Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Individuals vary widely in their responses to therapy with most drugs. Indeed, response to cardiovascular drug therapy, and antiarrhythmics in particular, is so highly variable that studying the underlying mechanisms has elucidated important lessons for understanding variable responses to drug therapy in general.
By disrupting gene product function, single nucleotide changes can produce dramatic changes in physiology; the long QT syndromes and inherited errors of metabolism, such as alkaptonuria, are examples. Indeed, recognition that inborn errors of metabolism arose from defective biotransformation of endogenous substrates led to the suggestion more than a century ago that exogenous substrates (drugs) might similarly be aberrantly metabolized and might produce unusual actions in affected patients. This pharmacogenetic paradigm has been validated by the identification of individual patients and families with defects in the genes encoding specific drug-metabolizing enzymes. The term pharmacogenomics encompasses the idea that variability in drug responses across individuals or populations reflects the combined influences of many DNA variants across individual genomes.
The series of events that occur between administration of a drug and the manifestation of its beneficial or adverse effects include two key steps ( Fig. 53.1 ). First, the drug must be delivered to its molecular site of action (e.g., receptor or ion channel). The magnitude of the effect at the target is determined in part by drug concentration; study of the time dependence of the drug concentration (and metabolites) achieved in plasma, tissue, or other sites, such as urine or bile, is termed pharmacokinetics. The second major process that determines drug action has been termed pharmacodynamics and broadly includes the processes that must occur between the interaction of a drug with a specific molecular target and manifestations of drug action at the molecular, cellular, whole-organ, and whole-patient levels. Because drugs act in a complex (and often abnormal) biologic milieu, considerable intersubject variability in drug effects can arise from pharmacodynamic mechanisms.
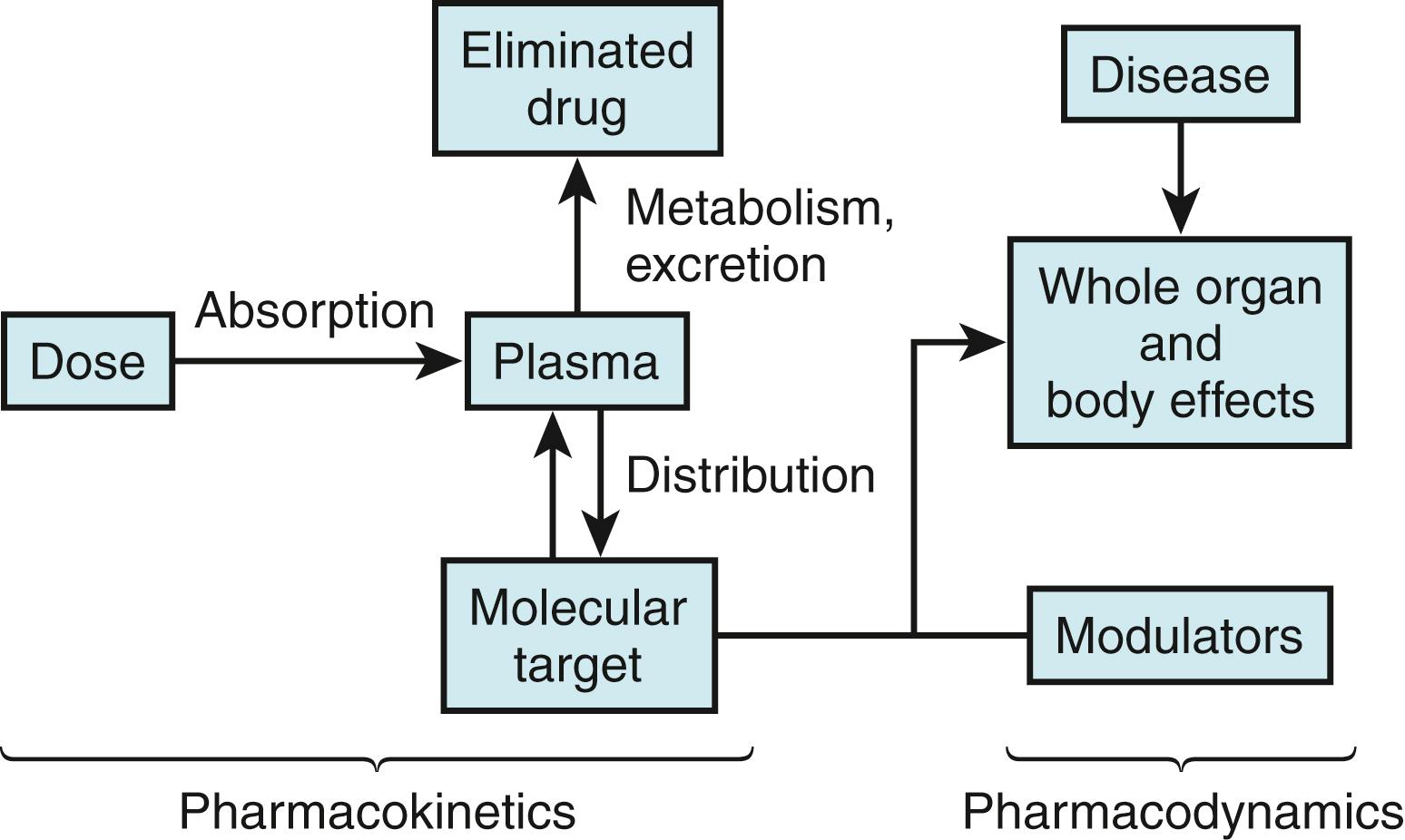
