Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Percutaneous nephrostomy (PCN) was first described by Goodwin in 1955 when a hydronephrotic kidney was accessed using a trocar needle during fluoroscopy. PCN is now widely used as the basis for a variety of therapeutic procedures ranging from simple urinary decompression to stone removal and stent insertion. PCN is usually a simple procedure with low complication rates and very high success. It is also one of the most frequently required procedures in emergency interventional radiology, so it is essential that all interventionists have a good understanding of the indications, contraindications, and techniques involved for successful PCN insertion.
Indications for performing PCN are as follows.
Relief of urinary obstruction due to:
malignancy (urothelial tumors, prostate carcinoma, or extrinsic compression/invasion by nonurological tumors)
urinary calculi
Pelvi ureteric junction (PUJ) obstruction
benign ureteric strictures
retroperitoneal fibrosis
inadvertent surgical ureteric occlusion
access for ureteric stent insertion.
Urinary diversion due to:
ureteric transection
ureteric perforation
bladder perforation
urinary fistulae.
Obstruction is most commonly caused by urinary calculi or malignancy. PCN allows rapid decompression of an obstructed system when retrograde stent insertion is not possible. It is often a life-saving procedure in patients with urosepsis or acute renal failure. In patients who have hypotension and leukocytosis, mortality from urosepsis may be reduced from 40% to 8% with emergency PCN.
It is very important to always consider retrograde stenting. In expert hands, it is just as safe as PCN and gives equal rapid relief of obstruction and resolution of infection. However, bladder and pelvic malignancy may make retrograde access impossible. Recent cross-sectional imaging can help to gauge the likelihood of successful retrograde stent insertion. Patients with significant tumor overlying the ureteric orifices are much more challenging. In this scenario, it would be appropriate to either proceed directly to PCN and antegrade stent insertion or be prepared to attend the operating theater to insert a PCN if the urologist fails to uncover the ureteric orifice. The presence of pelvic tumor (especially nonurological) should not deter an attempt at retrograde stent insertion because tumors frequently cause extrinsic compression of the ureter and are easily stented provided the ureteric orifice can be located. Patients will always prefer an internal stent rather than a PCN with its associated discomfort and inconvenient drainage bag.
A trial of nephrostomy can sometimes be useful in the transplant kidney to see if hydronephrosis and deteriorating function are due to obstruction or other causes.
Nephrostomy is also indicated for urinary diversion in the presence of urinary fistulae or rarely hemorrhagic cystitis. Fistulae are often iatrogenic, and in my experience are commonly due to complex nonurological pelvic surgery where the ureter has failed to be identified and has been damaged during resection of tumor or even in benign disease. This particular complication occurs less frequently if a retrograde stent is inserted before surgery, making the ureter more easily identifiable. Illuminated ureteric stents have been available for many years and reduced the incidence of ureteric damage to almost zero.
Fistulae may also occur after urological surgery such as ileal conduit formation, partial nephrectomy, cystectomy and prostatectomy, and renal transplantation. They can occur due to an inflammatory process or even appear many years after pelvic irradiation. Trauma can also damage the renal collecting system and ureters, requiring urinary diversion.
Management aims to completely divert the urine from the site of the leak. A Foley catheter is usually placed, followed by bilateral PCN or even unilateral PCN if only one side is involved. In some circumstances, the urine is not sufficiently diverted by PCN and ureteric occlusion may be required.
CTIVU and magnetic resonance urography have replaced PCN performed for diagnostic reasons in most circumstances. This is especially true in the evaluation of stone disease post-PCN or percutaneous nephrolithotomy (PCNL). However, antegrade pyelography after therapeutic PCN is useful to assess the location and severity of strictures either in the ureter, at ureteroileal anastomoses, or in transplant ureter anastomoses. This can usefully be combined with computed tomography (CT) or cone-beam CT when fluoroscopy cannot determine the nature of obstruction. PCN is sometimes used as a therapeutic trial in transplant kidneys
PCN is also performed before other interventional procedures such as stone removal with PCNL or stent insertion. Stent insertion should ideally be performed at the time of PCN performed for obstruction, except in severe sepsis where intervention should be minimized because of the risk of septicemic shock. This negates the need for a second visit to the interventional theater for stent insertion.
