Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Surgical site is typically hostile after resection and/or radiation for an underlying condition.
Healthy tissue transfer is usually necessary to mitigate wound-healing challenges.
A proactive surgical approach can help lessen impact of complications.
Access video and video lecture content for this chapter online at Elsevier eBooks+ ![]()
![]()
Perineal reconstruction poses the challenge of healing a problematic area with multiple site-specific obstacles. Although a number of benign conditions exist which can require resurfacing perineal skin, more frequently reconstruction is needed after treatment of underlying cancer. Modern treatment of malignancies in this region has evolved to more frequently use neoadjuvant radiation therapy, which can yield a high rate of complications at the time of surgical intervention. Local effects of radiation therapy and close proximity (combined in many cases with ostomies) to bowel, reproductive organs and bladder, can all complicate reconstructive flap inset, contaminate the surgical site, and threaten surgical outcomes. Successful reconstruction takes into account these impediments and minimizes the consequences of complications in this hazardous region.
The evolution of perineal reconstruction over the last century parallels the development of the reconstructive ladder. Since Miles described abdominoperineal resection in 1910 for carcinoma of the rectum, numerous methods of reconstructing the perineum have been described. In his original paper, the perineal wound was simply packed with gauze, with closure by secondary intention or delayed primary closure. Brunschwig’s introduction of pelvic exenteration provided the potential for oncologic cure, but left an even larger pelvic defect with significant associated morbidity. This lead to significant morbidity associated with an open perineal wound, and Altmeier subsequently introduced the concept of primary closure of the perineal wound with continuous suction drainage. Although there was some degree of success with both primary and secondary closure of the perineum, certain perineal wounds seemed predisposed to breakdown, particularly those that were irradiated and contaminated. Management of the draining perineal wound proved difficult; split-thickness skin grafts were attempted with limited success.
The advent of axial pattern flaps ushered in the next rung on the reconstructive ladder for perineal reconstruction. Although first applied for coverage of exposed bone in the lower extremity, muscle flaps were soon utilized throughout the body, including the perineum. The gluteus maximus flap was described as a potential cure for a chronic perineal sinus. The rectus abdominus flap subsequently emerged to be a workhorse in perineal reconstruction. Transverse, vertical, and oblique skin paddles have been designed and used effectively. The gracilis muscle has also been described extensively for use in the perineum.
More recently, fasciocutaneous flaps have been reported for perineal reconstruction. Wee introduced the concept of neuro-pudendal thigh flaps for vaginal reconstruction, creating sensate lining for the vaginal wall. The anterolateral thigh flap has emerged as a versatile flap that can be applied to perineal reconstruction as well as complex pelvic wounds. Additionally, the study of angiosomes and perforasomes has identified local and regional flaps of the perineum, which play a role in resurfacing of cutaneous infectious processes and malignancies.
Finally, advances in microsurgery have allowed free flaps to be performed routinely with reliable results. Although the utility of free flaps in perineal reconstruction has been somewhat limited by the ample locoregional flaps available, there have been reports of free flaps for coverage of large defects in the perineal area, particularly in conjunction with sacral defects. Moreover, microsurgical techniques developed over the last decade have allowed perforator flaps to be applied to this region of the body as well, based on essentially every regional vascular system. In summary, significant advances have been made over the past century for perineal reconstruction. The remainder of this chapter will describe in detail some of the flaps routinely used today for perineal coverage, and touch on promising advances in technique that are on the horizon.