These principles of pharmacokinetics and pharmacodynamics have been recognized for decades, and it is now apparent that they are manifestations of the highly regulated function of individual gene products. Thus metabolism of a drug occurs by the interaction of the drug with specific drug-metabolizing molecules, and the absorption, distribution, and renal and biliary excretion reflect the cellular drug uptake and efflux by specific transporter molecules. The variability in the function and expression of metabolizing and transporting molecules, which are regulated by a host of genetic and environmental factors, determines pharmacokinetic variability. Similarly, variability in the biologic milieu in which drugs act can be conceptualized as variability in the function of multiple molecules, including the target molecules with which drugs interact to produce their beneficial and adverse effects.
Some DNA variants are rare, cause specific “monogenic” diseases, and have conventionally been termed mutations. More common variants are known as polymorphisms and may or may not alter function or expression of the encoded protein. As we begin to understand that each human harbors millions of DNA variants , —some common and some extremely rare—the distinction between “mutation” and “rare variant” becomes increasingly unclear, and more generic language, such as rare and common polymorphisms, is being adopted. One critical aspect of modern genomics is that DNA tends to be highly ancestry specific. Variants implicated in traits such as variable drug responses in one ethnic group may be absent in another, or different variants in the same gene may also contribute.
A change in a single nucleotide, known as a single-nucleotide polymorphism (SNP), is the most common type of DNA variant. Others include nucleotide insertions or deletions (indels) and duplication or deletion of large stretches of DNA, termed copy number variations (CNVs). Only about 1% of the genome is protein coding (this subset of DNA is termed the exome ), and protein function can be altered if a polymorphism results in a change in primary amino acid sequence (a nonsynonymous polymorphism ). To generate a protein, DNA is copied (transcribed) to pre-mRNA, from which noncoding sequence is then removed by RNA splicing; variants in noncoding regions can alter splicing and thus mRNA and protein sequence. In addition, noncoding variants can alter protein abundance through multiple mechanisms (e.g., by changing mRNA stability or, more commonly, by regulating the rate of mRNA transcription). Such regulation can arise because of polymorphisms in the promoter (the region that directly controls gene transcription, often directly upstream of exon 1) or in more distant genomic regions. One emerging view is that polymorphisms may be physiologically silent until an environmental stressor is superimposed; drug administration is one obvious example, and others include adrenergic stress or acute myocardial ischemia.
