Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The contributions to this chapter by Dr. Peter Pytel, Department of Pathology, University of Chicago, in several previous editions of this book are gratefully acknowledged.
The peripheral nerves and skeletal muscles permit purposeful movement and provide the brain with sensory information about our surroundings. Both the anatomic distribution of lesions and their associated signs and symptoms are helpful in classifying neuromuscular diseases. The following discussion of neuromuscular disorders is organized along anatomic lines from proximal peripheral nerves to distal neuromuscular junctions and skeletal muscle.
The two major functional elements of peripheral nerves are axonal processes and their myelin sheaths, which are made by Schwann cells. Axonal diameter and myelin thickness correlate with each other and with the conduction velocity of electrical impulses along the nerve. These characteristics distinguish different types of axons, which mediate distinct sensory inputs and motor function. Light touch, for example, is transmitted by thickly myelinated large-diameter axons with fast conduction velocities, whereas temperature sensation is transmitted by slow, lightly myelinated or unmyelinated thin axons. In the case of myelinated axons, one Schwann cell makes and maintains exactly one myelin segment, or internode, along a single axon ( Fig. 20.1 A ). Adjacent internodes are separated by the nodes of Ranvier along which saltatory conduction occurs. Any given nerve contains axons of different sizes and axons serving different functions. These are arranged in fascicles ensheathed by a layer of perineurial cells. Perineurial cells form a barrier between endoneurium on the inside of the fascicle and epineurium on the outside.
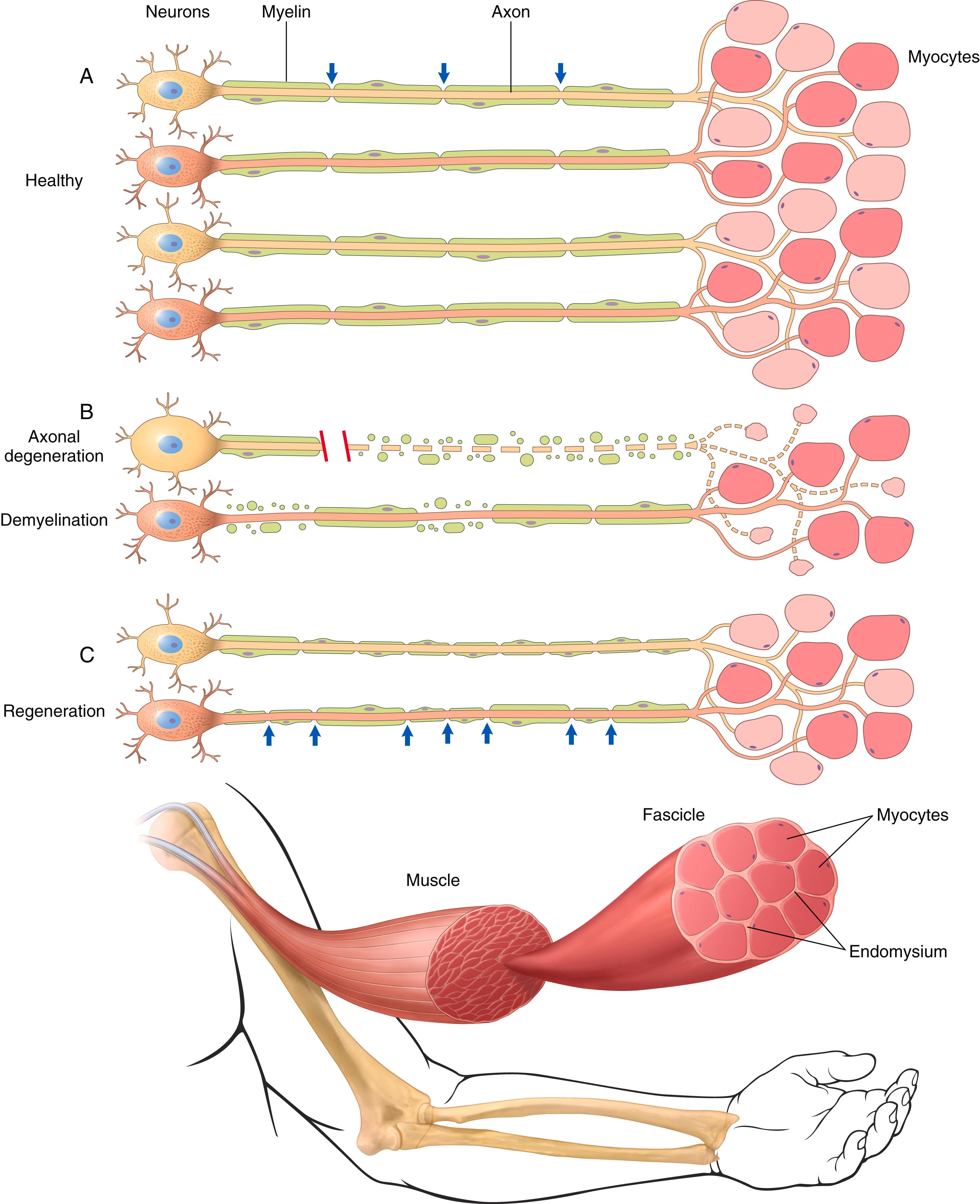
Peripheral neuropathies are often subclassified as axonal or demyelinating, even though many diseases exhibit mixed features. Axonal neuropathies are caused by insults that directly injure the axon. The entire distal portion of an affected axon degenerates (called Wallerian degeneration ). Axonal degeneration is associated with secondary myelin loss ( Fig. 20.1 B). Regeneration takes place through axonal regrowth and subsequent remyelination of the distal axon ( Fig. 20.1 C). The morphologic hallmark of axonal neuropathies is a decrease in the density of axons, which in electrophysiologic studies correlates with a decrease in the signal amplitude of nerve impulses.