The only absolute contraindication to performing PCN is uncorrected coagulopathy. A recent consensus states that international normalized ratio should be less than 1.5 and platelet count greater than 50,000/μL. Aspirin need not be withheld, but clopidogrel should be withheld for 5 days before the procedure. Patients taking therapeutic low-molecular-weight heparin should withhold one dose before the procedure. Transfusion of platelets or plasma may be necessary to correct coagulation. Clearly, in an emergency situation, the risk of bleeding should be balanced with the risk of delaying PCN.
Severe hyperkalemia should be corrected by dialysis before the procedure. The level of hyperkalemia you should accept is a local decision. However, we will not perform PCN with a potassium > 6.5 mEq/L due to a potential risk of cardiac arrythmia.
Another contraindication to PCN is terminal illness with imminent death. In this scenario, PCN will not alter life expectancy. Indeed, the decision to proceed to PCN in patients with terminal illness should be made with the patient being fully informed of the outcome and the potential need for longer-term PCN if antegrade stent insertion fails. Some patients may prefer not to go through with the procedure in this situation.
Untreated severe sepsis, perhaps paradoxically, is a contraindication to PCN. This is due to the risk of PCN worsening bacteremia. Severely septic patients should always be resuscitated with fluids and antibiotics before PCN to make sure that they are as well as possible. Ultimately, renal decompression by PCN or retrograde stent insertion is the only definitive treatment and the decision as to when this should be performed should be made jointly with the clinician.
The patient should be seen by the interventional radiologist before PCN and consent obtained. The deemed acceptable incidence of complications was reviewed by the Society of Interventional Radiologists standards of practice group in 2016.
The main risks that should be discussed are:
hemorrhage (<4%)
septic shock (<10%)
other organ damage (<1%)
failure (almost never).
Before the procedure, the following preparations should be made.
Antibiotic prophylaxis should be given on local microbiology advice.
Coagulation studies and complete blood count should be performed.
A safe-surgery checklist based on the WHO checklist should be reviewed.
Intravenous sedation should be considered, especially if antegrade stent insertion is to be performed.
There are many ways to perform a nephrostomy and some people would much prefer one technique over another, depending on their individual skill set and available equipment:
Ultrasound and fluoroscopy
Ultrasound only
Fluoroscopy only
CT guidance
The most common technique for access is with ultrasound used to puncture the calyx in a hydronephrotic kidney. Fluoroscopy is then used for guidewire and drainage tube insertion. Ultrasound can be used alone in dilated systems when stenting is not intended. This is useful in an emergency situation, such as in the intensive care unit if the patient cannot be moved to the interventional radiology theater. Sometimes the system is already opacified because of contrast medium administered for CT or even from an interventional radiology unit. The calyx can then be directly punctured using fluoroscopy similar to when performing access for PCNL. Rarely CT may be required, if the kidneys cannot be seen with ultrasound due to body habitus or abnormal anatomy.
The patient needs to be positioned to make the intended kidney easiest to access. This is usually prone but may be with the intended side raised. There is often debate about which calyx to puncture, with popular thinking being that a lower pole calyx makes antegrade stenting more difficult due to the angles involved. This is not the case if stent insertion is performed with a sheath and I would suggest that you puncture whichever calyx is closest and looks easiest to target. A successful PCN is the most important result! I would only use a supracostal approach if accessing an upper pole calyx containing a stone for PCNL, or if the anatomy is grossly abnormal. Supra–12th-rib punctures are safer than supra–11th-rib punctures. I usually access the kidney at least one hand’s-breadth lateral to the midline with an oblique approach through the relatively avascular plane of Brodel. This is where the smaller end vessels from the anterior and posterior arteries converge and is thought to give the fewest hemorrhagic complications ( Fig. 100.1 ). Sometimes it may be necessary to use a more lateral approach if the patient will require the PCN long term. This makes it easier for them to manage drainage bag changes. Lateral punctures do increase the chance of colonic perforation, but the colon should be easily avoided if ultrasound guidance is employed ( Fig. 100.2 ).
