Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pericardium derives its name from the Greek term peri, meaning “around,” and kardia, meaning “heart.” It is composed of two layers. The inner layer (visceral pericardium) consists of a single layer of mesothelial cells, collagen, and elastin fibers separated from epicardium by fat. The outer layer (parietal pericardium) is mostly acellular and consists of collagen and elastin fibers. The pericardial space between these two layers contains a small amount of serous fluid. The pericardium surrounds all the cardiac chambers except the left atrium, which is extrapericardial. Superiorly, the fibrous envelope extends to the base of great vessels.
Although not essential for survival, the pericardium has some important functions. It acts as a barrier to prevent spread of infections, inflammation, and neoplasia. The fluid in the pericardial space allows free movement of the heart throughout the cardiac cycle, limits its acute distension, and helps in ventricular coupling. The parietal layer imparts characteristic physical properties to the pericardium. At low level of stretch, it is very elastic but as the stretch increases, the tissue stiffens abruptly and become resistant to further stretch. As a result, the pericardium exhibits a nonlinear pressure-volume curve with an initial flat portion and a steep ascent later ( Fig. 57 . 1 ).
The pericardium is afflicted in variety of diseases, both localized to the heart and as part of systemic illnesses. Pericardial diseases cause distinctive hemodynamic changes and some of the classic physical findings in cardiology. Although clinical overlap is common, most of the cases can be categorized as having pericarditis, pericardial effusion with or without tamponade, and pericardial constriction. Other structural abnormalities such as congenital absence of pericardium and pericardial cysts are rare ( Table 57 . 1 ). This chapter provides an overview of common pericardial diseases in children.
| Pericarditis and Pericardial Effusion | Pericardial Constriction | Congenital |
|---|---|---|
| Infectious Viral Bacterial Mycobacterial HIV associated Fungal Noninfectious Inflammatory Connective tissue disorder Postpericardiotomy Autoinflammatory disorders Drug induced Neoplastic Primary Secondary Radiation induced Traumatic Iatrogenic Postcardiac intervention Postradiofrequency ablation Miscellaneous Chronic renal failure Hypothyroidism |
Tubercular pericarditis Bacterial pericarditis Radiation induced Postcardiac surgery Autoimmune disorder Idiopathic |
Pericardial cyst Absent pericardium |
Pericarditis is the most common pericardial pathology encountered in clinical practice. Based on the duration of illness, it can be classified as acute, recurrent, or chronic. Acute pericarditis is usually self-limiting, lasting less than 2 weeks. In some cases, the inflammation may continue up to 3 months beyond which it is labelled as chronic pericarditis. Concomitant involvement of the myocardium is referred to as “myopericarditis.”
Acute pericarditis is an acute inflammation of the pericardium. The exact incidence of acute pericarditis in childhood is not known. Most cases of acute pericarditis are idiopathic or due to viral infections. Some cases labeled as idiopathic pericarditis may actually be due to viral infections.
Older children and adolescents present with acute onset of sharp, substernal chest pain radiating to the trapezius ridge. The pain worsens with inspiration and on lying down and subsides with leaning forward. The pain in young children is variable though postural and respiratory variation is preserved. Pericardial friction rub is the most characteristic physical finding. This high frequency, superficial scratching sound is similar to the sound made when walking on crunchy snow. It results from the friction between inflamed pericardial layers and typically consists of three components corresponding to ventricular systole and early and late diastolic filling. It is best heard during inspiration, along the left sternal border with the patient leaning forward. The rub is often intermittent necessitating frequent auscultation. It disappears with the accumulation of significant pericardial fluid. Nonspecific symptoms and obvious difficulties in eliciting clinical signs make the diagnosis challenging in infants and young children.
Electrocardiography (ECG) is perhaps the most important diagnostic tool. It is useful in the diagnosis at an early stage of the disease. ECG shows sequential changes in ST and PR segments. The ST-segment elevation is usually concave upwards and is seen in all the leads except in leads V1 and aVR, which show depressed ST-segment ( Fig. 57.2 ). There is generalized PR-segment depression except in leads V 1 and aVR. Based on ECG findings, four stages of acute pericarditis are described, although each patient may not have this stage-wise transition. In stage 1, ST-segment is elevated with PR depression. In stage 2, ST-segment normalizes and T wave is flattened. T wave is inverted in stage 3, while in stage 4, ECG is nearly normalized. In some patients, ST-segment changes are limited to only a few leads. Occasionally, PR-segment depression is the only ECG manifestation. The ECG may be completely normal in 10% of patients with acute pericarditis. The ECG also sometimes provides clues toward specific diagnosis of acute pericarditis. For example, presence of atrioventricular block may indicate Lyme disease, while low voltage complexes and electrical alternans indicate significant pericardial effusion. Chest radiograph is essentially normal in an uncomplicated acute pericarditis. Echocardiogram is done mainly to detect pericardial effusion. Pericardial effusion is present in about two-thirds of patients with pericarditis. In the vast majority, the effusion is small and is of no concern. Large pericardial effusion is seen in about 3% of patients. A new onset of left ventricular dysfunction should alert to the possibility of myopericarditis.