Demyelinating neuropathies are characterized by damage to Schwann cells or myelin and relative axonal sparing, resulting in abnormally slow nerve conduction velocity but preserved amplitude. Demyelination may occur discontinuously, affecting individual internodes along the length of an axon in a random distribution. This process is termed segmental demyelination (see Fig. 20.1 B). Morphologically, demyelinating neuropathies show a relatively normal density of axons and features of segmental demyelination and repair. This is recognized by the presence of axons with abnormally thin myelin sheaths and short internodes (see Fig. 20.1 C).
Many different diseases may be associated with peripheral neuropathy ( Table 20.1 ). We next discuss selected entities that are prototypical for a specific type of polyneuropathy or that are particularly common.
| Etiologic Category | Causative Disorders/Agents |
|---|---|
| Nutritional and metabolic | Diabetes |
| Uremia | |
| Vitamin deficiencies—thiamine, vitamin B 6 , vitamin B 12 | |
| Toxic | Drugs (e.g., vinblastine, vincristine, paclitaxel, cisplatin, oxaliplatin, bortezomib, colchicine, isoniazid) |
| Toxins (e.g., alcohol, lead, aluminum, arsenic, mercury, acrylamide) | |
| Vasculopathic, infiltrative | Vasculitis |
| Amyloidosis, sarcoidosis, lymphoma | |
| Inflammatory | Autoimmune diseases such as lupus, Sjögren, mixed connective tissue disorder |
| Guillain-Barré syndrome | |
| Chronic inflammatory demyelinating polyneuropathy (CIDP) | |
| Infections | Herpes zoster |
| Leprosy | |
| HIV | |
| Lyme disease | |
| Inherited | Charcot-Marie-Tooth neuropathy, type I, type II, and X-linked |
| Others | Paraneoplastic, some leukodystrophies |
Diabetes is the most common cause of peripheral neuropathy, usually developing with long-standing disease. Up to 80% of those who have had the disease for more than 15 years show evidence of peripheral neuropathy. Diabetic neuropathies include several forms that can occur singly or together.
Distal symmetric sensorimotor polyneuropathy is the most common form of diabetic neuropathy. Sensory axons are more severely affected than motor axons, resulting in a clinical presentation dominated by paresthesias and numbness. This form of diabetic polyneuropathy exhibits features of both axonal and demyelinating injury. The pathogenesis of diabetic neuropathy is complex and not completely understood; accumulation of advanced glycosylation end products due to hyperglycemia, increased levels of reactive oxygen species, microvascular changes, and changes in axonal metabolism have all been implicated. Strict glycemic control is the best form of therapy.
Autonomic neuropathy is characterized by orthostatic hypotension and changes in bowel, bladder, cardiac, and/or sexual function.
Lumbosacral radiculopathy (diabetic amyotrophy) usually manifests with asymmetric pain, numbness, weakness, and muscle atrophy that typically starts in one lower extremity and may spread to the other.