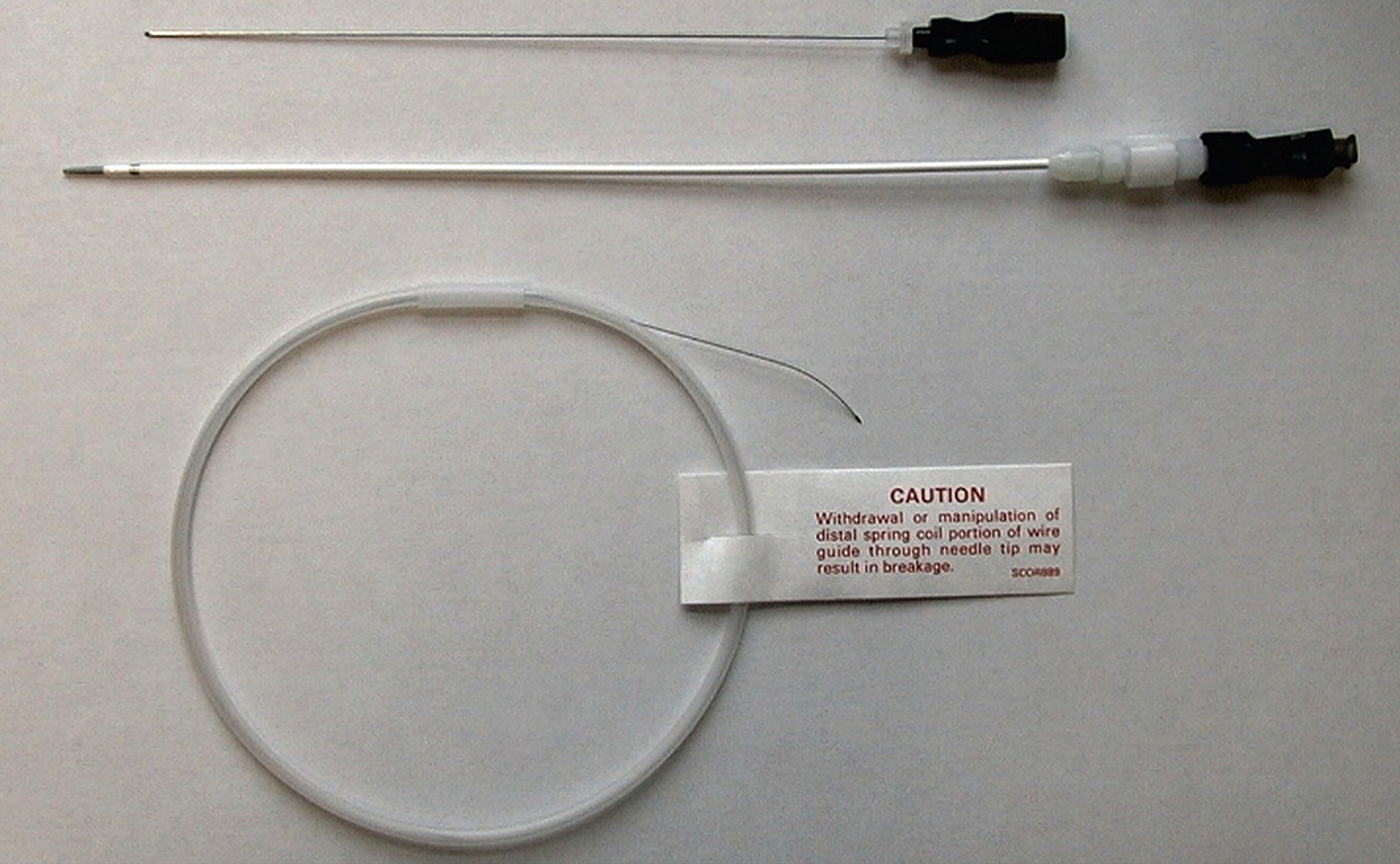
Access sets and other equipment for PCN insertion can be seen here:
The initial puncture can be made with either a larger 18G noncutting needle or a smaller 22G Chiba-type needle depending on equipment availability and your personal preference. I use an 18G diamond-shaped tip noncutting needle with a metal stylet and trocar (Cook Medical [Bloomington, IN] and Boston Scientific [Marlborough, MA], respectively) ( Figs. 100.3, 100.4 ). This needle is available with an echogenic tip, making it easier to see, does not flex easily and therefore maintains its course, and may be less likely to cause bleeding due to the nature of its tip.


