Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Postoperative febrile morbidity is related to infection in approximately 20% of cases and noninfectious causes in 80% of cases.
Infection in older adults will not always present with classic findings. The amount of temperature elevation may not reflect the severity of the infection. Not uncommonly, the first signs of infection in older adults will be mental status changes. Also, the degree of leukocytosis may not reflect infection, being blunted or absent.
Minimum urine output is approximately 0.5 mL/kg per hour. The use of a 20-mL/hour benchmark for all women is only an approximation and should be adjusted for the patient’s weight.
Because of the shifts in water balance, the postoperative hemoglobin at 72 hours is a more accurate measurement of operative and postoperative blood loss than a hemoglobin at 24 hours.
After subtracting the effects of the operative blood loss from the preoperative hemoglobin, a further reduction in hemoglobin of 1 to 2 points reflects a postoperative hemorrhage of approximately 500 mL.
Women should be transfused when their hemoglobin falls to less than 7 or sooner if they are symptomatic or have significant cardiac or pulmonary comorbidities.
Microatelectasis is a common occurrence developing during almost all pelvic surgeries and is persistent 24 hours postoperatively in approximately 50% of women. Studies have demonstrated that there is no association between fever and the amount of atelectasis diagnosed radiologically.
Radiographic diagnoses are approximately 60% accurate for bacterial or viral pneumonia in women with laboratory-proved pneumonia.
Rapid loss of 20% of a woman’s blood volume produces mild shock, and a loss of greater than 40% of blood volume results in severe shock.
Approximately 15% to 45% of surgical blood loss is absorbed onto drapes, pads, and other areas. Thus blood levels in the suction bottle are inaccurate markers of total operative blood loss.
Massive blood loss has been defined as hemorrhage that results in replacement of 50% of circulating blood volume in less than 3 hours.
Returning to the operating room to control hemorrhage is often a difficult decision. However, when indicated, this decision should be carried as soon as possible in conjunction with volume replacement.
The extent of wound or pelvic hematomas is determined by the potential size of the compartment into which the bleeding occurs. Retroperitoneal or broad ligament hematomas may contain several units of blood.
Superficial phlebitis is the leading cause of an enigmatic postoperative fever during the third, fourth, or fifth postoperative day.
The clinical management of mild superficial thrombophlebitis includes rest, elevation, and local heat. Moderate to severe superficial thrombophlebitis may be treated with nonsteroidal antiinflammatory agents.
Venous thrombosis and pulmonary embolism are the direct causes of approximately 40% of deaths after gynecologic surgery.
Signs and symptoms of pulmonary emboli are nonspecific; however, the most common symptoms are chest pain, dyspnea, apprehension, tachypnea, rales, and an increase in the second heart sound over the pulmonic area.
Intermittent in-and-out catheterization is preferable to continuous drainage with a Foley catheter for women with intermediate-term voiding dysfunction.
Although symptoms of urinary incontinence may present within a few hours of the operative procedure, most fistulas present 8 to 12 days after operation and occasionally as late as 25 to 30 days after the operation.
If there is a suspicion that trauma to the bladder has occurred during an operative procedure, continuous catheter drainage for 3 to 5 days usually results in spontaneous healing.
Approximately 25% of adult women experience postoperative nausea and vomiting.
Normal return of bowel function after abdominal surgery can take 3 to 7 days. The left colon takes the longest to resume function, approximately 72 hours after surgery. If return of bowel function does not occur by 7 days, a diagnosis of mechanical bowel obstruction or another cause for the ileus should be considered.
Postoperative oral feeding is safe and efficacious. This practice is preferred because it facilitates recovery and shortens hospital stay.
The difference between small bowel obstruction and adynamic ileus is subtle because adynamic ileus can be associated with partial obstruction of the small intestine. The use of diatrizoate (Gastrografin) contrast can be both diagnostic and therapeutic.
Second- and third-generation cephalosporins are the antibiotics associated with the highest risk of developing Clostridium difficile diarrhea.
The incidence of postoperative wound infection is increased eightfold when the woman’s preoperative weight exceeds 200 pounds. The thickness of subcutaneous tissue is the greatest risk factor for wound infection in women undergoing abdominal hysterectomy.
Necrotizing fasciitis involves the subcutaneous tissue and superficial fascia. It rapidly expands in the subcutaneous spaces. This condition is a surgical emergency and patients should have operative debridement as soon as possible.
The incidence of wound dehiscence is approximately 1 in 200 gynecologic operations. Wound infection is found in approximately 50% of women with wound disruption.
The classic feature of an impending wound disruption is the spontaneous passage of copious serosanguineous fluid from the abdominal incision.
Most postoperative pelvic infections are polymicrobial, usually from endogenous vaginal flora, and approximately 60% to 80% involve anaerobic organisms.
Common causes of femoral neuropathy are continuous pressure from self-retaining retractors, particularly in thin women, or exaggerated hip flexion or abduction in the dorsal lithotomy position.
Discharge instructions should be given in verbal and written forms, and the gynecologist should anticipate the most common questions.
Appendixes A, B, and C are available at ExpertConsult.com .
The goal of postoperative care is the restoration of a woman’s normal physiologic and psychological health. The postoperative period includes the time from the end of the procedure in the operating room until the woman has resumed her normal routine and lifestyle.
Postoperative complications may occur at any time; however, early recognition and management will often preclude larger problems. Thus attention to postoperative details cannot be overemphasized. Complications increase the duration of the postoperative stay in the hospital and increase the risk of hospital readmission. Because many procedures are now performed using minimally invasive techniques, patients will usually leave less than or close to 24 hours after surgery, which is often before the signs and symptoms of a complication present. Before discharge, it is important that the patient receive education regarding expectations, signs and symptoms of infection and other complications, and appropriate contact information.
Significant risk factors in any surgical population include underlying cardiac and pulmonary disease, smoking, obesity, prior or current abdominal/thoracic surgery, and type of anesthesia. General caveats of postoperative management emphasize attention to the particular needs of each woman. Studies conflict on whether age alone is an independent risk factor for perioperative morbidity and mortality. Older patients tend to have more underlying disease, placing them at higher risk for perioperative complications. Unfortunately, this alone does not completely account for their worse outcomes. In one large population-based study, even healthy elderly patients continued to have higher morbidity and mortality ( ). It is likely that elderly patients respond differently to perioperative physiologic stressors and pharmacologic interventions. Individualization is especially important in the postoperative care of geriatric women. Special nursing attention and minimal doses of narcotics help prevent confusion and disorientation. Ongoing verbal communications with the nursing staff help eliminate misunderstandings that might result in less than ideal postoperative care.
Surgical stress invokes several physiologic responses meant as the body’s defenses. Many of these responses may be more problematic than the actual surgery. For example, some women will respond to the insulin resistance from surgical trauma with severe hyperglycemia, which is detrimental to healing. Peri- and postoperative management strategies are aimed at minimizing or preventing these adverse effects, such as prevention of thromboembolism, or selective use of beta blockade in older patients to prevent cardiac complications ( Fig. 25.1 ) ( ).
This chapter discusses major issues of management during the period from the end of surgery until the return to normal physiologic and psychological function. However, much of the data regarding postoperative complications only involve the period up through postoperative day 30. Problems and complications arise over the whole spectrum of the postoperative time frame and are interrelated. Thus the clinician must be aware at all times of a woman’s changing status during recovery. For simplicity, this chapter is organized around organ systems and their potential complications.
The exact definition of postoperative febrile morbidity varies greatly among authors. Diurnal fluctuations are characteristic of the normal daily body temperature patterns of humans. Most definitions use a temperature greater than 38° C a day after surgery as the indicator of febrile morbidity. It is not unusual for gynecologic patients to have a mild temperature elevation during the first 72 hours of the postoperative period, especially during the late afternoon or evening. Up to 75% of patients develop a temperature greater than 37° C, which is usually not associated with an infectious process. The incidence of postoperative febrile morbidity after benign hysterectomy ranges from 14% to 16% with as few as 3% having a documented infectious source ( ; ).
Fever is the most common morbidity in the postoperative patient. Common causes of a fever include atelectasis, pneumonia, urinary tract infection (UTI), nonseptic phlebitis, wound infection, and operative site infection. Two intraoperative factors that dramatically increase the risk of postoperative fever are an operative time longer than 2 hours and the necessity for intraoperative transfusion. Increased intraoperative blood loss is associated with a 3.5-fold relative risk (RR; 95% confidence interval [CI], 1.8 to 6.8) of developing a postoperative fever ( ).
The physician’s primary goal in examining the postoperative patient with fever is to determine the cause. Approximately 20% of postoperative fevers are directly related to infection and 80% are related to noninfectious causes ( ). Some conditions necessitate active intervention, whereas others are self-limiting. Thus it is imperative not to treat a postoperatively febrile patient empirically with broad-spectrum antibiotics. Protocols limiting antibiotic use to high-risk patients (bowel operation, preoperative infection, immunodeficiency, indwelling vascular access, mechanical heart valves, or intensive care unit [ICU] admission) or those with persistent fevers greater than 101° F for more than 48 hours have been shown to be safe ( ).
The pathophysiology of postoperative fever is primarily related to the release of cytokines. The cause of a postoperative fever may be simple and common, such as atelectasis or dehydration, or unusual, such as malignant hyperthermia or septicemia. The temporal relationship of the onset of a woman’s febrile response to common postoperative complications is depicted in Table 25.1 .
| Causes | Day | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 Week or Longer | |
| Atelectasis Pneumonia Wound infection Streptococcal or clostridial Other bacterial Ovarian abscess Cuff cellulitis Phlebitis Superficial Deep Urinary tract infection Ureteral or bladder injury Bowel injury |
|||||||
The initial workup for a postoperative fever should focus on the most common problems. Medical students memorize the “five Ws” in the differential diagnosis of a postoperative fever: w ind (atelectasis), w ater (UTI), w ound (infection or hematoma), w alking (superficial or deep vein phlebitis), and w onder drugs (drug-induced fever). The proper workup of a postoperative fever, similar to that of any problem in medicine, involves the three classic steps of history, physical examination, and laboratory evaluations, with major emphasis placed on the physical examination. The physical examination emphasizes the following: examination of the lungs for atelectasis and pneumonia; the wound and operative site for infection or hematoma formation; the costovertebral angles for tenderness, which may suggest pyelonephritis; and superficial veins in the arms for superficial phlebitis and deep veins in the legs for deep vein phlebitis.
The findings of the history and especially the physical examination influence the extent of laboratory tests ordered. The three most commonly ordered laboratory tests are complete blood cell count (CBC), chest radiography, and urinalysis, although a study by Schwandt and colleagues emphasized that chest radiography and urine cultures are best ordered only for specific clinical signs, not as reflex orders ( ). Other common tests include culture and Gram stains of body fluids, including sputum, urine, and blood. One study of more than 300 women who were febrile after hysterectomy did not identify a single positive blood culture, whereas another study found a 9.7% positive blood culture rate in more than 500 patients, suggesting a role for judicious use of blood cultures ( ; ). Women with persistent and undiagnosed fevers may need imaging studies, such as pelvic ultrasound or computed tomography (CT) to detect problems such as compromised ureters, abscesses, or foreign bodies, but routine imaging is likely low yield and may result in indeterminate results because of postoperative changes.
Each major complication will be discussed in detail later in the chapter; however, several specific generalizations concerning the type and characteristics of fever patterns should be emphasized. Fever is a common postoperative finding and rarely is the cause of the fever a serious infection. Atelectasis is thought to be the cause of approximately 90% of fevers occurring in the first 48 hours after operation. Patients who develop fever as a result of an indwelling catheter, such as intravenous (IV) lines or Foley catheters, are afebrile for several days and then experience an abrupt temperature spike. In contrast, wound or pelvic infections, which are usually clinically diagnosed from the fourth to seventh postoperative days, usually are associated with a low-grade fever that begins early in the postoperative period. An empiric trial of IV heparin for 72 hours is often a diagnostic and therapeutic trial for pelvic thrombophlebitis in refractory cases of postoperative fever of unknown origin.