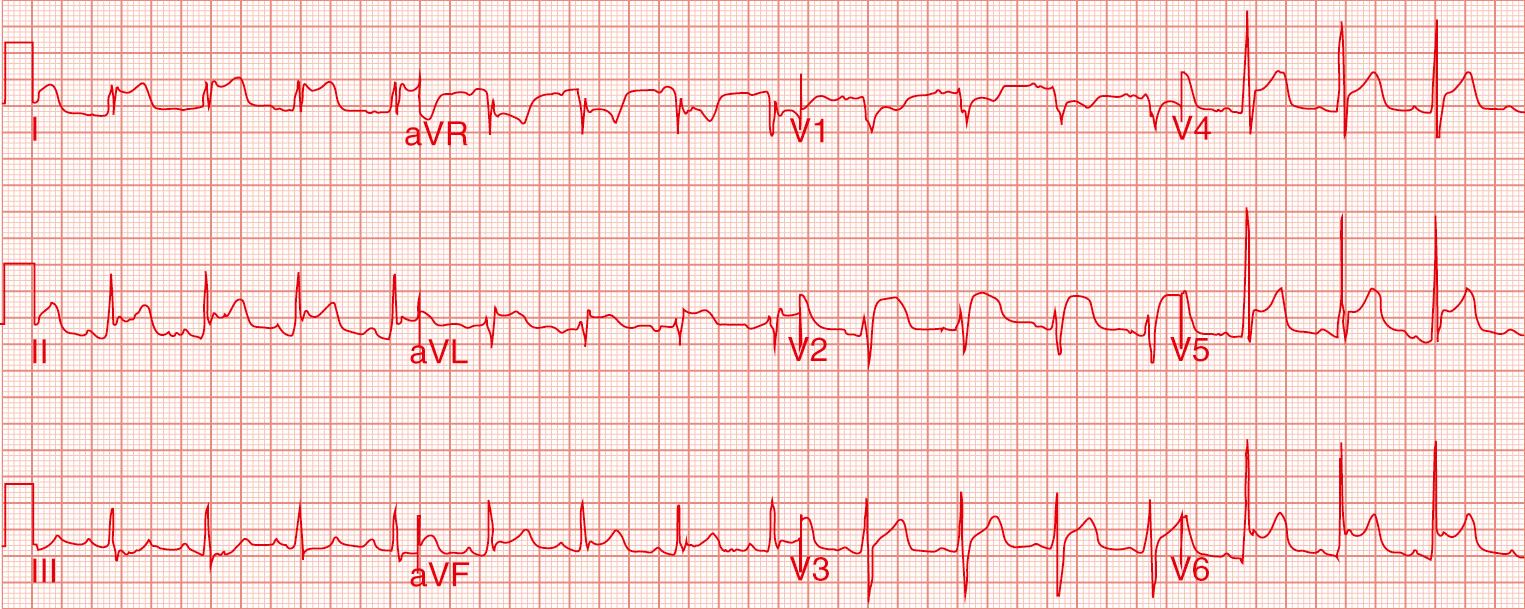
Acute pericarditis is diagnosed clinically if any two of the following abnormalities are present: pericarditic chest pain, pericardial rub, new onset ST-T changes on ECG, and new onset or worsening of pericardial effusion. In addition, C-reactive protein, erythrocyte sedimentation rate, leucocyte count, and other markers of inflammation indicate acute inflammation of the pericardium. Elevated troponin and other markers of myocardial injury suggest concomitant myocarditis. Data from adults suggest that high-sensitivity C-reactive protein (hs-CRP) is elevated in three-fourths of patients with acute pericarditis and predicts a higher rate of recurrence. In most cases, hs-CRP levels normalize within a week and in almost all cases by 4 weeks. The level of hs-CRP is useful in guiding the duration of therapy.