If you are less confident and may need multiple passes to access the collecting system, you may prefer to use a 22G needle and access set. There are numerous access kits on the market, usually comprising a 22G needle, 0.018-inch wire and introducer and central stiffener. These allow initial access with the smaller needle and wire. The introducer is then inserted over the wire. This and the central stiffener are removed to allow insertion of a 0.035-inch wire over which the PCN can be inserted. The 0.018-inch wire can be left in situ with the 0.035-inch wire inserted alongside it. This leaves the 0.018-inch wire as a safety wire and the 0.035-inch wire as the working wire for tube insertion ( Figs. 100.5, 100.6 ).
Local anesthetic should slowly be infiltrated into the skin, followed by further anesthetic with a longer needle all the way to the renal cortex. My preferred anesthetic is 1% lidocaine mixed with 8.4% sodium bicarbonate in the ratio 9 mL lidocaine to 1 mL bicarbonate. The sodium bicarbonate neutralizes the acidity of the lidocaine, making it much less painful to inject. Sodium bicarbonate should not be mixed with bupivacaine because it forms a precipitate.
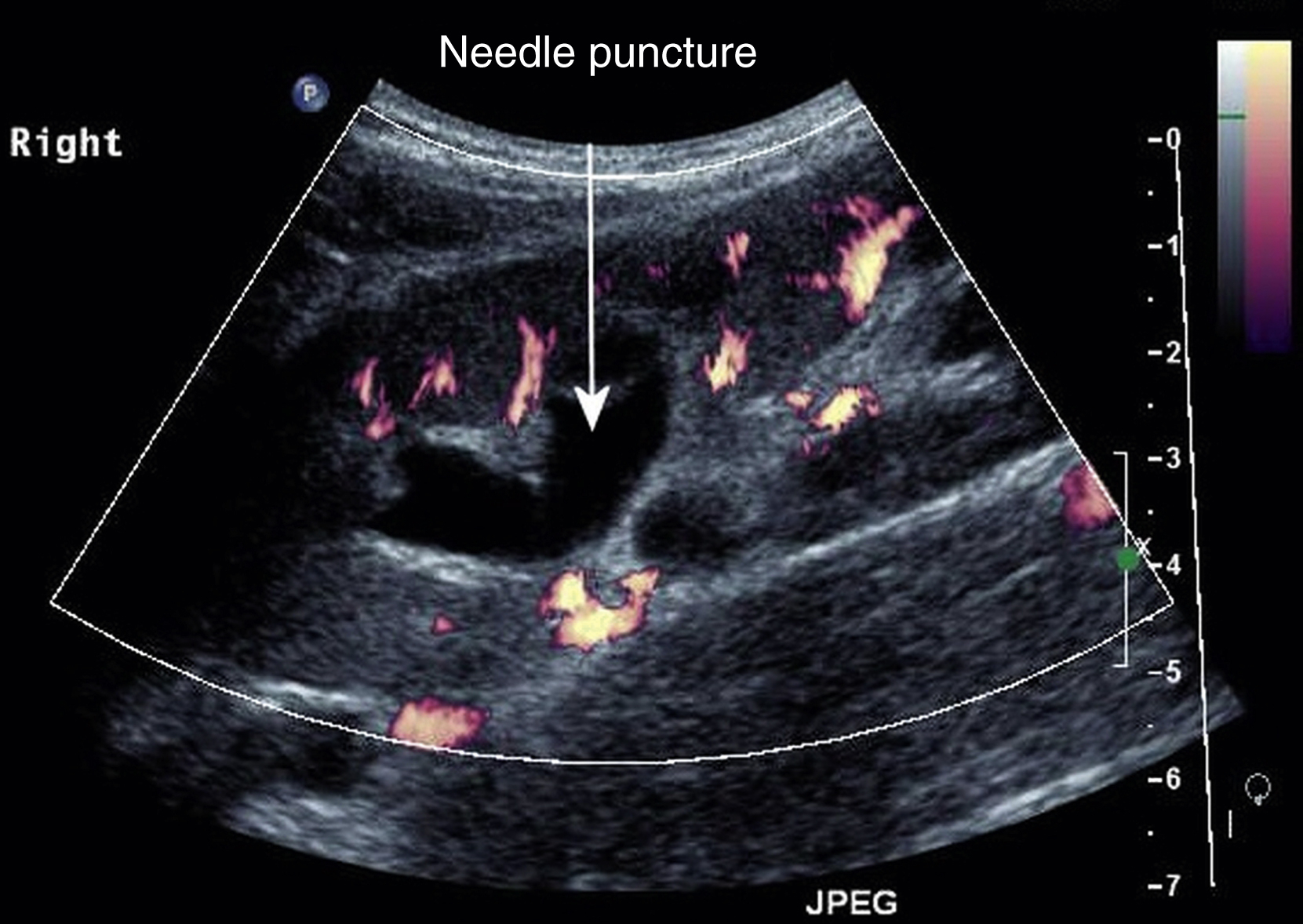
I use ultrasound longitudinally so that the track of the needle can be seen all the way to the kidney ( ).
The initial sequence should always be:
Puncture
Decompress the system
Opacify the system
As previously discussed, choose a calyx that is closest to the skin and provides an easy angle of approach in the avascular plane. Observe your needle path all the way to the renal cortex. Some initial resistance will be felt at this point with some give as it enters the cortex. There may be a further tactile “pop” as the needle enters the collecting system. The stylet is then removed, and urine should drain freely. A syringe should then be attached, taking care not to dislodge the needle, and urine should be aspirated to decompress the system. It is important to aspirate at least as much urine as contrast to be injected. For this reason, the system should be opacified with neat contrast medium using the smallest amount needed to confirm calyceal entry and allow guidewire and catheter navigation. The urine remaining in the system will immediately dilute the contrast, giving optimal concentration. A guidewire can then be inserted through the metal sheath. The sheath is removed and the PCN inserted over the wire.