Importantly, infection in the older woman will not always present with classic findings. The amount of temperature elevation may not reflect the severity of the infection. Not uncommonly, the first signs of infection in older adults will be mental status changes. Additionally, the degree of leukocytosis, being blunted or absent may not reflect infection.
A woman with a drug-induced fever feels better and does not look as ill as her temperature course indicates. The tachycardia associated with the elevated temperature is usually much less than usually anticipated with a similar temperature elevation secondary to inflammation or infection. The presence of eosinophilia suggests a drug-induced fever. However, drug fever is rare and is usually a diagnosis of exclusion. Presumptive evidence of a drug-induced fever is established when the fever disappears after discontinuation of the drug. The most commonly implicated drugs include allopurinol, carbamazepine, lamotrigine, phenytoin, sulfasalazine, vancomycin, minocycline, dapsone, and sulfamethoxazole. The risk of developing a drug-induced fever is higher in elderly and patients with human immunodeficiency virus (HIV).
Superficial thrombophlebitis often produces an enigmatic fever. Often there is tenderness at the IV site. IV catheters should be removed at the first sign of tenderness or erythema, but routine replacement (exchange) to prevent thrombophlebitis is not indicated ( ). Transfusion reactions can also cause febrile events. Leukocyte or platelet antibodies usually cause these reactions. As long as a major blood type incompatibility is not found, treatment is usually conservative.
It is common practice to repeat the basic fever workup at regular intervals until the diagnosis is established. The woman should be reexamined and selective laboratory tests reordered. Rare causes of postoperative fever include pulmonary embolism (PE), thyroid storm, and malignant neoplasms. These diagnoses usually present with other signs and symptoms as well as temperature elevation. It is important to consider that fever is a potentially beneficial physiologic response of the patient. Therefore unless the woman is symptomatic secondary to the elevated temperature, it is not necessary to order antipyretic medications. Cellular damage usually occurs when the core temperature exceeds 41° C. Active cutaneous cooling does not reduce core temperature effectively and may have undesirable effects, such as increasing the metabolic rate and activating the autonomic nervous system.
Bleeding is one of the most worrisome postoperative complications. Significant arterial bleeding in the first 24 hours often necessitates reoperation. This complication is discussed later in the chapter, along with the management of shock and pelvic hematoma.
Vital signs should be ordered at frequent intervals during the first 24 hours because changes in vitals are often the first manifestation of hypovolemia. A 4-hour interval for vitals is appropriate for most floor patients. Most women will have sufficient intravascular volume to compensate (during the early phases of hemorrhage) through the redistribution of blood flow from less vital to more vital organs, and as a result, low urine output may be the earliest sign of a decrease in intravascular volume. Thus after an operation, sizable amounts of unrecognized intraperitoneal or retroperitoneal bleeding are sometimes present without the woman having subjective symptoms or appreciable changes in her vital signs. This may be the case particularly in young, healthy women. Typical teaching for appropriate postoperative urine output is at least 0.5 mL/kg per hour; however, many enhanced recovery protocols allow for permissive oliguria to help reduce overuse of IV fluids. There should remain a low threshold to check a CBC or evaluate a patient if oliguria is seen in the postoperative period. A consistent orthostatic decrease in blood pressure of more than 10 mm Hg can indicate a decrease of 20% of the blood volume, and therefore orthostatic vitals can be considered as part of the evaluation for postoperative hypovolemia caused by blood loss. Measuring the hemoglobin at two intervals during the postoperative course is helpful to allow for observation of change over time. It should be noted that a hemoglobin drawn within 24 hours after an operation may not truly reflect postoperative blood loss and a repeat hemoglobin evaluation 48 to 72 hours postoperatively will more accurately reflect the hemoglobin nadir from blood loss.
The normal physiologic response to the stress of the operation and tissue destruction is a release of aldosterone, cortisol, and antidiuretic hormone (ADH). The higher levels of aldosterone seen as a result of surgical stress produce an increase in sodium and water retention, whereas increased levels of ADH promote free water retention. This has been called the ebb phase of postoperative physiology. It is common for women to have notable lower extremity edema for the first few postoperative days as a result of these hormonal shifts and the use of perioperative IV fluids. Depending on the type and amount of intraoperative and postoperative IV fluids, the hemoglobin on the first postoperative day may be misleading and reflect fluid changes rather than intraoperative or postoperative hemorrhage. The hemoglobin from the third postoperative day is a more accurate measurement of postoperative change. If the patient is doing well, stress hormone levels decline, and water retention stops, the patient will begin to experience a brisk diuresis beginning around the third postoperative day. After the effects of the operative blood loss are subtracted from the preoperative hemoglobin, each further reduction in hemoglobin of 1 to 2 points reflects a postoperative hemorrhage of approximately 250 to 500 mL. Historically, patients were transfused to maintain a hemoglobin greater than 10, but mounting evidence supports the practice of more restrictive transfusion parameters with individualized consideration for transfusion. In hemodynamically stable patients who are asymptomatic from anemia, a hemoglobin level less than 7 is usually an indication for transfusion; however, a patient with cardiovascular disease and a hemoglobin level less than 8 should be considered for transfusion. In a patient with a hemoglobin of 8 to 10, transfusion is not indicated unless the patient is symptomatic, there is ongoing bleeding, or there is concern for acute coronary syndrome ( ). The morbidity and mortality associated with a surgical procedure are directly related to the amount of intraoperative and postoperative blood loss and not the corresponding level of preoperative anemia.
Alterations of pulmonary function are an expected physiologic change in women having general anesthesia and operations that enter the peritoneal cavity. Respiratory complications can occur in up to 10% to 30% of patients undergoing major abdominal surgery and can contribute significantly to the morbidity and mortality of surgery. The use of minimally invasive surgery for hysterectomy has resulted in fewer postoperative respiratory complications (<2%), which is attributed to less acute pain from the operative incision ( ). Although postoperative respiratory status is improved, intraoperative ventilation is more challenging for anesthesia providers to manage with insufflation and Trendelenburg positioning.
The term atelectasis is derived from two Greek words that mean “imperfect expansion.” The severity of atelectasis ranges from lack of expansion of a small group of terminal bronchioles and alveoli to complete collapse of a lung. In most patients, atelectasis is the failure to maintain patency of the small pulmonary airways and alveoli. Atelectasis is the most common cause of postoperative temperature elevations. Studies have demonstrated that there is no association between fever and the amount of atelectasis seen radiographically. The incidence of atelectasis depends on the number of predisposing risk factors and the vigor with which the clinical diagnosis is established.
Of all postoperative respiratory complications, 90% are related to atelectasis. The immediate postoperative period is characterized by a decrease in functional residual capacity and lung compliance ( Fig. 25.2 ). Thus the work of breathing is increased. Microatelectasis is most common when small airways (<1 mm in diameter) become blocked by secretions. When small airways remain closed by a combination of mucous plugs and bronchospasm, the gas distal to the obstruction is absorbed. This process results in atelectasis. These changes occur during the first 72 hours after an operation. When atelectasis becomes progressive and involves a large area of lung tissue, there is an associated decrease in oxygen saturation and a decrease in arterial oxygen pressure (Po 2 ). This is associated with a normal to low arterial carbon dioxide pressure (Pco 2 ).
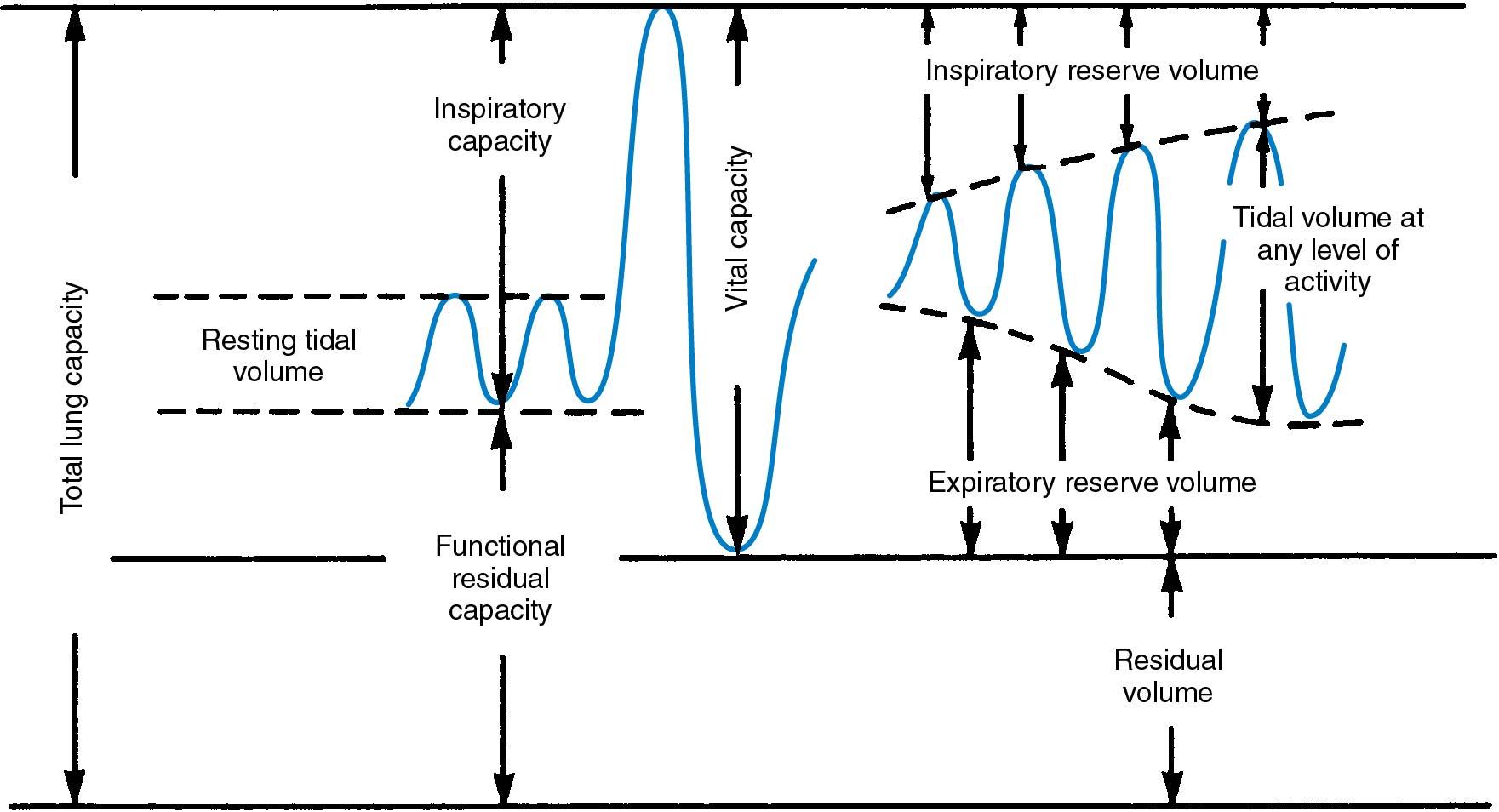
Pulmonary and nonpulmonary factors that favor premature airway closure and development of atelectasis are listed in Box 25.1 . The supine position decreases the functional residual capacity by approximately 20% compared with the erect position; thus early ambulation should be encouraged. Obesity, smoking, age older than 60 years, prolonged operative time, presence of a nasogastric tube, and coexisting medical conditions, such as cardiac or lung disease and pulmonary infection, all predispose women to atelectasis. Insufflation of the abdomen for laparoscopic and robotic surgery also contributes to postoperative atelectasis by collapsing the dependent portion of the lung bases if adequate ventilation pressures are not used.