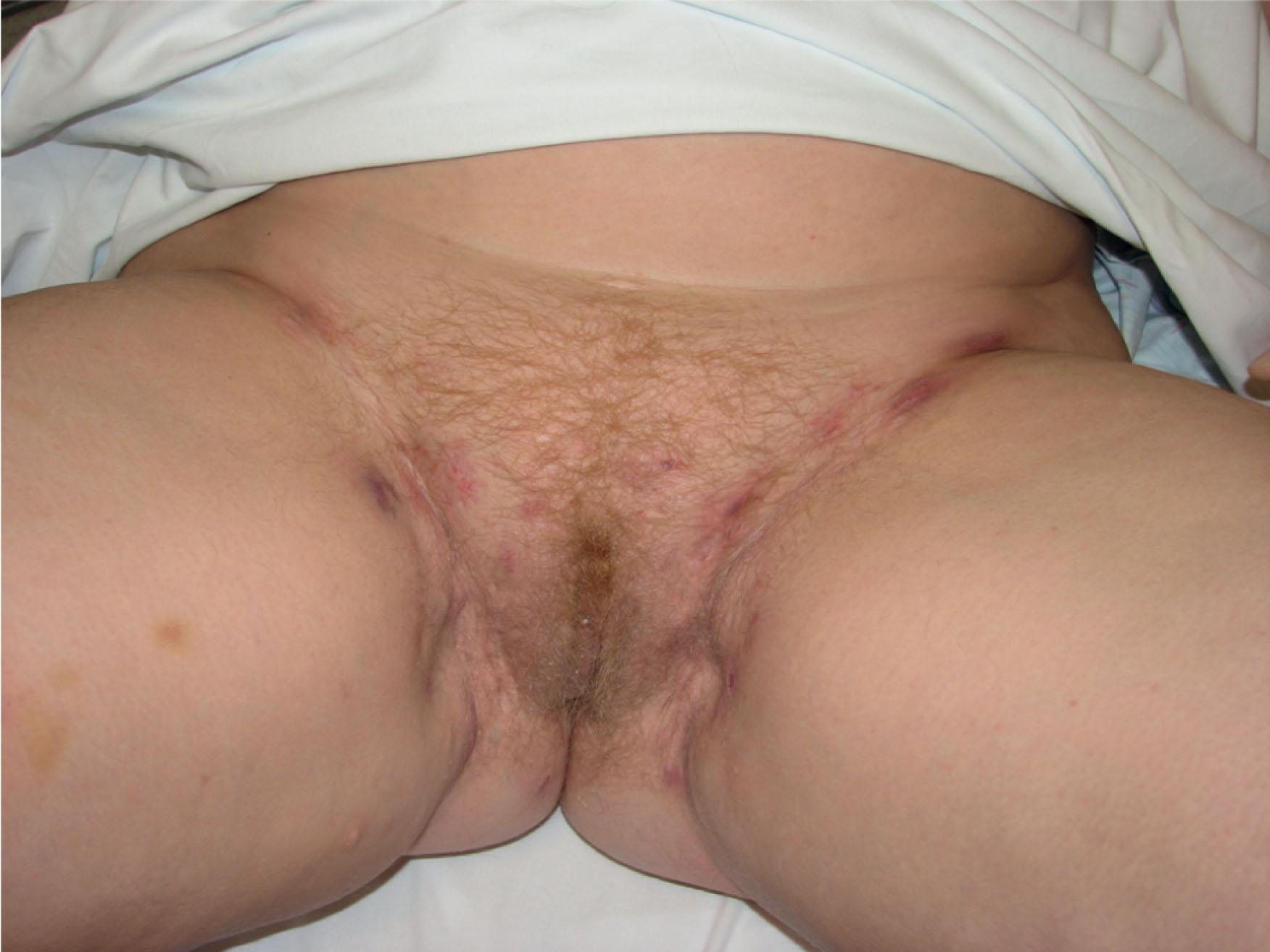
The processes requiring reconstruction of the perineum can broadly be broken into superficial and deep processes ( Table 17.1 ). Benign conditions primarily include hidradenitis suppurativa, infectious destructive processes such as fasciitis or Fournier’s gangrene, and trauma, but can range to other rarer dermatologic conditions such as pyoderma gangrenosum and vasculitic ulcers. Malignant superficial lesions usually include vulvar cancers and cutaneous lesions in the area, such as extramammary Paget’s disease ( Fig. 17.1 ). These entities have in common the depth of involvement, which typically leaves only an external cutaneous deficit on the perineum (see Fig. 10.18 ). Radiation treatment for these given wounds is unusual so the surrounding skin is typically healthy. Once the primary process is addressed and resolving, the defect can begin secondary healing. Negative pressure therapy and skin grafts can often play a useful role in shortening the time to healing. Some authors advocate locoregional flaps to hasten treatment for superficial defects, although this has yet to gain universal utility. Challenges to healing in this area include a high bacterial bioburden in many of the causative etiologies, an inherently moist surgical site, and pressure, tension, and shearing forces, which threaten to disrupt the site as the patient begins to mobilize. Outcomes are more favorable in this group.