Guillain-Barré syndrome is a rapidly progressive acute demyelinating disorder affecting motor axons, resulting in ascending weakness. Symptoms typically progress over a period of 2 weeks and by 4 weeks after the onset, vast majority of patients have reached the nadir of the disease. It is one of the most common life-threatening diseases of the peripheral nervous system. About two-thirds of Guillain-Barré syndrome cases are triggered by an infection that provokes the generation of microbe-specific T cells and antibodies, which then cross-react with antigens in the nerve sheath. Although both T cell–mediated and antibody-mediated responses are involved, the former are believed to play a dominant role. Associated infectious agents include Campylobacter jejuni, Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus (HIV), Zika virus, and most recently SARS-CoV-2. The injury is most extensive in the nerve roots and proximal nerve segments and is associated with mononuclear cell infiltrates rich in macrophages. Treatments include plasmapheresis (to remove offending antibodies), intravenous immunoglobulin infusions (which suppress immune responses through unclear mechanisms), and supportive care, such as ventilatory support. Patients who survive the initial acute phase of the disease usually recover with time.
CIDP is an inflammatory peripheral neuropathy, characterized by symmetrical mixed sensorimotor polyneuropathy that progresses for 2 months or more. Both motor and sensory abnormalities are common, such as weakness, difficulty in walking, numbness, pain, and tingling. Like Guillain-Barré syndrome, CIDP is immune mediated, but unlike Guillain-Barré syndrome it follows a chronic, relapsing-remitting, or progressive course. It occurs with increased frequency in patients with paraproteinemias, lymphoid neoplasms, and HIV infection. The peripheral nerves show segments of demyelination and remyelination.
There are diverse other causes of peripheral neuropathy (see Table 20.1 ), some of which merit brief discussion.
Drugs and environmental toxins , such as alcohol, various drugs used in cancer chemotherapy (e.g., taxanes, platinum), and arsenic, that interfere with axonal transport or cytoskeletal function produce peripheral neuropathies. The longest axons are most susceptible; hence, symptoms appear first and are most pronounced in the distal extremities.
Systemic vasculitis. Peripheral nerves are damaged in different forms of systemic vasculitis ( Chapter 8 ), including polyarteritis nodosa, cryoglobulinemia, and eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome). Overall, peripheral nerve damage is seen in about one-third of patients with vasculitis at the time of presentation. The most common clinical picture is that of a painful asymmetric mixed sensory and motor peripheral neuropathy that randomly affects individual nerves. Patchy involvement is also apparent at the microscopic level, as single nerves may show considerable interfascicular variation in axonal damage.
Inherited diseases of peripheral nerves are a heterogeneous but relatively common group of disorders. Hereditary motor and sensory neuropathies, sometimes included under the umbrella of Charcot-Marie-Tooth disease, are by far the most common inherited peripheral neuropathies, affecting up to 1 in 2500 people. They can be demyelinating or axonal. Most manifest in adulthood and follow a slowly progressive course that may mimic that of acquired polyneuropathies. The most common causes are mutations in the genes encoding myelin-associated proteins.
Amyloid neuropathies are caused by deposition of amyloid fibrils in the peripheral nerves. Light chain amyloidosis (in the setting of multiple myeloma or monoclonal gammopathy of uncertain significance) and familial transthyretin–related amyloidosis are the most common types of amyloid neuropathy. They present with progressive weakness, numbness, and neuropathic pain, with one of their characteristics being progressive autonomic manifestations, such as orthostatic hypotension, early in the course of the disease. Treatment of light chain amyloidosis includes chemotherapy to eradicate the plasma cell clones that secrete the pathogenic light chains and sometimes autologous stem cell transplantation. Liver transplantation (to eradicate the source of mutant transthyretin) has been used for the treatment of familial transthyretin related amyloidosis for 3 decades, but more recently silencing the transthyretin gene expression through gene therapy methods has become the first line of disease-modifying treatment. Several drugs that stabilize transthyretin to prevent its aggregation are also available.
Between 30% and 40% of neuropathies are labeled idiopathic (or cryptogenic) after a workup fails to reveal a cause. Most occur in older adults (more than 55 years old) and present as slowly progressive, length-dependent, painful axonal neuropathy. Some cases of idiopathic neuropathy have been attributed to prediabetes and metabolic syndrome (a constellation of dysglycemia, hypertension, hyperlipidemia, and obesity). Treatment mainly consists of management of neuropathic pain with topical products, antiepileptics, antidepressants, and analgesics.