Supine position
Obesity
Increased abdominal girth (ileus, pneumoperitoneum)
Breathing at low lung volumes
Bindings around the chest and abdomen
Incisional pain
Sedative narcotic drugs
Prolonged effect of paralyzing drugs
Immobility
Excessively high concentrations of oxygen for prolonged periods
Interstitial edema
Loss of surfactant with air space instability
Airway obstruction
Inflammatory with swelling of bronchial and interbronchial tissue
Constriction of bronchial smooth muscle
Retained secretions
In normal breathing, periodic, involuntary, deep inspirations help expand all areas of the lung. Incisional pain, the supine position, narcotics, and abdominal distention contribute to a pattern of monotonous shallow breathing without spontaneous deep sighs in the postoperative period. As a result of the incisional pain, chest wall breathing dominates over abdominal breathing. The resultant decrease in the movement of the diaphragm contributes to the development of atelectasis. A further decrease in functional residual capacity, a decrease in surfactant, and a depression of mucociliary transport, all contribute to ventilation-perfusion ( ![]() ) mismatches and reduced (
) mismatches and reduced ( ![]() ) ratios. The results are gas trapping, atelectasis, and vascular shunting. In most individuals, microatelectasis is patchy and localized to small areas. However, the severity of atelectasis varies and may involve a complete lung. Distribution of pulmonary blood flow is influenced by gravity. A greater proportion of pulmonary blood flows to dependent areas of the lungs in the supine patient. Increased blood flow to dependent areas in combination with atelectasis in dependent areas results in impaired oxygenation and decreased elimination of carbon dioxide. Elevation of the head of the bed in patients with obesity may help improve vital capacity and ventilation, thus decreasing atelectasis.
) ratios. The results are gas trapping, atelectasis, and vascular shunting. In most individuals, microatelectasis is patchy and localized to small areas. However, the severity of atelectasis varies and may involve a complete lung. Distribution of pulmonary blood flow is influenced by gravity. A greater proportion of pulmonary blood flows to dependent areas of the lungs in the supine patient. Increased blood flow to dependent areas in combination with atelectasis in dependent areas results in impaired oxygenation and decreased elimination of carbon dioxide. Elevation of the head of the bed in patients with obesity may help improve vital capacity and ventilation, thus decreasing atelectasis.
Atelectasis may present as the classic triad of fever, tachypnea, and tachycardia developing within the first 72 hours after an operation. On physical examination, decreased breath sounds and inspiratory rales may be heard. These findings are most prominent over the lung bases. If the atelectasis is not reversed with incentive spirometry and deep breathing, there will be an increase in productive cough and leukocytosis. Chest radiographs may demonstrate a patchy infiltrate with elevations of the diaphragm.
Atelectasis usually resolves spontaneously by the third to fifth postoperative day. Nevertheless, major efforts are made to prevent atelectasis, especially in high-risk individuals. Atelectasis prevention is based on the encouragement of uneven ventilation and the production of episodes of prolonged inspiration to increase functional residual capacity. Thus the patient is encouraged to walk, take deep breaths, cough, turn from side to side, remain semierect rather than supine, and use an incentive spirometer regularly. Early mobilization and ambulation have been documented to be as effective as chest physical therapy in the prevention of pulmonary complications. Keeping pain relief to a level at which the woman will be able to cooperate and not have monotonous shallow breathing is also helpful; however, excessive narcotic use can result in respiratory depression. Although neither the use of postoperative continuous positive airway pressure (CPAP) or incentive spirometry has been shown to reduce postoperative pulmonary morbidity, simple bedside incentive spirometry can be used to prevent and reverse atelectasis ( ; ). The primary risk of atelectasis is progression to pneumonia.
Postoperative pneumonia is commonly associated with atelectasis because bacterial infections often begin in collapsed areas of the lungs. Predisposing factors to the development of pneumonia include chronic pulmonary disease, heavy cigarette smoking, alcohol abuse, obesity, advanced age, nasogastric tubes, long operative procedures, gram-negative bacterial infections, postoperative peritonitis, and debilitating illnesses.
The symptoms and signs of pneumonia are fever, cough, dyspnea, tachypnea, and purulent sputum. When pain occurs, it may be felt in the back or chest. The classic physical finding of pneumonia is coarse rales over the infected area. The patient usually has a higher temperature and more systemic toxicity than a woman with atelectasis. Leukocytosis is pronounced in most patients, although it may be delayed or attenuated in older women. Chest radiographs often demonstrate diffuse patchy infiltrates of the lung. Radiographic diagnoses are approximately 60% accurate for bacterial or viral pneumonia in women with laboratory-proven pneumonia. Gram staining of the sputum helps differentiate between bacterial colonization and infection. In cases of pneumonia the smear contains a large number of inflammatory cells with both intracellular and extracellular bacteria.
The management of pneumonia is similar to the management of atelectasis, with the addition of parenteral antibiotics. Antibiotic choice is based on the type of pneumonia diagnosed. Most postoperative patients will fall into one of the following categories: hospital-acquired pneumonia (HAP) occurring 48 hours or more after hospital admission or ventilator-acquired pneumonia (VAP) developing 48 to 72 hours after endotracheal intubation. The concept of health care–associated pneumonia (HCAP) occurring in a nonhospitalized patient with extensive health care contact was added as a category in 2005 and removed in 2016 in favor of a more stringent individual risk assessment and the use of hospital “antibiograms” to help guide treatment in each individual facility ( ). Risk factors for multidrug-resistant pathogens are listed in Box 25.2 . Most patients with HAP and VAP who do not have significant risk factors for multidrug resistance and can be treated with empiric antibiotics using one of the following regimens: (1) cefepime 1–2 g intravenously every 8 hours, (2) piperacillin-tazobactam 4.5 g intravenously every 6 hours, (3) levofloxacin 750 mg intravenously daily, (4) imipenem 500 mg intravenously every 6 hours, (5) meropenem 1 g intravenously every 8 hours, or (6) aztreonam 2 g intravenously every 8 hours. However, in the setting of risk factors for multidrug resistance (see Box 25.2 ), one should choose one of the following: (1) an antipseudomonal cephalosporin (such as cefepime 2 g intravenously every 8 hours or ceftazidime 2 g intravenously every 8 hours), (2) an antipseudomonal carbapenem (such as meropenem 1 g intravenously every 8 hours or imipenem 500 to 1000 mg every 6 hours), or (3) piperacillin-tazobactam 4.5 g intravenously every 6 hours. In patients with severe penicillin allergies who cannot have these regimens, aztreonam 2 g intravenously every 6 to 8 hours should be used. In addition, the provider should add either an antipseudomonal fluoroquinolone (ciprofloxacin or levofloxacin), an aminoglycoside (gentamycin, tobramycin, or amikacin) or one of the polymyxins (Colistin or Polymyxin B). Finally, methicillin-resistant Staphylococcus aureus (MRSA) coverage should be provided with vancomycin (15 to 20 mg/kg intravenously every 8 to 12 hours) or linezolid (600 mg every 12 hours).
Prior intravenous antibiotic use within 90 days
Septic shock at time of VAP
ARDS preceding VAP
Five or more days of hospitalization prior to the occurrence of VAP
Acute renal replacement therapy prior to VAP onset
Prior intravenous antibiotic use within 90 days
Prior intravenous antibiotic use within 90 days
Prior intravenous antibiotic use within 90 days
ARDS , Acute respiratory distress syndrome; HAP, hospital-acquired pneumonia; MDR , multidrug resistant; MRSA , methicillin-resistant Staphylococcus aureus; VAP , ventilator-associated pneumonia.
Postoperative patients can also develop aspiration pneumonia as a result of loss of protective airway reflexes during intubation and extubation, or related to postoperative nausea and vomiting. The most common pathogens in aspiration pneumonia are upper airway pathogens such as Streptococcus pneumoniae, Haemophilus influenza, Staphylococcus aureus, and gram- negative rods. The Infectious Disease Society of America and the American Thoracic Society in 2019 recommended that aspiration pneumonia is best treated with amoxicillin or doxycycline for outpatients with no comorbidities or risk factors for resistant bacteria. Previously, macrolides were recommended as first-line antibiotics, but the new guideline lists macrolides as alternative options as a result of therapy failures in patients with macrolide-resistant S. pneumoniae and increasing macrolide resistance in United States; therefore, in that setting, azithromycin and clarithromycin are still options but erythromycin has been removed because of resistance ( ). For outpatients with comorbidities or risk factors, combination therapy is recommended with amoxicillin/clavulanate or cephalosporin plus a macrolide or doxycycline. One can also consider monotherapy with levofloxacin or moxifloxacin in this setting. For inpatients, combination therapy with a beta-lactam (ampicillin plus sulbactam 1.5 to 3 g every 6 hours, cefotaxime 1 to 2 g every 8 hours, ceftriaxone 1 to 2 g daily, or ceftaroline 600 mg every 12 hours) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily) is recommended. Monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily) can also be used in low-risk patients but should be used in combination with a beta-lactam for high-risk patients. Additional anaerobic coverage in patients with aspiration pneumonia is not recommended.
Approximately 1 in 3000 surgical procedures are complicated by aspiration pneumonitis produced by the aspiration of gastric fluid (sterile and highly acidic). The aspiration produces a severe chemical pneumonitis. Aspiration and its complications are a cause of approximately 30% of anesthetic mortalities. Risk factors for aspiration pneumonia include older age, obesity, hiatal hernia, or emergency surgery associated with a full stomach. The morbidity from aspiration is secondary to both particulate matter entering into the lungs and the caustic nature of gastric acid. The combination of these insults leads to a destructive inflammatory response. When aspiration is significant and severe, adult respiratory distress syndrome often develops. Secondary infection usually complicates aspiration pneumonitis, and broad-spectrum antibiotics should be given when this diagnosis is entertained. Preventive measures include avoiding the routine use of nasogastric suction, perioperative antacid ingestion, and the administration of H2 blockers, as well as judicious use of narcotics and sedatives.
Sleep apnea has become a significant concern because of the rising incidence of obesity and morbid obesity in the United States. The increased soft tissues of the head and neck can lead to airway compromise that leads to intermittent apnea and hypoventilation while a woman sleeps. The increased weight of the extra adipose tissue on the neck, chest, and abdominal wall lead to a decrease in pulmonary compliance. As a result, the relative hypoxia may induce systemic and pulmonary hypertension. Patients may also develop chronic hypercapnia as the respiratory drive shifts from a CO 2 -driven response to a hypoxia-driven response in patients with sleep apnea. It is important to note that when patients with morbid obesity are given higher levels of oxygen or narcotics, they are at increased risk for apnea. These patients develop an increased sensitivity to narcotics that shuts down the respiratory drive. Patients with chronic hypoxia from any cause will often have an increased sensitivity to narcotics, but it is particularly problematic in the patient with obesity who is dependent on low levels of oxygen for respiratory stimulation. These patients should be given oxygen as needed; however, during the postoperative period, when narcotics are given, the goal should be to keep the oxygen saturation in the 94% range. At saturation levels of 96% to 99% these patients may lose respiratory drive and become hypercarbic and acidotic ( ).
Preoperatively patients who are thought to be at risk for sleep apnea may be queried by asking them the “STOP-BANG” questions. These eight questions can predict sleep apnea with a high degree of sensitivity. The questions include the following: Do you s nore? Do you often feel t ired? Has anyone o bserved you stop breathing? Are you being treated for high blood p ressure? Is your b ody mass index >35? Are you older than a ge 50? Is your n eck size greater than 16 inches? Is your g ender male? A “yes” answer to zero to two questions implies that one is low risk for obstructive sleep apnea (OSA); three to four “yes” answers places one at intermediate risk for OSA; five to eight ”yes” answers places one at high risk for OSA ( ).