| Process | Etiology | Complicating factors | Reconstructive options |
|---|---|---|---|
|
|
|
|
|
|
|
|
Deep defects are primarily the result of malignancy, including colorectal cancer, urologic and gynecologic cancers, most of which originate from and involve pelvic structures. Abdominoperineal resection or exenteration has been demonstrated to provide the best cure in these cases. Typically, this extensive resection is accompanied by perioperative radiation therapy. Treatment of these more complex defects must be individualized for the missing and exposed structures. The bony pelvis effectively splints open the pelvic outlet, so contraction cannot occur, and seromas are common. Further impediments to healing such as fistulas and radiation changes in the area generally mean these defects are frequently contaminated, prone to infection, and will not heal without the addition of a non-radiated tissue transfer. After extensive resection, large areas of dead space and loss of support allow the abdominal viscera to fall into the irradiated pelvic basin. This can lead to pelvic hernias, adhesions, obstruction, and fistulas, all of which can be disastrous to treat secondarily. Nearby organs such as the bladder or vagina may also be resected in part or whole as part of the treatment course. Finally, the surgical incision is inherently moist, and mobilizing the patient can cause pressure or shearing stresses, further compounding the difficulty of healing a radiated closure at that location. Surgical outcomes reflect the many challenges posed in this group.
Patients with superficial disease may present at various stages in their treatment. As a rule, reconstruction should not be initiated until the primary process is identified and resolved with repeated surgical debridement or excisions; underlying medical conditions or autoimmune disorders should be treated. Typically, areas of external pudendal skin are involved and will require resurfacing in some fashion (see Fig. 17.1 ).
Patients with deep disease are typically treated in conjunction with a multidisciplinary oncologic team for an underlying malignancy. Often the extent of the defect is not obvious until after the surgical resection is performed, and must be anticipated in advance. Preoperative consultation is important to manage patient expectations, outline the possible extent of surgery, and prepare for postoperative adversity. Such cases may involve more extensive external pudendal defects ( Figs. 17.2 & 17.3 ) or deeper structures ( Fig. 17.4 ). In the event that vaginal resection is necessary, preferences regarding vaginal versus simple perineal reconstruction should be discussed, along with a thorough dialogue about sexual function. Further details and management of vaginal reconstruction are discussed in depth in Chapter 15 .