There remains a paucity of randomized controlled trials to guide management of childhood pericarditis. Routine testing for detecting viral etiology is not recommended as the yield is low and it is not cost effective. A specific cause is identifiable in only 17% of patients and is more frequently found in cases having a subacute course with fever, large pericardial effusion, and poor response to nonsteroidal antiinflammatory drugs (NSAIDs). Initial management should focus on screening for specific causes, prompt detection of pericardial effusion, and alleviation of symptoms. For symptomatic relief, the patient should receive NSAIDs. There are no studies comparing different NSAIDs for the treatment of acute pericarditis in children. Ibuprofen in a dose of 20 to 30 mg/kg per day in 2 to 3 divided doses is preferred considering its better side-effect profile. Indomethacin, naproxen, and acetylsalicylic acid are other alternatives. Children with prompt response to NSAIDs and having mild pericardial effusion can be managed on an out-patient basis. Others with severe symptoms, poor response to therapy, or those having large pericardial effusion should be hospitalized.
Patients with poor response to NSAIDs have traditionally been treated with corticosteroids. Their use, however, is associated with higher rate of recurrence, particularly if a short course of high-dose steroid with rapid tapering is used. Therefore a lower initial dose of 0.25 to 0.5 mg/kg per day of prednisolone with gradual tapering over 8 to 12 weeks is preferred. Colchicine is more effective than corticosteroids in reducing the risk of recurrences. The recommended dose of colchicine is 0.5 to 1 mg/day for 3 months. These doses are generally well tolerated. Gastrointestinal side effects result in drug discontinuation in approximately 10% of patients. Some authors recommend using both corticosteroid and colchicine in patients with poor response to NSAIDs. In such cases, NSAIDs are initially withheld and are resumed once corticosteroids are tapered.
Acute idiopathic pericarditis is usually self-limiting and complete resolution is expected within 2 to 3 weeks. Female gender, presence of large effusion, and failure of NSAIDs predict a higher risk of complications. In a large series of 453 adult patients with acute pericarditis, 3.1% developed cardiac tamponade and 1.5% developed pericardial constriction.
Recurrent pericarditis is defined as the reappearance of pericardial inflammation after an initial attack of acute pericarditis with an asymptomatic period of 4 weeks or longer. The recurrence is thought to be related to a repeat episode of acute pericarditis or an autoimmune response. While recurrent pericarditis is seen in 15% to 30% adults, it is uncommon among children. Symptoms are usually not as severe and findings of pericardial rub, ECG changes, and pericardial effusion are less frequent.
The pericardial pain is treated using NSAIDs. Colchicine or corticosteroids are used for pericardial inflammation. The risk of future recurrences increases to 50% after the first recurrence if treated with corticosteroids. Colchicine, on the other hand, is useful in preventing future recurrences. A longer course of colchicine therapy for 6 to 12 months is usually recommended. Pericarditis refractory to NSAIDs, steroids, and colchicine is challenging and may need immunosuppressive agents such as azathioprine, cyclophosphamide, or intravenous immunoglobulins. Newer agents such as interleukin-1β inhibitor, anakinra, and tumor necrosis factor-α inhibitors are also effective in refractory pericarditis. Pericardiectomy may be considered for debilitating symptoms despite advanced immunosuppression.
Recurrent pericarditis may be part of a generalized autoinflammatory disease resulting from abnormal activation of the immune system. Most extensively studied autoinflammatory syndromes are familial Mediterranean fever (FMF) and tumor necrosis factor receptor-1–associated periodic syndrome (TRAPS). Clinically, both the conditions present with fever, rash, polyserositis, arthralgia, and arthritis. FMF is an autosomal-recessive disorder due to mutation in a gene on chromosome 16p13 and presents during adolescence and adulthood. Colchicine is effective in aborting and preventing its recurrence.
TRAPS result from mutation in tumor necrosis factor receptor (TNFRSF1A) gene located on chromosome 12p13 and has an autosomal-dominant inheritance. TRAPS typically has childhood onset. Unlike FMF, the inflammation in TRAPS is mediated by tumor necrosis factor and interleukin-1. Steroids may be useful in treating an episode but the inflammation responds poorly to colchicine. Tumor necrosis factor-α inhibitor (etanercept) is shown to reduce frequency and severity of flares. Refractory cases respond to interleukin-1 inhibitors (anakinra).
All causes of pericarditis can potentially result in pericardial effusion. Pericardial effusions can be classified based on its onset (acute, subacute, or chronic), distribution (loculated or circumferential), composition (exudative, transudative, blood, or pus), and hemodynamic impact (none, cardiac tamponade, or effusion constrictive).
In most clinical conditions with inflammation, infection, or injury, the pericardial fluid is exudative while in cases with fluid overload and elevated systemic venous pressure, it is transudative. The hemodynamic consequences of pericardial effusion are determined by the amount of fluid and rapidity of accumulation. Gradual accumulation of fluid shifts the pericardial pressure-volume curve to the right. As a result, even large effusion has no compressive effect on cardiac chambers and consequently may remain asymptomatic. The nonlinear pressure-volume curve of pericardium results in an almost vertical rise in pericardial pressure after an initial slow ascent (see Fig. 57.1 ). This late steep rise in pressure is responsible for “last drop” phenomenon: the final increment of fluid producing critical cardiac compression while the first decrement during pericardial drainage leads to the largest relative decompression.