Shock is defined as a condition in which circulatory insufficiency prevents adequate vascular perfusion of vital organs. Systemic hypotension results in poor tissue perfusion and reduced capillary filling. Prolonged hypotension can result in oliguria, progressive metabolic acidosis, and multiple organ failure. Shock may be produced by hemorrhage, cardiac failure, sepsis, and anaphylactic reactions. Hypovolemic shock is the most common cause of acute circulatory failure in gynecologic patients. Cardiogenic shock and septic shock are less common. Shock from postoperative hemorrhage is usually seen in the first several hours after surgery. In the perioperative period, hypovolemia may be secondary to several factors, including preoperative volume deficiency, underreplaced blood loss during surgery, extracellular fluid loss during surgery, inadequate fluid replacement, and, most commonly, continued blood loss after the surgical procedure. Tachycardia is the classic cardiovascular physiologic response to hypotension. Progressive hypovolemia results in diminished urine output.
The majority of perioperative cases of shock are related to hemorrhage secondary to inadequate hemostasis. The development of shock from acute blood loss depends on the rate of bleeding; for example, slow venous oozing may produce a large amount of blood loss but not produce shock. Rapid loss of 20% of a woman’s blood volume produces mild shock, whereas a loss of greater than 40% of blood volume results in severe shock. Even with the extensive use of suction equipment, the actual measurement of intraoperative blood loss is imprecise. Massive blood loss has been defined as hemorrhage that results in replacement of 50% of the circulating blood volume in less than 3 hours. The most common cause of postoperative bleeding is a less than ideal ligature or hemorrhage from a vessel that has retracted during the operation. Bleeding may come from an isolated artery or vein or may be more generalized when the bleeding is secondary to a clotting abnormality.
In addition to hemorrhage, hypotension in the immediate postoperative period may be secondary to the residual effects of anesthesia or oversedation. For example, older patients often experience prolonged vasodilation secondary to the sympathetic blockade produced by epidural or spinal anesthesia. The differential diagnosis of postoperative hypotension and tachycardia should include conditions such as pneumothorax, PE, aspiration, myocardial infarction, and acute gastric dilation. However, in the postoperative period the index of suspicion should be highest for bleeding.
In patients in whom ineffective coagulation is noted, the differential diagnosis includes medication-induced anticoagulation, sepsis, fibrinolysis, diffuse intravascular coagulation, and a previously unrecognized coagulation defect, such as von Willebrand disease. Coagulopathies can also develop from excessive transfusion and dilution of fibrinogen and other clotting factors. Blood product replacement for massive hemorrhage should incorporate adequate use of coagulation factors via fresh-frozen plasma (FFP) or cryoprecipitate and platelets because thrombocytopenia, impaired platelet function, and a decrease in factors V, VIII, and XI commonly occur with massive transfusions as a result of dilution of these factors with use of packed red cells alone. Further, hemorrhagic shock results in progressive acidosis, which interferes with assembly of coagulation factor complexes, and hypothermia, which causes platelet dysfunction and decreased activity of thromboxanes. Hypofibrinogenemia is the first to develop, followed by deficiencies of other coagulation factors. Thrombocytopenia is often the last defect to be recognized in the coagulopathy cascade, but the timing of its development varies among individuals. Thus most hospitals have a massive transfusion protocol, which includes replacing packed red cells, plasma, and platelets in a 1:1:1 ratio. In cases of submassive hemorrhage, transfusion of platelets should be determined by serial platelet counts ( Box 25.3 ). Similarly, the transfusion of FFP can be performed as needed to match a clotting deficiency as measured by the prothrombin time (PT) and activated partial thromboplastin time (aPTT). Each unit of FFP can be expected to raise increase clotting protein levels by 2.5% to 10%.
Recent (within 24 hr) platelet count <10,000/mm 3 (for prophylaxis)
Recent (within 24 hr) platelet count <50,000/mm 3 with demonstrated microvascular bleeding (oozing) or a planned surgical/ invasive procedure
Demonstrated microvascular bleeding and a precipitous fall in platelet count
Adult patients in the operating room who have had complicated procedures or have required more than 10 units of blood and have microvascular bleeding. Giving platelets assumes that adequate surgical hemostasis has been achieved.
Documented platelet dysfunction (e.g., prolonged bleeding time >15 min, abnormal platelet function tests) with petechiae, purpura, microvascular bleeding (oozing), or surgical or invasive procedure
Unwarranted indications:
Empirical use with massive transfusion when patient is not having clinically evident microvascular bleeding (oozing)
Prophylaxis in thrombotic thrombocytopenic purpura, hemolytic-uremic syndrome, or idiopathic thrombocytopenic purpura
Extrinsic platelet dysfunction (e.g., renal failure, von Willebrand disease)
Two early signs of hypovolemia caused by hidden internal bleeding include tachycardia and decreased urine output. The body’s adrenergic response to hemorrhage includes perspiration, tachycardia, and peripheral vasoconstriction. Urine output decreases to less than 0.5 mL/kg per hour (20 to 25 mL/hr) as a result of poor perfusion of the kidneys. With further loss of blood, agitation, weakness, and skin pallor can appear; the extremities may feel cold and clammy; and, ultimately, systolic blood pressure drops to less than 80 mm Hg. Again, because of adaptive cardiovascular changes, it takes a rapid loss of approximately one-third of the blood volume to produce significant hypotension.
Postoperatively, occult intraperitoneal and retroperitoneal bleeding often occurs without significant local symptoms. Extraperitoneal bleeding may present as bleeding from the vaginal cuff. Abdominal distention, muscle rigidity, and shoulder pain are late signs of intraperitoneal hemorrhage. The diagnosis of clinically significant postoperative bleeding may be confirmed by serial changes in hemoglobin levels; however, it is important to caution that marked changes in hematocrit and hemoglobin levels require time to develop. Imaging studies may demonstrate hematomas or increased intraperitoneal free fluid.
The management goals of postoperative shock are to replace, restore, and maintain the effective circulating blood volume and establish normal cellular perfusion and oxygenation ( Box 25.4 ). To accomplish this goal, an adequate cardiac output and appropriate peripheral vascular resistance must be maintained. The first priority is to provide adequate ventilation because poor respiratory gas exchange is the most common cause of death in these patients. The second, almost simultaneous priority is rapid fluid replacement with adequate amounts of blood and crystalloid solution (normal saline or lactated Ringer solution). The 3:1 rule suggests a ratio of 3 mL of crystalloid solution for every 1 mL of blood loss. The optimal fluid replacement is a fluid evenly distributed throughout multiple body compartments. Randomized trials of crystalloid and colloid resuscitation solutions have shown no clear survival benefit to the use of colloids (albumin, gelatin, dextran, and hydroxyethyl starch), but a reduced rate of tissue edema, abdominal compartment syndrome, and hyperchloremic metabolic acidosis is demonstrated. The substantial cost of these agents should be weighed against potential benefits ( ). Crystalloids should be considered the initial resuscitation fluid of choice in hemorrhagic shock; colloids are appropriate for resuscitation in conjunction with crystalloids when blood products are not immediately available. Guidelines for transfusion of packed red blood cells (PRBCs) are listed in Box 25.5 .
Restore circulating blood volume.
Maintain oxygenation.
Correct coagulopathy.
Maintain body temperature.
Correct biochemical abnormalities.
Prevent pulmonary and other organ dysfunction.
Treat underlying cause of hemorrhage.
*The exact priority depends on the circumstances.
Hemoglobin <8 g/dL or acute blood loss in an otherwise healthy patient with signs and symptoms of decreased oxygen delivery with two or more of the following:
Estimated or anticipated acute blood loss of >15% of total blood volume (750 mL in 70-kg man)
Diastolic blood pressure <60 mm Hg
Systolic blood pressure drop >30 mm Hg from baseline
Tachycardia (.100 beats/min)
Oliguria, anuria
Mental status changes
Hemoglobin <10 g/dL in patients with known increased risk of coronary artery disease or pulmonary insufficiency who have sustained or are expected to sustain significant blood loss
Symptomatic anemia with any of the following:
Tachycardia (>100 beats/min)
Mental status changes
Evidence of myocardial ischemia, including angina
Shortness of breath or dizziness with mild exertion
Orthostatic hypotension
Unfounded or questionable indications:
To increase wound healing
To improve the patient’s sense of well-being
7 g/dL < hemoglobin < 10 g/dL (or 21% < hematocrit < 30%) in otherwise stable, asymptomatic patient
Mere availability of predesignated autologous blood without medical indication
The goals of fluid replacement are to maintain a systolic blood pressure that is similar to preoperative readings and a urine output greater than 0.5 mL/kg per hour (usually >30 mL/hr). Table 25.2 lists types of blood components used for replacement therapy. Traditionally in patients with massive hemorrhage, replacement a ratio of 2 units of packed red blood cells to 1 unit of FFP has been the desirable ratio, but more recent literature in trauma surgery and massive hemorrhage suggests that a ratio of 1:1:1 for packed red cells, plasma, and platelets is superior ( ). For every 6 units of packed red blood cells, a 6-pack of platelets will be required, as well as 6 units of FFP to maintain these ratios ( ). Each unit or pack of platelets will raise the platelet level by 15,000/mm 3 . The platelet count should be maintained at greater than 50,000/mm 3 in a woman who is bleeding ( ). The importance of adequate transfusion is not only support of intravascular volume but also supply of oxygen.
| Product | Content | Indication | |
|---|---|---|---|
| Acceptable | Unacceptable | ||
| Red blood cells | Red cells | To increase oxygen-carrying capacity in anemic women; for orthostatic hypotension secondary to blood loss | For volume expansion; to enhance wound healing; to improve general well-being |
| Platelet concentrates | Platelets | To control or prevent bleeding associated with deficiencies in platelet number or function | In patients with immune thrombocytopenic purpura (unless bleeding is life-threatening) |
| Fresh-frozen plasma | Plasma, clotting factors | To increase the level of clotting factors in patients with demonstrated deficiency | For volume expansion; as a nutritional supplement; prophylactically with massive blood transfusion |
| Cryoprecipitate | Factors I, V, VIII, XIII, von Willebrand factor, fibronectin | To increase the level of clotting factors in patients with demonstrated deficiency of fibrinogen, factor VIII, factor XIII, fibronectin, or von Willebrand factor | Prophylactically with massive blood transfusion |
Coagulation studies, PT, and activated PT should be obtained regularly during the bleeding episode to help guide resuscitation. The term washout is used to describe the loss of clotting factors as a woman uses up her blood volume and it is replaced with PRBCs and crystalloid. Disseminated intravascular coagulation (DIC) is an intravascular consumption and is different than washout. However, both conditions require replacement and ongoing evaluation. Although some consider off-label use of recombinant factor VIIa (70 to 90 μg/kg) in episodes of persistent severe bleeding, meta-analyses suggest there is no mortality benefit to its use and potential increased risk of thromboembolism. This combined with cost limits the utility of this product ( , ).
The decision to return to the operating room to control hemorrhage is often difficult to make because the offending artery or vein is often unable to be identified at the time or reoperation. Additionally, friable inflamed postoperative tissues can result in further bleeding. If ongoing postoperative bleeding requires reoperation, this decision should not be postponed. It should be performed expediently after volume replacement and sometimes concomitantly. During this second operation, excellent anesthesia, a full selection of surgical instruments, and the value of good assistance cannot be overemphasized. Proper exposure is paramount for the success of this operation. Initially the old clots are removed and further bleeding is reduced by direct pressure over the presumed bleeding vessels while a systematic search is conducted in an effort to identify the individual vessels that are bleeding.
Bilateral ligation of the anterior divisions of the hypogastric arteries distal to the posterior parietal branch is an effective operation to control persistent postoperative pelvic hemorrhage. This procedure results in a reduction of pulse pressure, which allows a stable clot to form at the site where the pelvic vessels are injured. Classically, two ligatures are placed and tied around each hypogastric artery ( Fig. 25.3 ). The major potential complication of this procedure is injury to the hypogastric vein. If there is generalized oozing, thrombocytopenia, DIC, or factor VIII deficiency should be suspected. If these conditions are excluded, venous oozing from small vessels in the pelvis may be controlled by the local application of topical hemostatic agents such as microfibrillar collagen compounds, gelatin compounds, or topical thrombin compounds (e.g., Avitene, Gelfoam, Floseal).