The neuromuscular junction is a complex, specialized structure located at the interface of motor nerve axons and skeletal muscle that serves to control muscle contraction. Here, the distal ends of peripheral motor nerves branch into small processes that terminate in bulbous synaptic boutons. Nerve impulses depolarize the presynaptic membrane, stimulating calcium influx and the release of acetylcholine into the synaptic cleft. Acetylcholine diffuses across the synaptic cleft to bind its receptor on the postsynaptic membrane, leading to depolarization of the myofiber and contraction through electromechanical coupling. Disorders of the neuromuscular junction often result in structural changes in the neuromuscular junction but may also produce functional deficits without any significant visible morphologic alterations. Considered in this section are some of the more common or pathogenically interesting disorders that disrupt the transmission of signals across the neuromuscular junction.
Myasthenia gravis is an autoimmune disease with fluctuating muscle weakness that is caused by autoantibodies that target the neuromuscular junction. About 85% of patients with generalized myasthenia have autoantibodies against postsynaptic acetylcholine receptor (AChR), while most of the remaining patients have antibodies against sarcolemmal muscle-specific tyrosine kinase (MuSK). We focus here on the more common anti-AChR associated form, which has a prevalence of 150 to 200 per 1 million. There is a bimodal age distribution: early onset with a peak in the second and third decades (female predominance) and a late onset in the sixth to eighth decade (male predominance). Thymic abnormalities are common and take two forms: (1) thymic hyperplasia, actually a condition marked by the presence of reactive B-cell follicles (60% to 70% of cases); and (2) thymoma, a neoplasm of thymic epithelium (10% to 15% of cases) ( Chapter 10 ). Both are believed to perturb tolerance to self antigens, setting the stage for the generation of anti-AChR antibodies that damage the postsynaptic membrane. AChR autoantibodies are found in 85% of patients with generalized and 50% with ocular myasthenia gravis (see below). Autoantibodies to AChR are classified as binding, blocking, or modulating. Binding antibodies cause complement activation that damages the neuromuscular junction and destroys the AChR. Blocking antibodies prevent the binding of ACh to the AChR. Modulating antibodies cross-link the receptor subunits, resulting in internalization, and are associated with myasthenia gravis due to thymoma.
Clinically, myasthenia gravis frequently manifests with ptosis (drooping eyelids) or diplopia (double vision) because of weakness in the extraocular muscles. This pattern of weakness is distinctly different from that of most primary myopathic diseases, in which there is relative sparing of facial and extraocular muscles. There are two clinical forms of myasthenia gravis: ocular and generalized. In some patients, symptoms are confined to ocular muscles, while others have both ocular and generalized weakness, including weakness of the bulbar and respiratory muscles, which may necessitate mechanical ventilation. The severity of the weakness typically fluctuates, sometimes over periods of a few minutes. Characteristically, repetitive nerve stimulation results in a decrease in the amplitude of the response. On the other hand, cholinesterase inhibitors improve strength by increasing the concentration of acetylcholine in the synaptic cleft. Effective treatments besides cholinesterase inhibitors include steroids, other immunosuppressants, complement pathway inhibitors, intravenous immunoglobulin, plasmapheresis, and, in selected patients, thymectomy. The prognosis of myasthenia gravis has significantly improved with these advancements in treatment, with most of the patients having a normal lifespan. However, about 10% of myasthenic cases are treatment refractory, and some patients still succumb to complications of the disease such as respiratory failure.
Lambert-Eaton syndrome is caused by autoantibodies that inhibit the function of presynaptic calcium channels, thereby reducing the release of acetylcholine into the synaptic cleft. Patients with Lambert-Eaton syndrome experience limb and sometimes generalized muscle weakness, and there is improvement in weakness with brief muscle contraction or high-frequency repetitive nerve stimulation, which result in buildup of sufficient intracellular calcium to facilitate acetylcholine release. In about two-thirds of patients, Lambert-Eaton syndrome arises as a paraneoplastic disorder, particularly in patients with small cell lung carcinoma; in the others it is a primary autoimmune disease. Symptomatic treatment of Lambert-Eaton syndrome includes agents that block the presynaptic potassium channel, which increases the duration of action potentials in the presynaptic membrane. Unlike myasthenia gravis, cholinesterase inhibitors are not effective. Other forms of therapy include treatment of any underlying cancer and plasmapheresis or immunosuppression, which lower the concentration of the causative antibodies. The prognosis is worse than that of myasthenia gravis because of the frequent coexistence of an aggressive malignancy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here