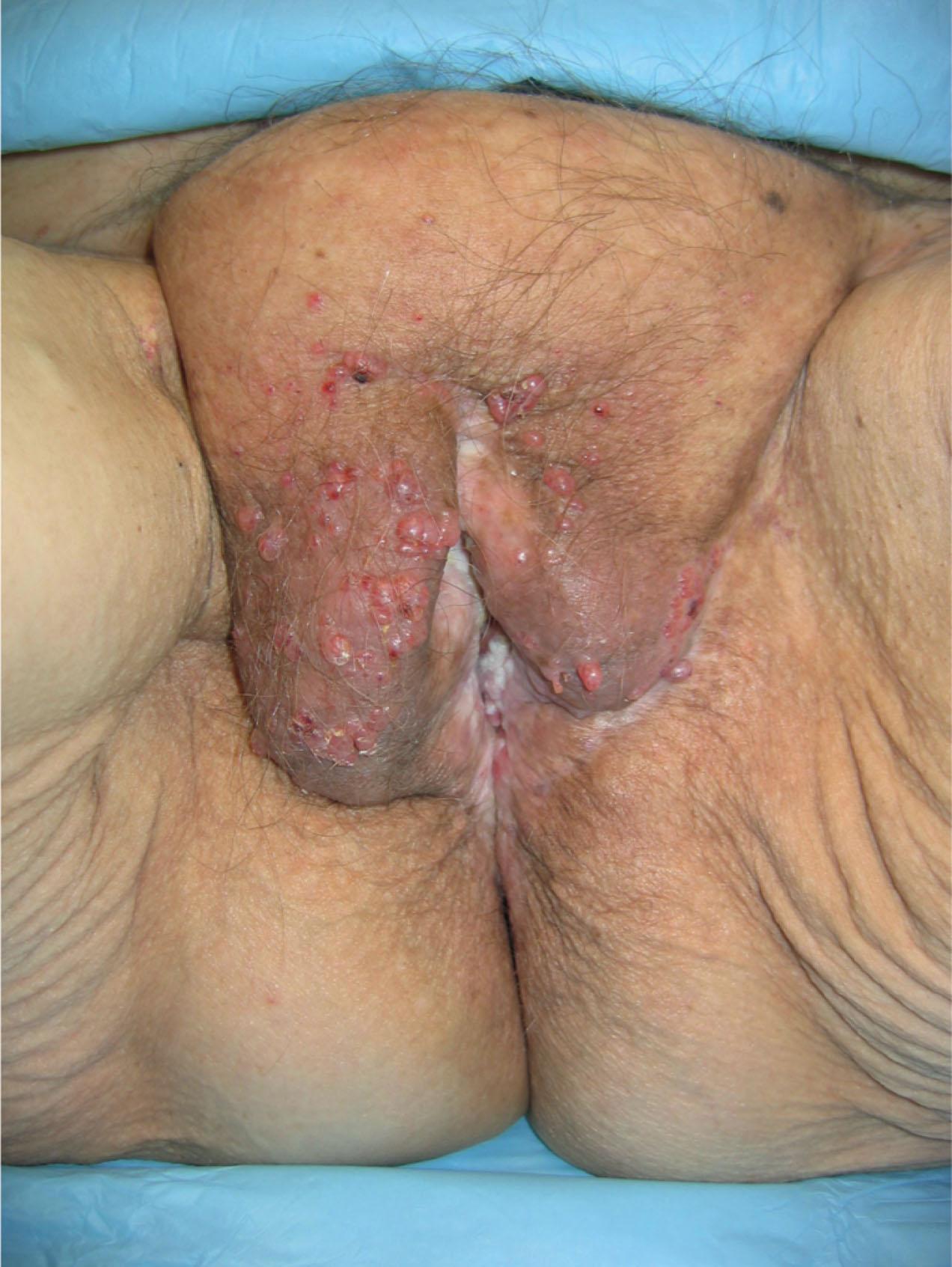
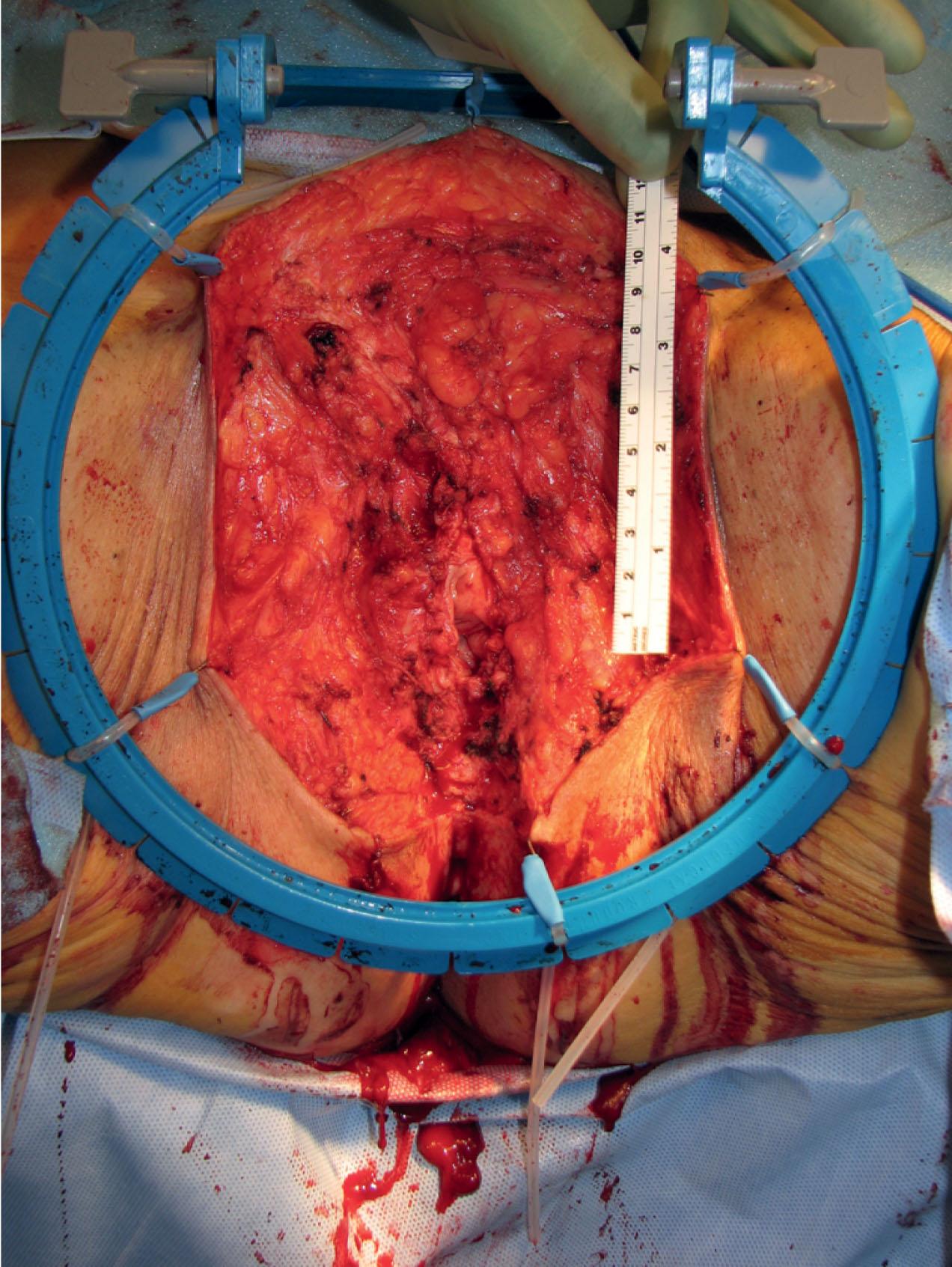
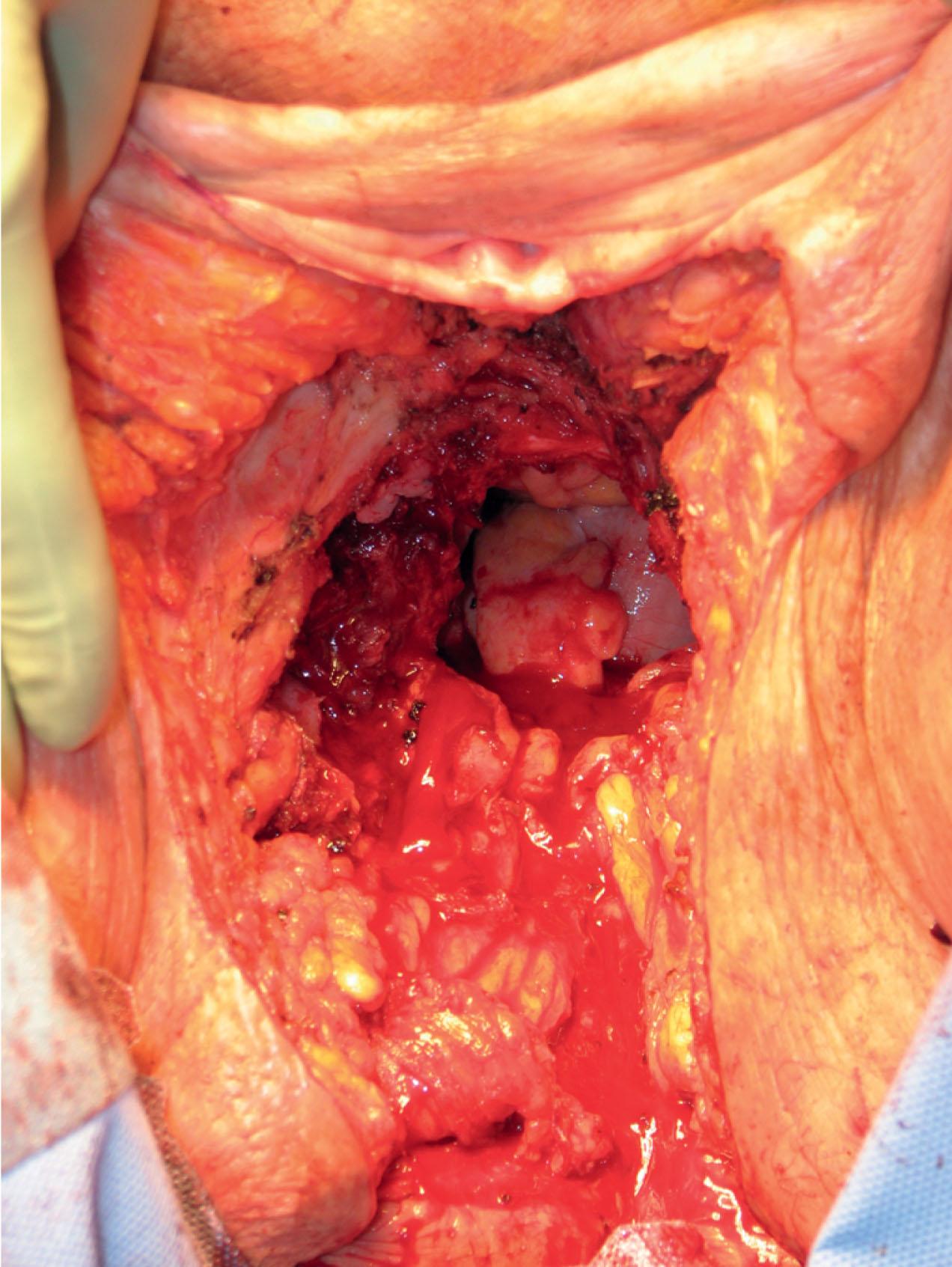
Patients with superficial defects are more straightforward in management; as their underlying process resolves, the defect begins to heal secondarily, even in response to a conventional wet to moist dressing regimen. Typically, a modest, shallow perineal defect is left. A number of publications support the use of a negative pressure therapy plan to accelerate the healing process. Within a matter of weeks, a healthy wound bed can be established, confirming the suitability of these patients for skin grafting. If the wound fails to begin the healing process despite wound healing optimization, a more complex approach and tissue transfer might be warranted. Especially for superficial malignancies such as vulvar cancer, reconstruction at the time of resection is feasible, and standard in many centers. Application of microsurgical principles for perforator flap dissection has been proposed in that setting.