Intraoperative rapid autologous blood transfusion is used extensively in cardiovascular and trauma surgery, but this technology is not often used by gynecologists. The major complication of rapid autologous transfusion is a 10% hemolysis rate. The risks of air embolism or infusion of particulate matter are minimal. Autologous blood does not contain platelets or clotting factors, so platelets and FFP have to be given concurrently for severe hemorrhage. Rapid autologous transfusion is contraindicated in advanced pelvic infection or malignancy.
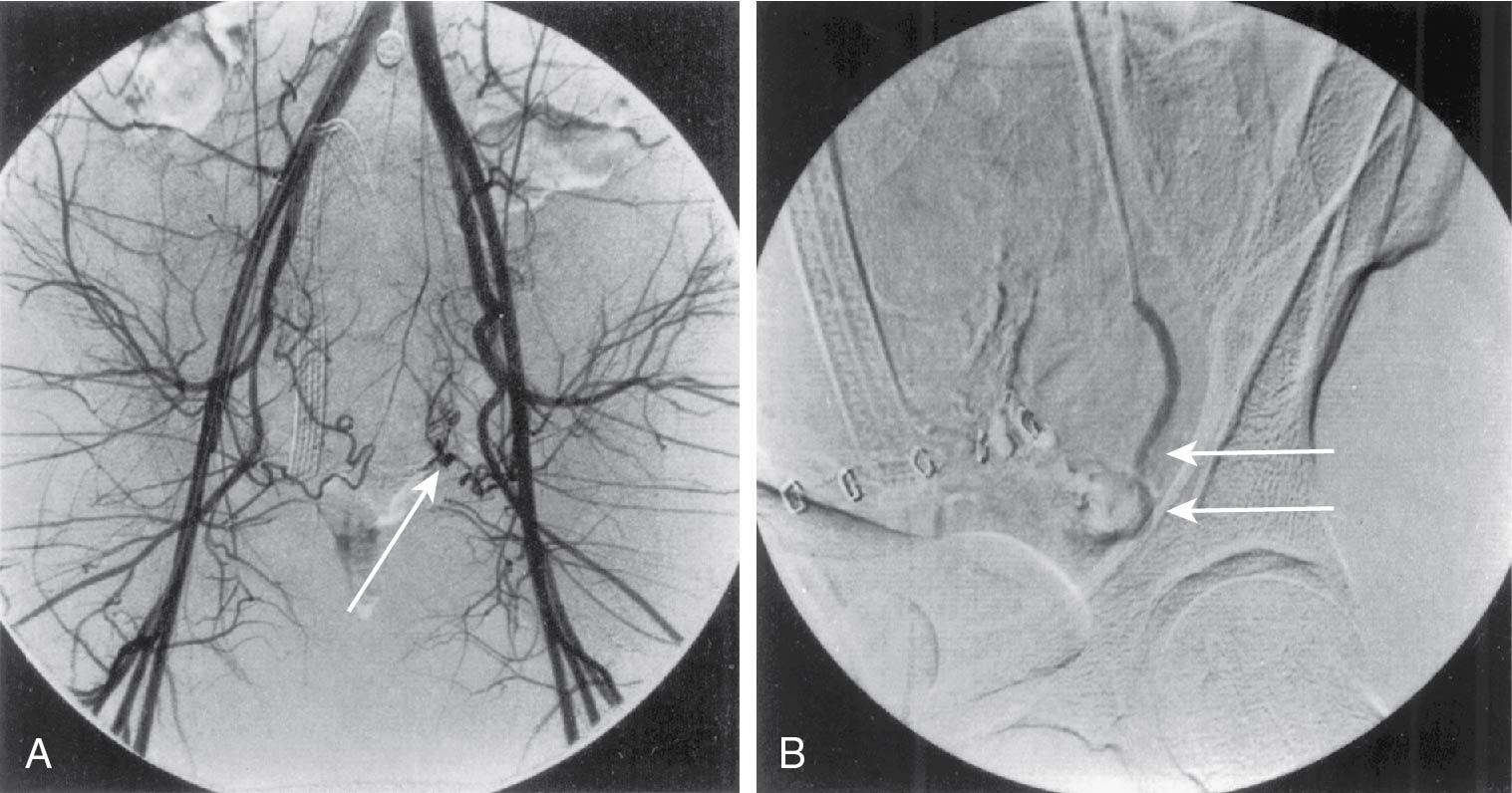
In many cases, angiographic embolization, instead of exploratory laparotomy, may be preferable for control of postoperative hemorrhage from an identifiable vessel ( Fig. 25.4 ). CT angiography has improved the rapid identification of bleeding vessels. Similarly, treatment of recurrent postoperative hemorrhage or hemorrhage late in the postoperative course (7 to 14 days) may be performed with angiographic arterial embolization. Absorbable gelatin sponges are used to produce vascular occlusion for 10 to 30 days. For permanent occlusion metal coils, polyvinyl alcohol particles, microspheres or various “glues” are used.
Management of delayed postoperative hematomas is challenging and controversial. The incidence of hematomas is inversely related to the extent to which meticulous hemostasis is obtained intraoperatively. Women who are given low-dose heparin or who take aspirin chronically are at a slightly higher risk of hematoma formation. Women on antiplatelet medication or anticoagulation are also at risk. Hematomas result from intermittent or slow, continuous venous bleeding and are almost always self- limiting. Eventually, the pressure of the expanding hematoma will exceed the venous pressure and a stable clot will form.
The extent of the hematoma is determined partially by the potential size of the compartment into which the bleeding occurs. Retroperitoneal or broad ligament hematomas may contain several hundred milliliters or even liters of blood. A wound or pelvic hematoma should be suspected when the patient’s hemoglobin continues to decline beyond the usual nadir on postoperative day 3 . Clinical examinations may reveal mild to moderate tenderness over the affected area. Generally by postoperative day 5, the hematoma will liquefy and may be easier to outline during bimanual examination. Distinguishing between an uninfected hematoma and a hematoma that has become secondarily infected is difficult before incision and drainage. Both clinical situations produce tenderness and fever secondary to the inflammation surrounding the hematoma. The diagnosis of a retroperitoneal hematoma may be made by physical examination; most helpful is a careful rectovaginal examination. However, radiologic imaging studies are indicated when a hematoma is suspected and cannot be palpated. Imaging is commonly used to make the diagnosis of a postoperative hematoma.
Hematomas smaller than 5 cm in diameter may be treated conservatively. Larger hematomas may be drained transcutaneously with CT or ultrasound guidance. If not treated, most large hematomas will become secondarily infected, even if treated with parenteral antibiotics. Effective drainage of most pelvic and broad ligament hematomas usually can be accomplished vaginally or radiographically. Small subcutaneous hematomas or fascial hematomas usually resolve spontaneously; however, they are associated with an increased incidence of wound infection and pain.
With any operation there is the potential risk of an unrecognized retained foreign body, sponge, or laparotomy pad. The exact incidence of this complication is difficult to establish but is estimated to be from 1 in 1200 to 1500 laparotomies, typically with correct sponge counts at the time of surgery. When this complication is discovered during the first postoperative week, the woman usually has a tender pelvic mass that is infected. When this mass is discovered after the immediate postoperative course, patients are often asymptomatic or may exhibit minimal tenderness. The possibility of a retained foreign body should be considered in the differential diagnosis of pelvic hematomas and abscesses. A retrospective study of retained sponges by Gawande and associates noted that retained foreign bodies are more commonly associated with a higher body mass index (BMI), emergency surgeries, and an intraoperative change in the type of procedure to be performed ( ).
Surgery is a time of hypercoagulability secondary to the stress response. As such, the surgeon must be aware of the potential complications of thromboembolism throughout the postoperative course. Prophylaxis against deep vein thrombosis (DVT) is discussed in Chapter 24 . However, prophylaxis must be continued throughout the hospital stay and, in certain high-risk cases, even after discharge. For example, patients with malignancy who undergo laparotomy, patients with previous blood clot or personal history of thrombophilia, and those who will have decreased ambulation may benefit from up to 4 weeks of low-molecular-weight heparin (LMWH) after leaving the hospital per guidelines from the American College of CHEST Physicians ( ). Studies in patients with hip replacements and with abdominal pelvic malignancies have shown significant reductions (50% to 66%) in the incidence of DVT with prolonged anticoagulation, up to 4 weeks postoperatively. There is insufficient evidence to make recommendations for prolonged thromboprophylaxis in patients with routine gynecologic concerns, except in high-risk situations such as gynecologic cancer surgery ( ). There is a reduced risk of venous thromboembolism (VTE) with minimally invasive hysterectomy compared with abdominal hysterectomy. Without specific guidelines, the length of time for thromboprophylaxis should be individualized and based on risk and bleeding assessment. Prophylaxis will not prevent all DVTs; thus part of daily rounds includes assessments for this complication.
Superficial thrombophlebitis is one of the most commonly occurring postoperative complications and is most often associated with IV catheters . Superficial thrombophlebitis is often overlooked or disregarded as a cause of postoperative fever. Physical examination will reveal superficial tenderness and erythema over the course of the superficial veins. Women with established superficial varicosities in the lower extremities are especially susceptible because of localized stasis or pressure during the operative procedure and inactivity during the first 24 hours after operation. Superficial thrombophlebitis is a benign process; however, it is associated with deep vein thrombophlebitis in approximately 5% of cases. Thus the finding of superficial thrombophlebitis does not eliminate the necessity to consider DVT as well. Some series have documented the association of inherited thrombophilias with superficial phlebitis, increasing the risk by 4- to 13-fold. Recurrent superficial phlebitis, in varying anatomic sites, may be a sign of occult malignant disease. Detailed basic investigations have identified fibrin sheaths surrounding IV catheters in 60% to 100% of patients studied. The exact fate of the several inches of clot and fibrin sheath after the removal of the IV catheter is uncertain. Venography studies have found that these clots and fibrin sheaths do not break up on catheter removal but initially remain in situ.
IV catheters are also an important source of nosocomial infections. Approximately 30% of all hospital-acquired bacteremias are secondary to IV lines. The most serious complication of IV catheter use is infection of the thrombus, producing suppurative phlebitis or catheter sepsis. It was previously recommended that the IV catheters be removed and replaced in intervals ranging from 72 to 96 hours, regardless of whether signs or symptoms of superficial phlebitis are present, but a 2013 Cochrane review found no evidence to support routine catheter exchange without evidence of inflammation, infiltration, or blockage ( ). Although routine exchange is not indicated, venous catheters should be removed at the first sign of induration, erythema, or edema.
The clinical management of mild superficial thrombophlebitis includes rest, elevation, and local heat. Moderate to severe superficial thrombophlebitis may be treated with a nonsteroidal antiinflammatory drug (NSAID), such as ibuprofen, or with low-dose heparin if refractory to supportive care. In the rare case of proximal progression of the inflammatory process, treatment with IV heparin and antibiotics is warranted.
Fifty percent of thromboembolic complications occur within the first 24 hours and 75% occur within 72 hours. Approximately 15% occur after the seventh postoperative day. Diagnosis of DVT by physical examination alone is insensitive, and thus imaging studies are essential for establishing the correct diagnosis. Venous thrombosis and PE are the direct causes of approximately 40% of deaths after gynecologic surgery. The incidence of fatal PE after gynecologic operations is approximately 0.2%. Because women often die within a few hours of the appearance of initial symptoms, emphasis must be placed on prevention rather than treatment of this complication. PE is not the only major consequence of deep venous thrombosis. Many women develop chronic venous insufficiency or postphlebitic syndrome of the legs as a major sequela. The resulting damage to valves of the deep veins produces shunting of blood to superficial veins, chronic edema, pain on exercise, and skin ulceration.