Cardiac filling is altered as the cardiac chambers compete with the pericardial fluid in the relatively fixed pericardial space. The filling pressures in cardiac chambers are elevated and are equal to intrapericardial pressure. Elevated filling pressures limit the early diastolic filling of the ventricles and manifests as a distinctive loss of “y” descent in jugular venous pressure (JVP). Due to fixed cardiac volume, the atriums can fill only during the phase of ventricular systole when the blood exits the heart. This explains preserved “x” descent in JVP in patients with cardiac tamponade.
Pericardial fluid allows normal transmission of thoracic pressure to cardiac chambers. As a result, during inspiration, the right ventricle is preferentially filled while constrains of fixed cardiac volume limit left ventricular filling. The filling of the left and right ventricle, therefore, is 180 degrees out of phase with each other (exaggerated ventricular interdependence). The left ventricular systolic pressure falls when the systolic pressure in the right ventricle rises during inspiration and the reverse happens during expiration (ventricular discordance). The presence of an atrial septal defect, ventricular dysfunction, and aortic regurgitation may mask the findings of ventricular interdependence by nullifying the effect of respiration on ventricular preload.
Experimental and clinical studies have shown that cardiac tamponade is not an “all-or-none” phenomenon. Instead, it represents a continuum from minimal hemodynamic impairment to a state of hemodynamic collapse. Clinical examination is sufficient to recognize hemodynamic collapse but is inadequate in identifying milder hemodynamic compromise. Usually, left- and right-sided filling pressures are elevated to 20 to 25 mm Hg. In cases with hypovolemia, filling pressure may be less than 10 mm Hg. Occasionally, cardiac tamponade may be regional, resulting from localized clot or loculated effusion when typical physical, hemodynamic, and echocardiographic findings are absent. Rarely, large pleural effusion and pneumopericardium can compress the heart mimicking tamponade physiology.
The clinical presentation depends on the speed of accumulation of pericardial fluid. Slowly accumulating effusion is often asymptomatic and detected incidentally. Rapid accumulation of even small amounts of fluid can cause symptoms. Initially, compensatory sympathetic stimulation causes tachycardia, diaphoresis, and peripheral vasoconstriction. With progressive accumulation of fluid, compensatory mechanisms prove inadequate and cardiac output declines. Patients who are unable to mount a normal adrenergic response, such as those receiving β-blocking agents, are more susceptible to acute hemodynamic collapse. Dyspnea is common though the exact mechanism remains unknown.
The JVP is markedly elevated with blunted “y” and preserved “x” descent. Unimpeded transmission of thoracic pressure to cardiac chambers and consequent preferential right ventricular filling permits normal inspiratory fall in JVP. Pulsus paradoxus, characterized by an abnormally large (>10 mm Hg) decline in blood pressure during inspiration, is another distinctive clinical feature. In reality, there is no paradox and it only reflects exaggerated ventricular interdependence. The fall in blood pressure provides an estimate of ventricular interdependence and is proportionate to the severity of cardiac tamponade. Pulsus paradoxus is not unique to tamponade, and may be present in pericardial constriction, pulmonary embolism, and other pulmonary diseases with wide respiratory variation in thoracic pressures. Clinical recognition of paradoxical pulse relies on respiratory variation in Korotkoff sounds and therefore cannot be measured by currently available oscillometric sphygmomanometers. As such, the measurement of paradoxical pulse is difficult in children due to high heart and respiratory rates. Respiratory variations in plethysmography waveform on pulse oximetry correlates well with invasive assessment of pulsus paradoxus and can be used at the bedside to ascertain the diagnosis and severity of tamponade.
The heart sounds are muffled. Cardiac impulse is difficult to localize. In some cases, compression of the lower part of the lungs causes bronchial breathing and dullness to percussion (Ewart's sign). In its severe form, cardiac tamponade is characterized by Beck's triad, which includes distant heart sounds, hypotension, and distended neck veins.