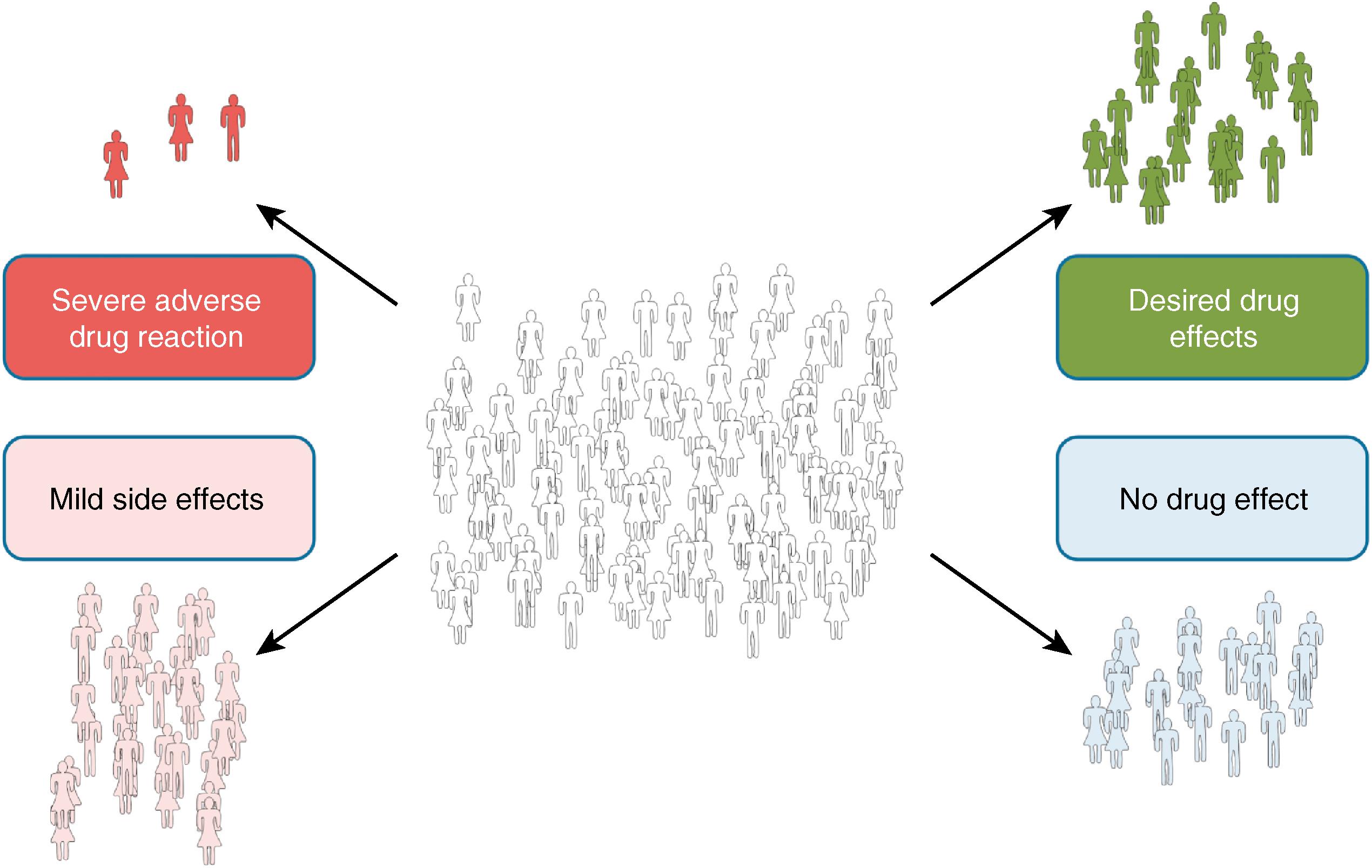
Drugs display variability in both efficacy and toxicity ( Fig. 53.2 ), and pharmacogenomic experiments have addressed both types of responses. Drug efficacy often reflects the combined effects of multiple pharmacokinetic and pharmacodynamic determinants; therefore identifying polymorphisms with large effect sizes contributing to efficacy has been challenging. Similarly, some drug toxicities reflect an extension of the biology of efficacy (e.g., excessive ventricular rate slowing with atrioventricular [AV] nodal blocking drugs), and thus the experimental challenges are similar. Other toxicities, however, are not predicted by what is known about the efficacy of a drug and seem to occur in a relatively unpredictable (often termed idiosyncratic ) fashion; examples include skin rashes, statin-related myopathy, and drug-induced arrhythmias. There really are no idiosyncratic drug reactions; a challenge to contemporary clinical pharmacology has been to define the underlying mechanisms. In some cases, single DNA variants with relatively large effect sizes have been identified.
Proving that a DNA variant contributes to a specific clinical phenotype (such as an unusual drug response) requires compelling statistical arguments and replication in multiple data sets ; a demonstration that a variant produces altered biologic properties in vitro can also serve as a supporting argument. The rapid proliferation of polymorphism databases has led to a very large number of false-positive associations between polymorphisms and variable human phenotypes, but these associations are often not reproduced.
Associating genetic variants with clinical phenotypes, including drug response, in humans has taken one of two broad approaches. The first is predicated on a perceived understanding of the fundamental physiology, pathophysiology, or pharmacology of the phenotype under study; this is termed a candidate gene approach. The second takes advantage of emerging high-throughput technologies by genotyping or sequencing large DNA regions (up to whole exomes and genomes) to then determine whether there is an association between any locus interrogated and the phenotype under study. To date, the most widely used method in this unbiased or hypothesis-free approach is the genome-wide association study (GWAS) paradigm. One clear emerging lesson of these genetic association studies is that any result requires further validation both by replication and by further experiments to test the underlying biology.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here