Historic incidence of DVT with gynecologic operations without prophylaxis varied from 7% to 45%, with an average of approximately 15% ( ). Since the institution of universal mechanical prophylaxis, VTE rates as low as 0.6% for open hysterectomies and 0.2% for minimally invasive hysterectomies have been reported ( ). The incidence of thrombosis is directly dependent on risk factors such as the type and duration of operation; age of the woman; history of thrombophilia or DVT; peripheral edema; surgical blood loss; restrictions in perioperative ambulation or immobility; and history or presence of obesity, malignancy, sepsis, diabetes, current oral contraceptive or hormone use, and conditions that produce venous stasis, such as ascites and heart failure ( Box 25.6 ) ( ). Older women and women with obesity have an increased incidence of thrombosis because of dilation of their deep venous system. There is a two- to fourfold increased risk for venous thrombosis in women taking postmenopausal estrogen therapy. The length of the surgical procedure also has an important influence on the development of thrombosis ( Table 25.3 ).
Active cancer
Acute medical illness (e.g., acute myocardial infarction, heart failure, respiratory failure, infection)
Advancing age
Antiphospholipid syndrome
Behçet syndrome
Central venous catheter
Chronic care facility stay
Congenital venous malformation
Dyslipoproteinemia
Heparin–induced thrombocytopenia
Hormone replacement therapy
Immobilization
Inflammatory bowel disease
Intravenous drug abuse
Limb paresis
Long-distance travel
Myeloproliferative diseases
Nephrotic syndrome
Obesity
Oral contraceptives
Other drugs
Antipsychotics
Chemotherapeutic agents
Tamoxifen
Thalidomide
Paroxysmal nocturnal hemoglobinuria
Pregnancy, puerperium
Previous venous thromboembolism
Prolonged bed rest
Superficial vein thrombosis
Surgery
Trauma
Varicose veins
Vena cava filter
| Risk Category | Risk Level | ||
|---|---|---|---|
| Low | Medium | High | |
| Age (yr) | 40 | 40 | 50 |
| C ONTRIBUTING F ACTORS | |||
| Operation | Uncomplicated or minor | Major abdominal or pelvic | Major, extensive |
| Weight | Moderately obese: 75-90 kg or >20% above ideal weight | Morbidly obese: >115 kg or >30% above ideal weight | |
| Previous venous thrombosis | |||
| Varicose veins | |||
| Cardiac disease | |||
| Diabetes (insulin-dependent) | |||
| Calf vein thrombosis (%) | 2 | 10-35 | 30-60 |
| Iliofemoral vein thrombosis (%) | 0.4 | 2-8 | 5-10 |
| Fatal pulmonary emboli (%) | 0.2 | 0.1-0.5 | 1 |
| Recommended prophylaxis | Early ambulation | Low-dose heparin or intermittent pneumatic compression | Low-dose heparin or intermittent pneumatic compression |
The process of thrombosis usually begins in the deep veins of the calf. It is estimated that 75% of pulmonary emboli originate from a thrombus in the leg. If one leg is involved, the contralateral leg will have a thrombus in approximately 33% of women. Usually the thrombus remains localized, it lyses spontaneously, and the local symptoms resolve. In approximately 1 in 20 cases the process extends centrally to the veins of the upper leg and pelvis. Involvement of the femoral vein often results in lower extremity swelling. Pulmonary emboli from calf veins alone are rare, with only 4% to 10% of pulmonary emboli originating from this area. In contrast, there is a 50% risk of a PE if thrombosis of the femoral vein is not treated.
In 1854, Virchow described the three key predisposing or precipitating factors in the production of thrombi: an increase in coagulation factors, damage to the vessel wall, and venous stasis. Subsequent studies have documented that all three events occur with gynecologic operations. Blood flow in the iliac vein decreases by approximately 55% during an operation. During an operation, there are several normal physiologic changes that produce hypercoagulability, including increases in factors VIII, IX, and X, number of platelets, platelet aggregation and adherence, fibrinogen, and, lastly, thromboplastin-like substance from tissue necrosis.
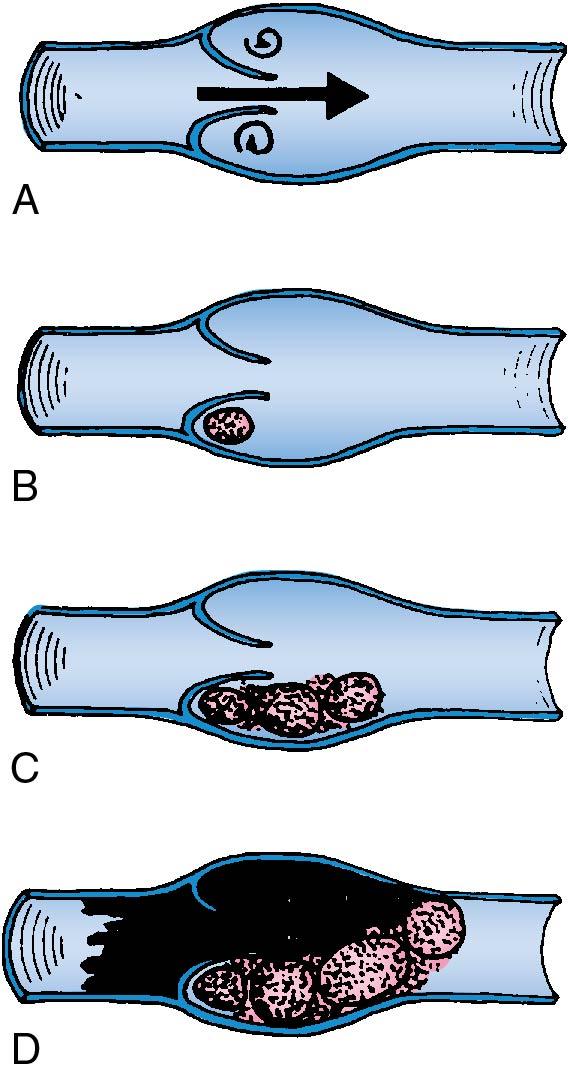
The site of initial formation of the thrombus is most often near the base of a valve cusp in the calf of the leg ( Fig. 25.5 ). The thrombus propagates and grows by repetitive layers of platelet aggregation and deposition of fibrin from fibrinogen. The most recently formed portion of the propagating thrombi are free floating (not attached to the vein) and are most likely to become pulmonary emboli. The body attempts to repair the area of thrombosis through an invasion of fibroblasts from the vein wall to encompass the base of the thrombus. Eventually the thrombus is attached to the vein wall, the area is reepithelialized, organization occurs, and symptoms resolve.
The signs and symptoms of DVT depend directly on the severity and extent of the process. Many localized cases of DVT in the calf are asymptomatic and are only recognized by a screening procedure such as duplex ultrasonography; however, even extensive areas of DVT may be asymptomatic and the first sign may be the development of a PE. In a woman who is asymptomatic, the pathophysiologic process may not totally obstruct the individual vein and drainage is obtained via associated competent collateral circulation.
Studies using 125 I-labeled fibrinogen to screen the legs have documented that approximately half of women who develop DVT after gynecologic surgery is totally free of symptoms ( ). Among women who develop signs and symptoms, approximately 68% have induration of the calf muscles, 52% have minimal edema, 25% have calf tenderness, and 11% develop a difference of more than 1 cm in diameter of the leg. The Homan sign is present in 10%, and differential pain over the calf with a blood pressure cuff is present in approximately 40%. The clinical diagnosis of iliofemoral thrombosis is much easier—the woman usually develops severe symptoms caused by obstruction of venous return. Usually there is an acute onset of severe pain and swelling.
The clinician must maintain a high degree of suspicion to begin the diagnostic workup for DVT. The clinical symptoms and signs of DVT are nonspecific. Common clinical findings may include persistent low-grade fever or unexplained tachycardia. The tachycardia is often more rapid than one would expect with a low-grade fever. The finding of a definite difference in leg circumference is supportive evidence of DVT but not a sensitive test; further, physical examination of the legs produces false-positive findings in approximately 50% of cases. Based on clinical examination, if the likelihood of DVT were low, the next step in nonsurgical patients, would be a d-dimer level; however, in the postoperative patient, d-dimer is not as reliable and should not be ordered. d-Dimer is a protein from cross-linked fibrin after it has been degraded by plasmin in the fibrinolytic process; thus d-dimer may be elevated because of trauma, surgery, intravascular hemolysis, pregnancy, and other inflammatory states. If the signs and symptoms are suggestive or the woman is at high risk, the next step should be imaging.
Imaging options for DVT include contrast venography (phlebography) and duplex ultrasound. Most clinicians obtain a duplex lower extremity ultrasound when suspicious for DVT. Duplex ultrasound is a combination of color Doppler and real-time B-mode ultrasound. It has a high sensitivity and specificity in symptomatic women. Real-time ultrasound imaging provides visualization of the larger veins, and sensitive Doppler ultrasound is focused simultaneously on the suspicious vessel. The technology depends on changes in venous flow for a positive diagnosis. The documented sensitivity of duplex ultrasonography in detecting proximal thrombi is 95% (95% CI, 92% to 98%) and the specificity is 99% (95% CI, 98% to 100%) ( ). The advantages of this method are that it is noninvasive, easy to use, highly accurate, objective, simple, and reproducible. Duplex ultrasonography may improve the diagnostic accuracy in larger veins. The main disadvantage of duplex ultrasound is its limited accuracy when investigating small vessels in the calf and vessels proximal to the inguinal canal. The inability to compress the deep vein by moderate pressure with the ultrasound probe is the most widely used criterion for the positive diagnosis of DVT.
The objectives of the clinical management of DVT associated with gynecologic surgery are early detection and early therapy. In reality, antithrombotic therapy is preventive medicine because the therapeutic agent interrupts progression of the disease (thrombus formation) but does not actively resolve the disease process. Anticoagulation with heparin (unfractionated or LMWH) is the initial treatment of choice for the diagnosis of DVT or PE ( Table 25.4 ). LMWH is as effective and has several advantages over unfractionated heparin, despite its increased cost. Ease of dosing, lower risk of bleeding complications, and reduced risk of heparin-induced thrombocytopenia are among the most notable. LMWH has effectively replaced unfractionated heparin as the gold standard for treatment of DVT. LMWH may be given subcutaneously with once or twice daily weight-based dosing; for example, for enoxaparin, the dosage would be 1.5 mg/kg daily or 1 mg/kg twice daily ( ). LMWH does not require monitoring in women with normal and stable renal function, LMWH has more stable pharmacokinetics, and there is actually a lower incidence of complications noted in some studies in terms of progression from DVT to pulmonary emboli. Additionally, studies comparing LMWH with unfractionated heparin have shown a greater effect with thrombus regression within the veins themselves. Testing of levels of LMWH is based not on the aPTT but on the anti–factor Xa activity level. Levels are calculated specifically for each LMWH. An aPTT of 1.5 times normal corresponds approximately to an anti–factor Xa activity level of 0.2. Therapeutic levels are between 0.4 and 0.8 . Bleeding usually occurs when levels of the anti–factor Xa activity level rise more than 1.0 to 1.2. If needed in patients with unstable renal status, levels may be checked approximately 4 hours after dosing.
| Drug | Method of Administration | Dosage * | Reported Risks (no./total no. [%]) | |
|---|---|---|---|---|
| Heparin-Induced Thrombocytopenia † | Major Bleeding | |||
| Unfractionated heparin | IV | Loading dose, 5000 U or 80 U/kg of body weight with infusion adjusted to maintain aPTT within therapeutic range | 9/332 (2.7) | 35/1853 (1.9) |
| LMW heparin | 0/333 (0) | 20/1821 (1.1) | ||
| Dalteparin | Subcutaneous | 100 U/kg every 12 hr or 200 U/kg daily; maximum, 18,000 U/day | ||
| Enoxaparin | Subcutaneous | 1 mg/kg every 12 hr or 1.5 mg/kg daily; maximum, 180 mg/day | ||
| Tinzaparin | Subcutaneous | 175 U/kg daily; maximum, 18,000 U/day | ||
| Nadroparin | Subcutaneous | 86 U/kg every 12 hr or 171 U/kg daily; maximum, 17,100 U/day | ||
* Doses vary in patients who are obese or who have renal dysfunction. Monitoring of anti–factor Xa levels has been suggested for these patients, with dose adjustment to a target range of 0.6 to 1.0 U/mL 4 hr after injection for twice-daily administration or 1.0 to 2.0 U/mL for once-daily administration. Even though there are few supporting data, most manufacturers recommend capping the dose for patients with obesity at the same dose as that for a 90-kg patient.