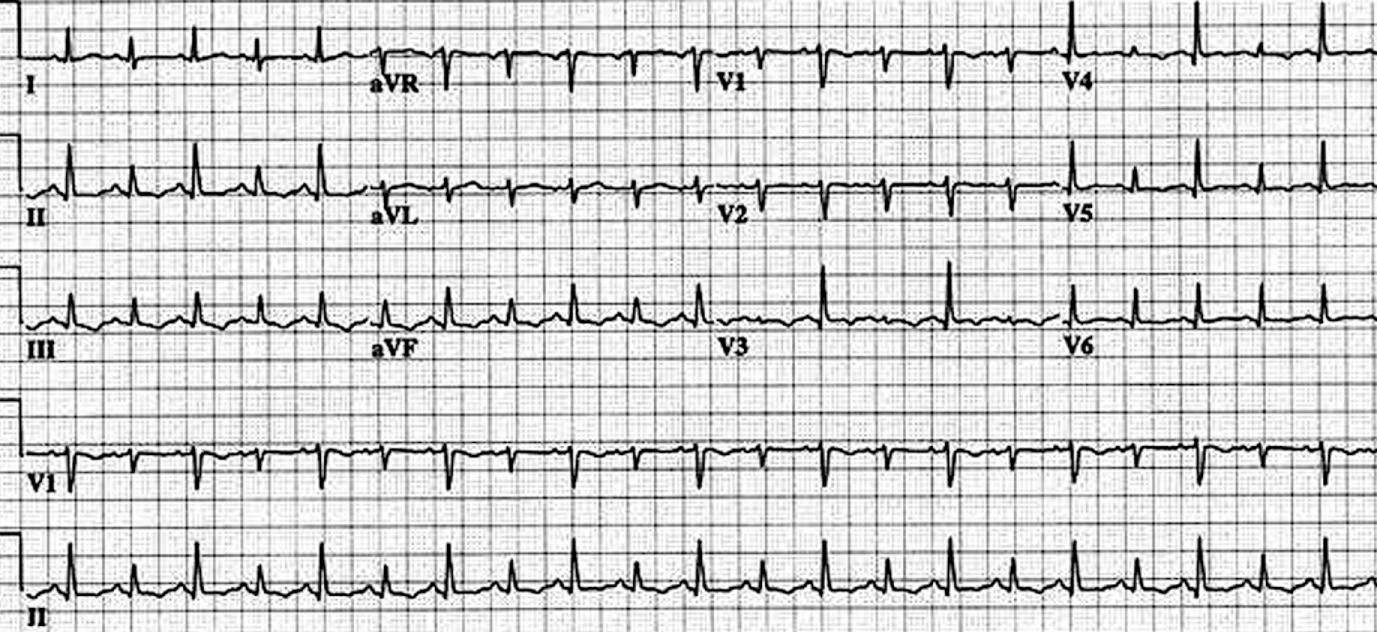
Electrocardiogram shows compensatory sinus tachycardia, low QRS voltages, and electrical alternans ( Fig. 57.3 ). Low QRS voltages are a nonspecific sign and may be seen in patients with emphysema, pneumothorax, and infiltrative myocardial diseases. Electrical alternans, on the other hand, is specific for large effusion albeit with poor sensitivity. Nonspecific ST-T changes may be present with coexisting pericarditis. The chest radiograph may be normal in patients with mild pericardial effusion. Large pericardial effusion present with a symmetrically enlarged cardiac silhouette with distinct margins assuming round, flask-like “water bottle” configuration. Lateral view radiograph shows a linear lucency separating the posterior chest wall and epicardial fat (fat pad sign).