In the case of deeper malignancies, such as colorectal, gynecologic, or urologic cancer, radiation therapy is part of the preoperative routine in most centers. This factor alone is the most significant indication for flap reconstruction at the time of resection. Large prospective series suggest that abdominal-based flaps demonstrate the most favorable outcomes and complication profile in this setting, even compared to thigh-based flap reconstruction. This must be balanced with the abdominal morbidity of the harvest. Some surgeons prefer to avoid using abdominal flaps if ostomies are necessary on both sides, although these can be inset through the external oblique musculature with no additional morbidity. A vertical skin pedicle provides the most discreet scar, but an oblique paddle can provide more tissue by extending off the muscle, effectively also extending its reach. Pre-existing abdominal wall defects might direct some surgeons to alternative flap options. Nevertheless, Butler has shown no increased incidence of abdominal wall problems after abdominal flap harvest. Additionally, when faced with large abdominoperineal resection (APR) or pelvic exenteration defects, systematic reviews and meta-analyses have demonstrated the efficacy of muscle flap closure as compared to primary closure in decreasing total and major perineal wound complications. Rarely, perineal resection can be performed without laparotomy, and this might predispose toward use of a non-abdominal flap option.
The complication profile of the gracilis flap is also reasonable, and represents a dramatic improvement over primary closure under these circumstances. Early studies comparing gracilis reconstruction to primary closure demonstrate a dramatic reduction in infectious complications from 46% to 12% with flap reconstruction. Limitations are the small muscle bulk and questionable skin island, but recent modifications may address the latter based on capturing additional perforators.
The anterolateral thigh flap represents an alternative option with documented excellent reliability for many applications. There is growing literature to support its use as a reliable regional option for perineal reconstruction. This is especially true if the abdomen cannot serve as a donor site. With the addition of the vastus lateralis muscle, it can also play a role for addressing the dead space left behind after large pelvic defects. However, more studies comparing efficacy between rectus abdominis flaps and anterolateral thigh flap with vastus lateralis may corroborate this in time.
Some surgeons favor a posterior thigh flap, harvested in similar fashion to the anterolateral thigh flap, but the axial nature of the perfusion off the inferior gluteal artery has been called into question. Recent series document a wound healing complication rate of over 50% using this method, which does not compare favorably to abdominal flaps.
For defects with less dead space or requiring less bulk, skin flap reconstruction (e.g., Singapore or perforator flaps) can be used, although outcomes data are more mixed and overall less favorable compared to abdominal flaps. Complications are reported to range from 7% to 62%. Recent outcome studies are not available directly comparing this method to other options in head-to-head fashion, but there is a growing role for local skin flaps in coverage of superficial wounds resultant from cutaneous infections or malignancies.
Massive defects in pelvic support may in fact require multiple flaps. Occasionally pelvic floor support is needed in the form of mesh reinforcement. In a hostile, radiated, often contaminated surgical site, prosthetic mesh is contraindicated. Newer bioprosthetic options provide a reinforcement alternative that is more robust in the face of complications.
Finally, if the two abdominal flaps and eight thigh-based flaps are unavailable or insufficient for the defect, there are several reports of distant flaps used in conjunction with microsurgical transfer. Composite thigh tissues can be transferred en bloc , and large surface area flaps such as the latissimus dorsi have been reported in extreme cases.
Laparoscopic or robotic techniques represent a new frontier in both extirpative and reconstructive surgeries. Performing the entirety of an abdominoperineal resection is now possible through incisions that total 2 cm in length. As techniques are developed for resection without an open laparotomy, the necessity for harvesting non-radiated tissue to fill the radiated pelvic basin remains. More challenging is avoiding a midline laparotomy incision for flap harvest, when extirpation avoids its use. Typically this entails harvest of the rectus muscle through the posterior sheath using either a laparoscopic or robotic approach. Laparoscopic tools are widely accessible, but robotic equipment is less widely available, and surgeons trained in using that for reconstruction are rarer still. Yet, plastic surgeons are no strangers to innovation, and it is certain that adoption of robotic techniques will be met by future plastic surgeons within the growing field of minimally invasive reconstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here