† The therapeutic range of activated partial thromboplastin time corresponds to heparin levels of 0.3 to 0.7 U/mL, as determined by anti–factor Xa assay. High levels of heparin-binding proteins and factor VIII may result in so-called heparin resistance. In patients requiring more than 40,000 U/day to attain a therapeutic aPTT, the dosage can be adjusted on the basis of plasma heparin levels.
If unfractionated heparin is desired as an IV infusion, weight-adjusted or fixed-dose infusions are acceptable. The weight-adjusted dose is 80 IU/kg per hour bolus followed by 18 IU/kg per hour. The fixed dose uses an initial loading dose of 5000 IU, followed by a continuous infusion of 1000 IU/hr ( ). The dosage of unfractionated IV heparin should be adjusted to prolong an aPTT to 2.5 times control values. Continuous heparin infusion is preferred over periodic bolus injections because there are fewer hemorrhagic complications (6% vs. 14%). The average half-life of heparin is 1 to 2 hours after IV injection. Failure to achieve adequate anticoagulation in the first 24 hours of therapy increases the risk of recurrent VTE 15-fold.
For women who are pregnant or have a malignancy, LMWH is the treatment of choice for long-term anticoagulation. Other women can be treated with oral warfarin or a direct oral anticoagulant (DOAC) such as dabigatran, rivaroxaban, apixaban, or edoxaban.
Standard dosing for warfarin is based on serial international normalized ratio (INR) laboratory values with a goal of 2.5 (range, 2 to 3). In general, a loading dose of warfarin is given with 5 to 10 mg nightly for two doses followed by INR-guided doses. Heparin or LMWH should be continued until the INR is greater than 2. The biologic half-life of warfarin is 2 to 3 days.
DOAC medications are a new class of anticoagulant that work directly on the coagulation cascade via inhibition of either factor Xa (apixaban, edoxaban, and rivaroxaban) or thrombin (dabigatran). They provide more consistent and reliable dosing than warfarin without laboratory monitoring and are widely used for DVT and PE in patients without malignancy. Their role in patients with malignancy is still an area of active research ( ), although they are commonly used in this setting.
In general, anticoagulation is continued for 3 to 6 months for adequate treatment and secondary prophylaxis of a provoked thrombus, but individualized treatment for continuation beyond this period is considered. For example, a provoked clot after an abdominal hysterectomy without other risk factors should be managed with 3 months of therapy ( ). Patients with large DVT, antiphospholipid antibody syndrome, or malignancy may require extended therapy, which may be performed indefinitely while the risk factor is present and in conjunction with a hematologist.
The primary risk of chronic anticoagulation therapy is the potential for major bleeding complications. Major bleeding occurs in approximately 4% of woman-years of therapy. Approximately 1% of patients on full-dose heparin develop thrombocytopenia (platelet count <100,000/mm 3 ). If thrombocytopenia develops, heparin should be discontinued because of the potential risk of paradoxical thrombosis.
Inferior vena cava filters may be used to protect against pulmonary emboli in patients who cannot receive anticoagulation. However, this practice is becoming less popular because many of the fluoroscopically placed temporary vena cava filters are just not removed, leading to more risk of complications. Routine perioperative use of vena cava filters has been shown to be of no benefit.
Routine screening for thrombophilia in all patients with VTE is not indicated. The presence of a hereditary thrombophilia does not alter therapeutic or prophylactic management of a patient and has not been associated with improved outcomes. Patients who may benefit from testing include those with recurrent thrombosis, patients with a family history of thrombosis, patients with thrombosis in unusual locations (hepatic vein, portal vein, mesenteric vein, or arterial), and possibly patients younger than age 45 years. Patients with a provoked thrombosis from cancer, hormonal therapy, and surgery do not require testing for thrombophilias.
There is no evidence that bed rest is helpful for patients with DVT or that immobilization will prevent PE. Patients with confirmed DVT may receive NSAIDs because coagulation factors will be monitored. Patients should also be prescribed support stockings, which should be worn for several months and for up to 2 years. The use of support stockings decreases the risks of postthrombotic syndrome. In a systematic review, women who used stockings up to 2 years after DVT had up to a 50% reduction in the incidence of postthrombotic syndrome ( ).
The accurate diagnosis of PE is essential for the prevention of morbidity from lack of treatment or unnecessary anticoagulation therapy. Autopsy studies have documented that pulmonary emboli are undiagnosed clinically in approximately 50% of women who experience this complication. Approximately 10% of women with a PE die within the first hour. The mortality of women with correctly diagnosed and treated pulmonary emboli is 8%, in contrast to approximately 30% if the disease is not treated. Most pulmonary emboli in gynecologic patients originate from thrombi in the pelvic and femoral veins. Predisposing risk factors are found in most women with PE.
No combination of symptoms or signs is pathognomonic for PE. They are nonspecific and similar to symptoms caused by other forms of cardiorespiratory disease. Many patients with PE will be asymptomatic. Common conditions considered in the differential diagnosis of PE include pneumonia, cardiac failure, atelectasis, aspiration, acute respiratory distress syndrome, and sepsis. Although the differential diagnosis is broad in scope, the symptoms should alert the physician to the possibility of a PE, thus allowing a proper diagnostic workup to establish or rule out the disease. Chest pain, dyspnea, and apprehension are the most common symptoms. The dyspnea is often of abrupt onset. The classic triad of shortness of breath, chest pain, and hemoptysis is seen in less than 20% of women with proved PE. Tachycardia, tachypnea, rales, and an increase in the second heart sound over the pulmonic area are the most commonly found signs of pulmonary emboli ( Table 25.5 ). Approximately 15% of women with pulmonary emboli have an unexplained low-grade fever associated with a PE. A high fever is rarely associated with a PE but may occur. The clinical manifestations of PE are produced primarily by occlusion of the large branches of the pulmonary arteries by embolic material with subsequent associated reflex bronchial constriction and vasoconstriction that intensify symptoms. More than 50% of clinically recognized pulmonary emboli are multiple. The most common location of pulmonary emboli is in the lower lobes of the right lung. Shock and syncope are associated with massive pulmonary emboli.
| Symptoms | Patients with Finding (%) |
|---|---|
| Predisposing factors * | 94 |
| Dyspnea | 84 |
| Pleuritic chest pain | 74 |
| Apprehension | 59 |
| Cough | 53 |
| Hemoptysis | 30 |
| Syncope | 14 |
| S IGNS | |
| Tachypnea | 92 |
| Rales | 58 |
| Accentuation of pulmonic valve closure | 53 |
| Tachycardia | 44 |
| Cyanosis | 20 |
* Prolonged immobilization, postoperative state, congestive heart failure, carcinomatosis.
Although imaging techniques are the gold standard for establishing the diagnosis of pulmonary emboli, several studies have found that clinical assessment is almost as accurate ( ). Laboratory studies that may help in diagnosis and management include electrocardiograms (ECGs), chest radiographs, blood gas analyses, assessment of troponin, d-dimer (not reliable in postoperative patients), and brain natriuretic peptide (BNP). Less than 15% of ECGs demonstrate significant changes of right ventricular strain, with T-wave inversion in V 1 to V 4 with a PE. Most women with pulmonary emboli demonstrate hypoxemia on blood gas determinations, but as with other routine tests, these findings do not occur invariably. Diminished pulmonary vascular markings may be a suggestive finding on a chest film, but they are fairly nonspecific. The chest radiograph may be helpful in the differential diagnosis by demonstrating other pulmonary processes. The most common findings on chest film examination in patients with a PE are infiltrate, pleural effusion, atelectasis, and enlargement of the heart or descending pulmonary artery.
In women who are in mild to moderate distress, fractionated or unfractionated heparin should be started while imaging studies are being ordered. If there is any question of severe distress or hemodynamic instability, thrombolytic therapy may be indicated for the appropriate patient. In stable patients, a stepwise approach is useful. Many clinicians will order Doppler studies of the lower extremities. If the Doppler studies are positive, the woman will be anticoagulated and no further workup for a thrombotic source is necessary. If the tests are negative, further imaging is still necessary, because in the postoperative patient, pelvic clots may be the origin of the pulmonary embolus, not lower extremity clots. The next two options are a ventilation-perfusion ( ![]() ) scan or CT pulmonary angiography (CTPA). CTPA has largely replaced
) scan or CT pulmonary angiography (CTPA). CTPA has largely replaced ![]() scanning as the most common imaging technique to establish or exclude the diagnosis of PE ( Fig. 25.6 ). CTPA uses IV contrast media to aid in the imaging of the pulmonary vessels. The procedure is minimally invasive and provides a volumetric image of the lung by rotating the detector at a constant rate around the woman. A cost-effective analysis from Doyle and colleagues found that CTPA was the most cost-effective first-line test to diagnose pulmonary embolus. A limitation of CTPA is a sixfold increase in radiation exposure compared with the
scanning as the most common imaging technique to establish or exclude the diagnosis of PE ( Fig. 25.6 ). CTPA uses IV contrast media to aid in the imaging of the pulmonary vessels. The procedure is minimally invasive and provides a volumetric image of the lung by rotating the detector at a constant rate around the woman. A cost-effective analysis from Doyle and colleagues found that CTPA was the most cost-effective first-line test to diagnose pulmonary embolus. A limitation of CTPA is a sixfold increase in radiation exposure compared with the ![]() scan. This leads to a slightly increased risk of breast cancer (<1%). Before
scan. This leads to a slightly increased risk of breast cancer (<1%). Before ![]() scanning, a chest radiograph should be obtained. If the radiograph shows other findings, such as atelectasis, the
scanning, a chest radiograph should be obtained. If the radiograph shows other findings, such as atelectasis, the ![]() scan has a much higher chance of being nondiagnostic and CTPA should be ordered instead.
scan has a much higher chance of being nondiagnostic and CTPA should be ordered instead.