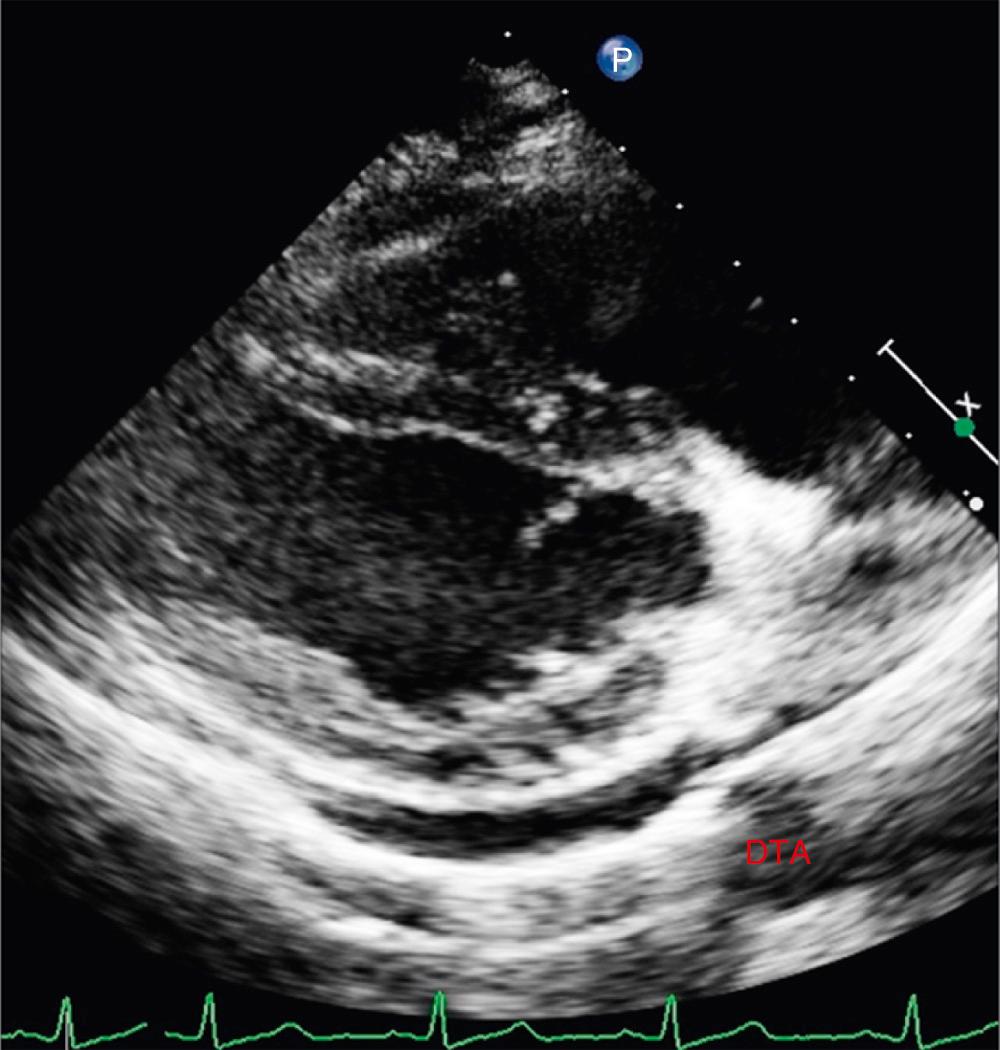
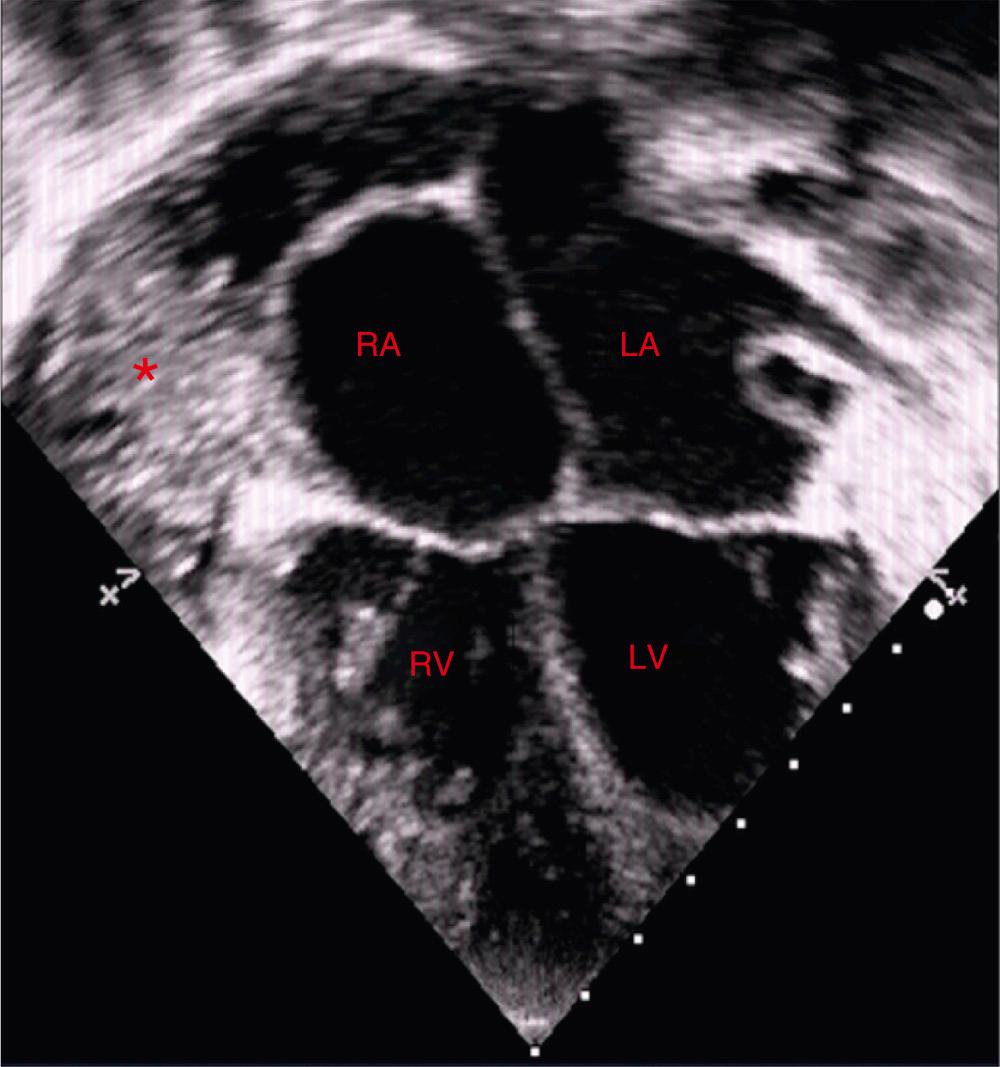
Echocardiography detects pericardial effusion with very high accuracy. In adults and adolescents, based on the width of echo-free space between visceral and parietal pericardium at end-diastole, circumferential pericardial effusion is classified as small (<10 mm), moderate (10 to 20 mm), large (>20 mm), and very large (>25 mm). There is no such standard for children. Occasionally, left-sided pleural effusion can mimic pericardial effusion. The presence of fluid between the heart and descending thoracic aorta in parasternal long-axis view localizes fluid to the pericardium ( Fig. 57.4 ; ). Echocardiography also aids in the assessment of hemodynamic impact of pericardial effusion. A dilated inferior caval vein with less than 50% inspiratory collapse is highly suggestive of cardiac tamponade. This finding may be absent in patients with hypovolemia and low-pressure tamponade. Echogenicity of pericardial fluid provides important information about the possible cause of effusion. Fibrinous strands within the fluid suggest acute inflammation and are common with bacterial pericarditis.
Pericardial fluid compresses cardiac chambers when pressure within the chamber falls below pericardial pressure. The collapse of right-sided chambers occurs earlier than the clinical detection of pulsus paradoxus. A brief collapse of thin-walled right atrium during late ventricular diastole may be normal. Nonetheless, right atrial collapse exceeding one-third of the cardiac cycle is nearly 