weeks of apremilast therapy, psoriasis patients with nail involvement had 22.5% to 29.0% improvement in the Nail Psoriasis Severity Index (NAPSI) score, compared with 6.5% to 7.1% improvement seen for the placebo population. Further, 40.9% to 46.5% of patients with severe scalp psoriasis who took apremilast 30 mg twice daily achieved a Scalp Physicians Global Assessment (ScPGA) of minimal or clear after 16 weeks. Significant improvements in palmoplantar psoriasis were also noted after 16 weeks of apremilast therapy; 46% of patients with a palmoplantar psoriasis physician global assessment (PPPGA) score of 1 or greater, indicating active disease at baseline achieved a PPPGA score of 0 (clear) compared with 25% of patients receiving placebo. It should be noted that this was a secondary analysis of patients in the phase III study of moderate-to-severe psoriasis, and that these patients did not have palmoplantar psoriasis as the primary feature of their disease. A separate study of patients with palmoplantar psoriasis as their primary disease failed to reach statistical significance at its primary endpoint.
Q18.4 In a study of psoriatic arthritis patients, 32.1% to 38.1% of patients on apremilast 30 mg twice daily achieved American College of Rheumatology (ACR)-20 compared with 18.9% to 19.0% of patients receiving placebo. At 52 weeks, an as observed statistical analysis showed that ACR20 was achieved in 54.6% patients receiving the same dosing.
Although not FDA approved for Behçet disease (BD), randomized clinical trials have shown that BD patients, receiving apremilast, had reduction in the number of oral ulcers, genital ulcers, reduction in pain from oral ulcers and improvement in quality of life measures at 12 weeks. In addition, small pilot studies have showed that apremilast may be of benefit in patients with discoid lupus erythematosus and lichen planus. Recent clinical studies have also shown that more patients with active ulcerative colitis, who receive apremilast, reach clinical remission within 12 weeks than do patients receiving placebo.
Individual reports of apremilast efficacy in treating various other disease states have been published as well; these include pityriasis rubra pilaris, vitiligo and SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome.
Apremilast is also being studied for efficacy and safety in many other dermatologic conditions, including frontal fibrosing alopecia, chronic itch, nummular eczema, hidradenitis suppurativa, AD, contact dermatitis, severe acne, alopecia areata, rosacea, prurigo nodularis, cutaneous sarcoidosis, and vulvodynia. The true efficacy of apremilast in treating these conditions is left to be determined. Indications for apremilast are summarized in Box 18.1 .
Psoriasis
Psoriatic arthritis
Behçet disease
SAPHO syndrome
Discoid lupus erythematosus
Lichen planus
Pityriasis rubra pilaris
Vitiligo
SAPHO , Synovitis, acne, pustulosis, hyperostosis, and osteitis.
Apremilast is metabolized in the liver, primarily by cytochrome P-450 (CYP)3A4, but also by CYP1A1 and CYP2A6. Coadministration with potent CYP inducers, including rifampin, phenobarbital, carbamazepine, and phenytoin should be avoided because it may result in decreased apremilast drug levels. A summary of drug interactions that should be considered when prescribing apremilast can be found in Table 18.1 . The apremilast half-life is approximately 6 to 9 hours, necessitating apremilast as twice daily dosing. The drug is excreted primarily through the urine, but a small portion is excreted through the feces.
| Drug Category | Drug Examples | Comments |
|---|---|---|
| Relatively High-Risk Drug Interactions a | ||
| Anticonvulsants | Phenytoin, carbamazepine, others | Strong CYP3A4 inducers; may lower levels of apremilast with loss efficacy, but no major adverse effects |
| Rifamycins | Rifampin, rifabutin | Strong CYP3A4 inducers: lower levels of apremilast with loss of efficacy, but no major adverse effects |
| Braf inhibitors | Dabrafenib | May increase serum concentrations of apremilast |
| --- | --- | Note—Apremilast is a CYP 3A4 substrate; however, primary source lists no major interactions with CYP3A4 inhibitors … would be “cautious” with strong inhibitors, such as selected azoles, macrolides |
| Lower-Risk Drug Interactions | ||
| Biologics | Tocilizumab | Minor reduction apremilast serum concentrations |
| Supplements | St. John’s wort | Strong CYP3A4 inducers: lower levels of apremilast with loss of efficacy, but no major adverse effects |
a Overall highest-risk drug interactions indicated in bold italics (none for apremilast).
Allergy to apremilast or to any of the ingredients in the formulation is the only absolute contraindication to its use. It has not been studied in pregnant or lactating patients or pediatric populations and, therefore risk in these scenarios cannot be ruled out.
Q18.5 The most common AE, occurring in up to 19% of patients, are GI in nature and include diarrhea, nausea and vomiting, and tend to occur during the first few weeks of therapy. The risk of GI AE may be increased in elderly patients and those that use certain medications, such as those that can cause volume loss and dehydration or hypotension. GI AE may be minimized with use of the titration starter pack.
Other AE occurring in greater than 5% of patients include upper respiratory tract infection, rhinorrhea, sneezing, congestion, abdominal pain, tension headache, and headache. Some apremilast patients experience weight loss; 10% to 12% of patients might lose 5% to 10% of baseline body weight, whereas more than10% body weight loss occurs in 2% of patients.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here