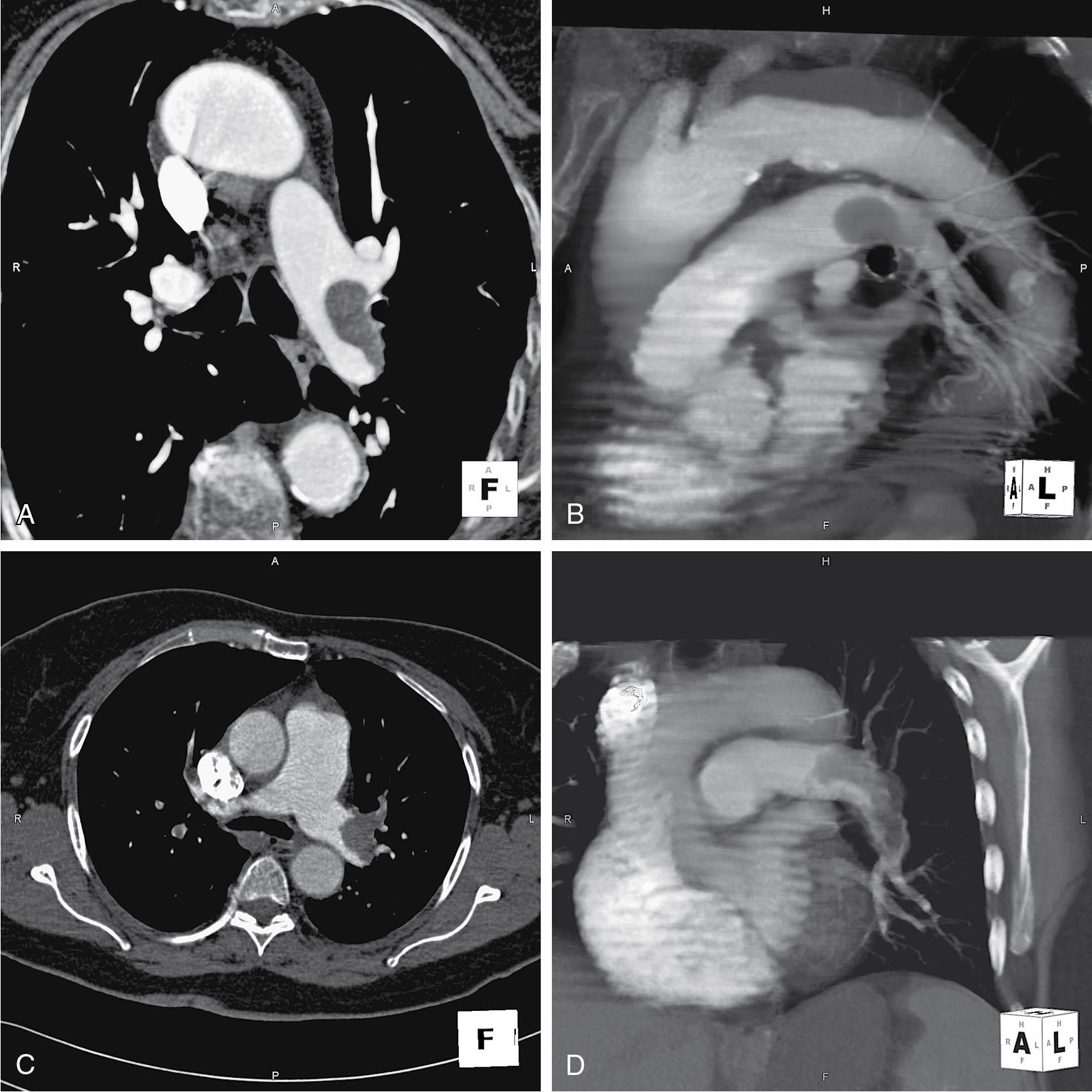
The ![]() scan is a safe and relatively easy-to-perform test. The scan involves the injection of small radiocolloid particles into the circulation. They are trapped in small vessels; their distribution depends on regional pulmonary blood flow. Ventilation scintigraphy uses radionuclides of technetium aerosol or xenon gas. The combination of lack of symmetry and a mismatch in the ventilation scan is the abnormality that leads to the diagnosis. Whereas a normal result effectively rules out the diagnosis of PE, 40% of patients with a suspected PE will have a normal scan. The multicenter Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) found that 4% of patients with normal or near-normal perfusion lung scans subsequently were discovered to have pulmonary emboli. This study emphasized a high sensitivity of 98% but a low specificity of 10% for
scan is a safe and relatively easy-to-perform test. The scan involves the injection of small radiocolloid particles into the circulation. They are trapped in small vessels; their distribution depends on regional pulmonary blood flow. Ventilation scintigraphy uses radionuclides of technetium aerosol or xenon gas. The combination of lack of symmetry and a mismatch in the ventilation scan is the abnormality that leads to the diagnosis. Whereas a normal result effectively rules out the diagnosis of PE, 40% of patients with a suspected PE will have a normal scan. The multicenter Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) found that 4% of patients with normal or near-normal perfusion lung scans subsequently were discovered to have pulmonary emboli. This study emphasized a high sensitivity of 98% but a low specificity of 10% for ![]() scans in the diagnosis of PE. The authors noted that almost all patients with acute PE had abnormal scans, but so did most patients without emboli.
scans in the diagnosis of PE. The authors noted that almost all patients with acute PE had abnormal scans, but so did most patients without emboli. ![]() scans have a high sensitivity but a variable specificity for the diagnosis of PE. For example, other cardiorespiratory diseases such as asthma may result in regional areas of decreased perfusion. If the scan documents multiple segments or lobar perfusion defects with a ventilation mismatch, the probability of pulmonary emboli is more than 85%.
scans have a high sensitivity but a variable specificity for the diagnosis of PE. For example, other cardiorespiratory diseases such as asthma may result in regional areas of decreased perfusion. If the scan documents multiple segments or lobar perfusion defects with a ventilation mismatch, the probability of pulmonary emboli is more than 85%. ![]() scans with less extensive perfusion abnormalities or matching ventilation defects do not reliably exclude the diagnosis of PE.
scans with less extensive perfusion abnormalities or matching ventilation defects do not reliably exclude the diagnosis of PE.
The management of the majority of pulmonary emboli is with anticoagulation with IV unfractionated heparin or full-dose LMWH, similar to the management of DVT ( Fig. 25.7 ). Prompt and early therapy with heparin provides anticoagulation and inhibits the release of serotonin from platelets, which potentially results in a decrease in the associated bronchoconstriction. Some women are candidates for thrombolytic therapy. The time window for effective use of thrombolysis is up to 14 days after the initial symptoms or signs of a PE, although it is rarely used beyond 48 to 72 hours. Thrombolytic therapy works by transforming plasminogen to plasmin. Use of thrombolytic therapy during the early postoperative period is contraindicated because of the increased risk of serious hemorrhage, except in very unique circumstances. Recombinant human tissue-type plasminogen activator (TPA) has been shown to be more rapid in onset and safer than urokinase for thrombolytic therapy. Thrombolytic therapy is the method of choice in patients with massive pulmonary emboli (angiographically, >50% obstruction of the pulmonary arterial bed) with associated moderate to severe hemodynamic instability, lobular obstruction, or multiple segmental profusion defects. Random trials of heparin versus thrombolytic therapy have shown that emboli clear more rapidly with initial thrombolytic therapy.
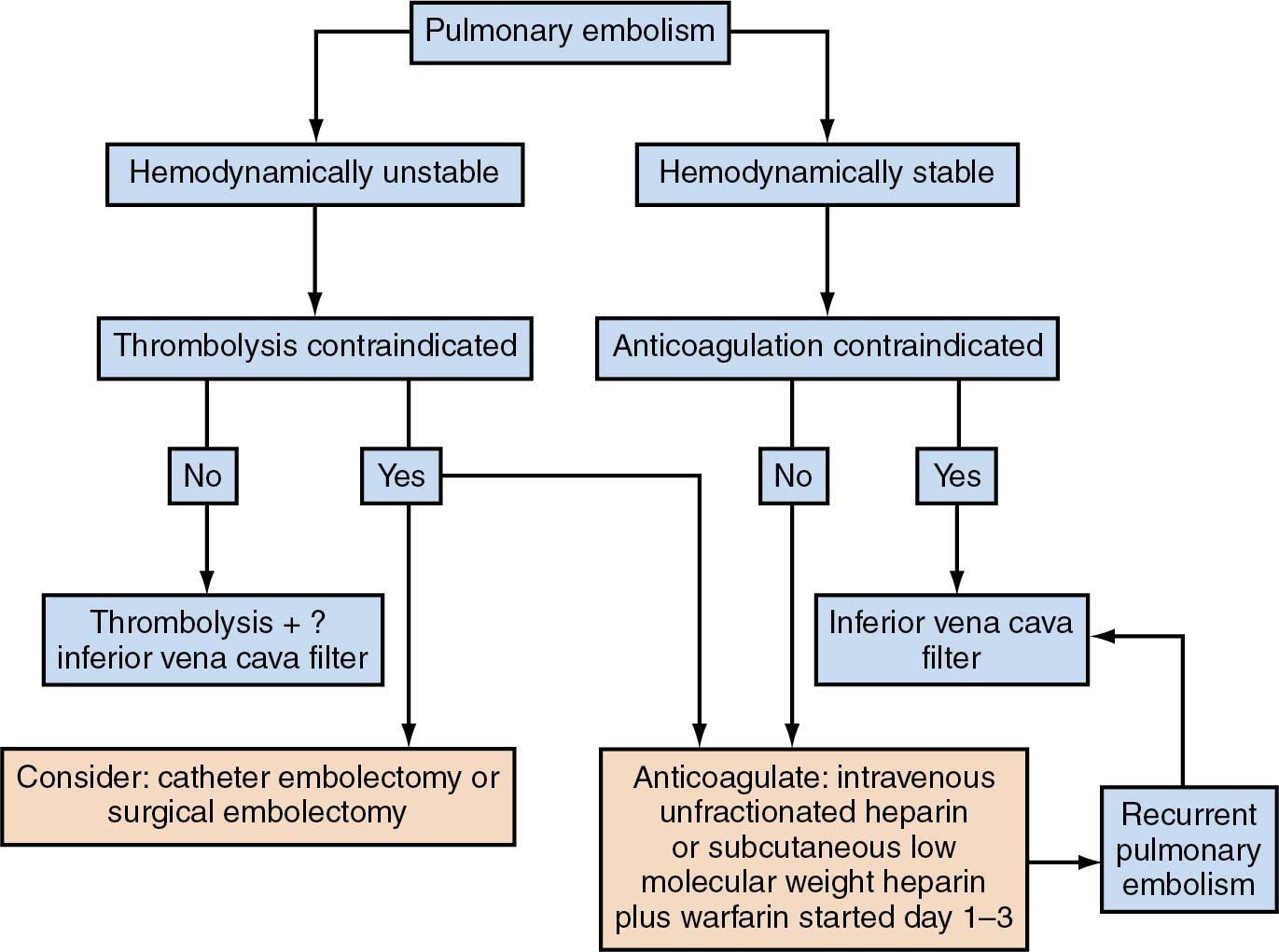
The Management Strategies and Prognosis of Pulmonary Embolus–3 trial (MAPPET-3) found that in severely affected patients (but not those in shock), thrombolytic therapy was superior to heparin. However, for all patients, particularly those with small emboli, the increased risks of intracranial bleeding may outweigh the benefits (1% to 3% of patients). Trials have evaluated thrombolytic therapy with heparin and found the combination superior to heparin alone. A thrombolytic agent is infused IV for the first 12 to 24 hours, and heparin therapy is continued for 7 to 10 days. The clinical assumption is that approximately 7 days are needed for the intravascular venous thrombus to become firmly attached to the vein’s sidewall. In patients who have heparin allergies or develop heparin-induced thrombocytopenia (HIT), thrombin inhibitors are an alternative therapy.
One additional potential adjunct for treatment is vena cava filters. The most widely accepted indication for vena cava filters is failure of medical management or a contraindication to heparin therapy. Approximately 35% of vena cava filters are placed for prophylactic indications. A randomized trial reported by Decousos and colleagues compared vena cava filters with LMWH or unfractionated heparin. They concluded that the initial beneficial effect of vena cava filters for the prevention of PE is counterbalanced by an excess of recurrent DVT, without any difference in mortality rates. Treatment of a massive PE in an unstable woman involves a choice of thrombolytic therapy, pulmonary artery embolectomy, transvenous catheter embolectomy, or filter placement in the inferior vena cava.
All patients with pulmonary emboli should have anticoagulation maintenance therapy for 3 or more months after assessment of the patient’s bleeding risk ( ). Options for treatment range from LMWH to warfarin or DOACs as discussed earlier. The risk of a woman developing a subsequent fatal PE during the 3 months of anticoagulation therapy is approximately 1 in 70 to 100.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here