100% sensitive and specific for cardiac tamponade. There can be right ventricle collapse during early diastole. The duration of right ventricular collapse is proportionate to the severity of tamponade, initially occurring only during inspiration and extending to expiration at later stages. The collapse of right-sided chambers may be absent in conditions with elevated pressure in the right ventricle as in pulmonary hypertension, pulmonary stenosis, or left ventricular dysfunction. Conversely, collapse may occur earlier in the setting of hypovolemia. In patients with posteriorly loculated pericardial effusion or pulmonary hypertension, there can be collapse of left atrium or left ventricle instead of right-sided chambers. A large pericardial effusion may cause beat-to-beat swinging of the heart.
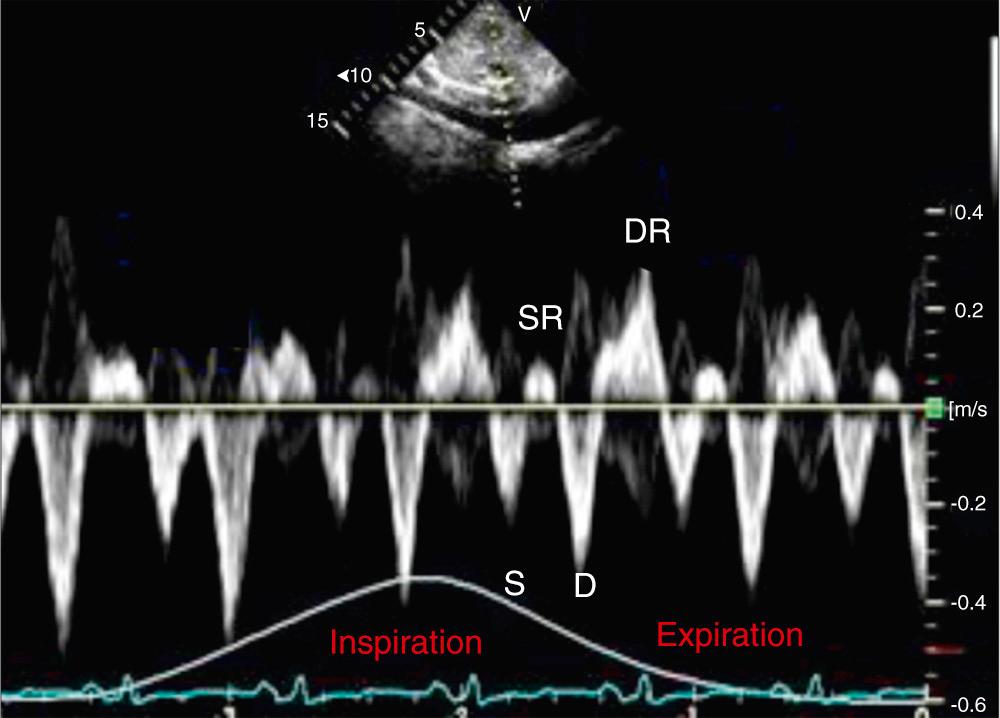
Doppler interrogation provides direct demonstration of altered ventricular filling. Doppler abnormalities are more sensitive than M-mode and two-dimensional echocardiographic abnormalities. Exaggerated ventricular interdependence on Doppler interrogation manifests as marked respiratory variation in mitral and tricuspid inflow velocities. The mitral inflow velocity, measured at the peak of the E wave, increases greater than 25% during expiration than during inspiration. On the other hand, the tricuspid inflow velocity increases during inspiration. Reduced filling of the right ventricle during expiration is also reflected as accentuated expiratory diastolic flow reversal in hepatic vein Doppler ( Fig. 57.5 ).
Cardiac computed tomography (CT) and magnetic resonance imaging (MRI) are not required routinely. Pericardial thickening and contrast enhancement suggests active inflammation. Attenuation values of pericardial fluid on CT allows distinction of transudate from exudate. An attenuation value of less than 10 Hounsfield units (HU) suggests transudate; 20 to 60 HU indicates purulent, malignant, or myxedematous collection; while greater than 60 HU suggests hemopericardium.
Established or impending tamponade is an emergency, with most patients requiring urgent pericardial drainage. Intravenous hydration with normal saline should be started immediately. Inotropic agents should be started in those with hypotension although their efficacy is limited.
Patients with no or minimal symptoms, even with a large collection, can be carefully observed without pericardial drainage. Even if drainage of pericardial effusion is needed, one-time closed pericardiocentesis is generally sufficient. The decision of pericardiocentesis must be individualized after careful clinical judgement. Medical therapy may be sufficient in some cases. For example, an effusion related to acute pericarditis and connective tissue disorder may respond promptly to NSAIDs and corticosteroids, respectively. Hypothyroidism-related effusion also responds quickly to thyroid replacement therapy. The use of NSAIDs and/or colchicine may be useful in children with recurrent large but asymptomatic pericardial effusion. A detailed evaluation for specific etiology helps in disease-specific management. However, similar to acute pericarditis, extensive testing is not cost effective.
The standard technique for closed pericardiocentesis involves subxiphoid insertion of needle into the pericardial space keeping the needle tip toward the left shoulder. Alternatively, based on the site of maximum pericardial effusion, an apical or left parasternal approach may be used. Aspiration of the pericardial fluid confirms correct positioning of the needle. Needle position can also be confirmed by echocardiographic visualization of microbubbles in the pericardial space following an injection of agitated saline ( Fig. 57.6 ; ). An injection of iodinated contrast under fluoroscopic vision is also useful for confirmation of needle position. Once the position is confirmed, an appropriate-diameter guidewire is placed through the needle over which a pigtail catheter is inserted. The catheter is manipulated to achieve the most dependent position for continuous drainage of pericardial fluid. The procedural success rate is 97% and major complications are seen in 1% to 2% of cases. Typically, the intrapericardial pigtail catheter is left in-situ for a variable period of time, sometimes several days, for continued drainage. The frequency of aspiration is dictated by the rate of reaccumulation. Open pericardiocentesis and creation of a pericardial window is preferable for refractory, recurrent pericardial effusions and hemopericardium.
A careful analysis of pericardial fluid is rewarding in most cases. Drainage of pus confirms the diagnosis of bacterial pericarditis, while serosanguinous fluid has limited diagnostic utility. Chylous effusion, rich in triglycerides, occurs after traumatic or surgical injury to the thoracic duct. Typical gold-paint cholesterol-rich effusion is seen in severe hypothyroidism. In addition to routine measurement of hematocrit, cell counts, sugar, and protein, the pericardial fluid is subjected to special tests based on possible etiology. In cases with a high suspicion of tuberculosis, pericardial fluid analysis should include measurement of adenosine deaminase and polymerase chain reaction–based detection of mycobacterium. In the appropriate clinical setting, pericardial fluid should also be subjected to measurement of tumor markers and malignant cells. Based on local expertise, fluoroscopy or pericardioscopy guided pericardial biopsy may be performed if the diagnosis is crucial for management.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here