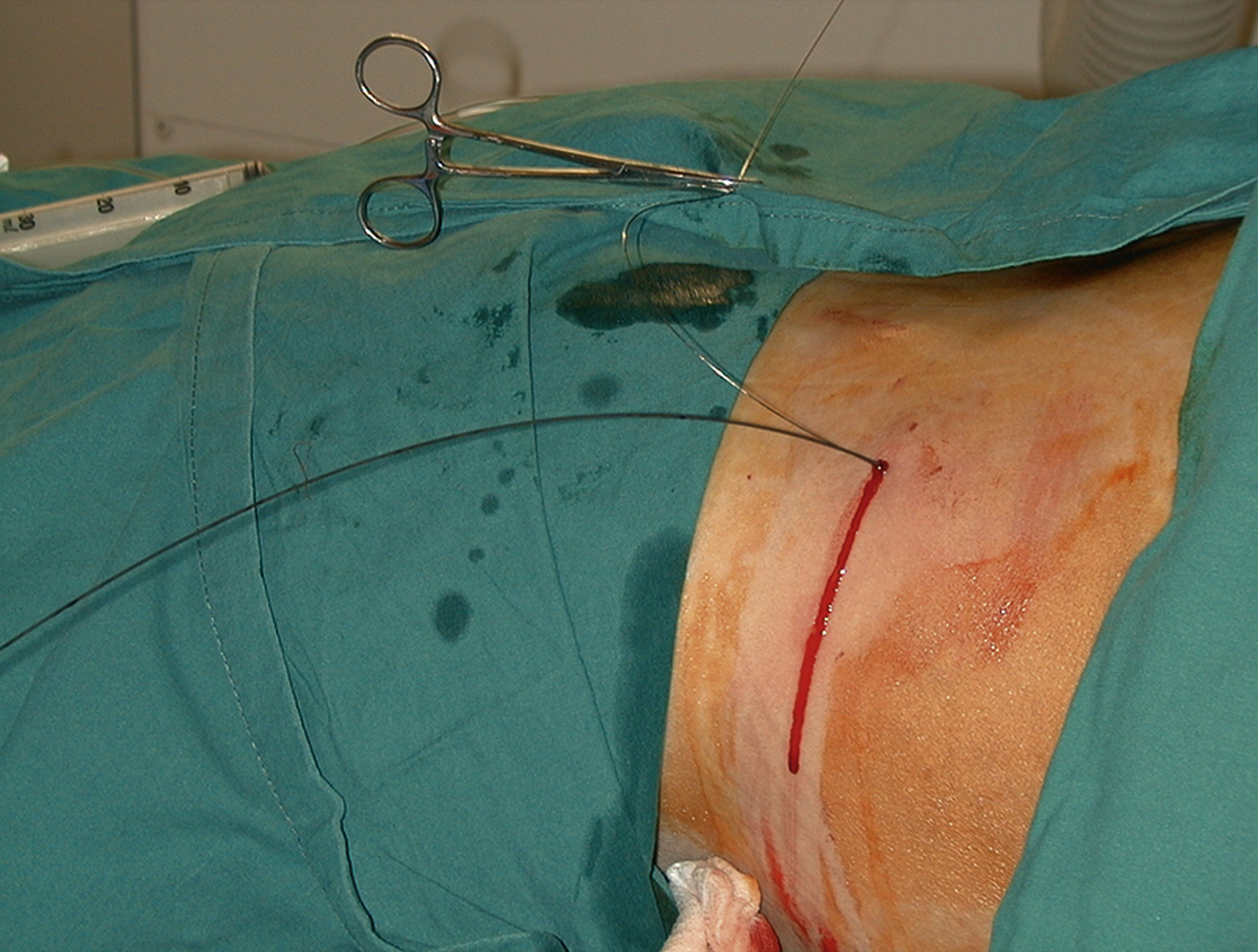
I initially use a hydrophilic guidewire through the metal outer sheath. I find this is easier to manipulate through the calyx into the renal pelvis and then can be steered down through the PUJ using a torque device into the ureter. If I am not planning to insert a stent, I usually change this wire for a Bentson or stiffer wire to insert the nephrostomy. The wire can be exchanged using a 5F dilator or catheter. Sometimes it is necessary to exchange for a stiffer wire, such as an Amplatz wire, to allow tube insertion in larger patients or where scar tissue makes traversing the tract difficult ( ).
It is important to be aware of the unsuspected presence of pus. Patients may well be laid prone for a period of time before the system is accessed. This allows pus/debris to settle anteriorly in the system so that the initial urine aspirated may appear clear. When contrast is injected in this situation, it appears to flow slowly like treacle [molasses] or lava ( ).
I routinely insert 8.5F locking pigtail catheters for nephrostomy. These are of adequate size to allow good drainage and the locking mechanism helps to prevent accidental removal. Smaller tubes down to 7F are also sufficient for most purposes. Occasionally we insert a large 12F tube if it is to be left in for a longer period or if the patient frequently suffers from tube blockages.
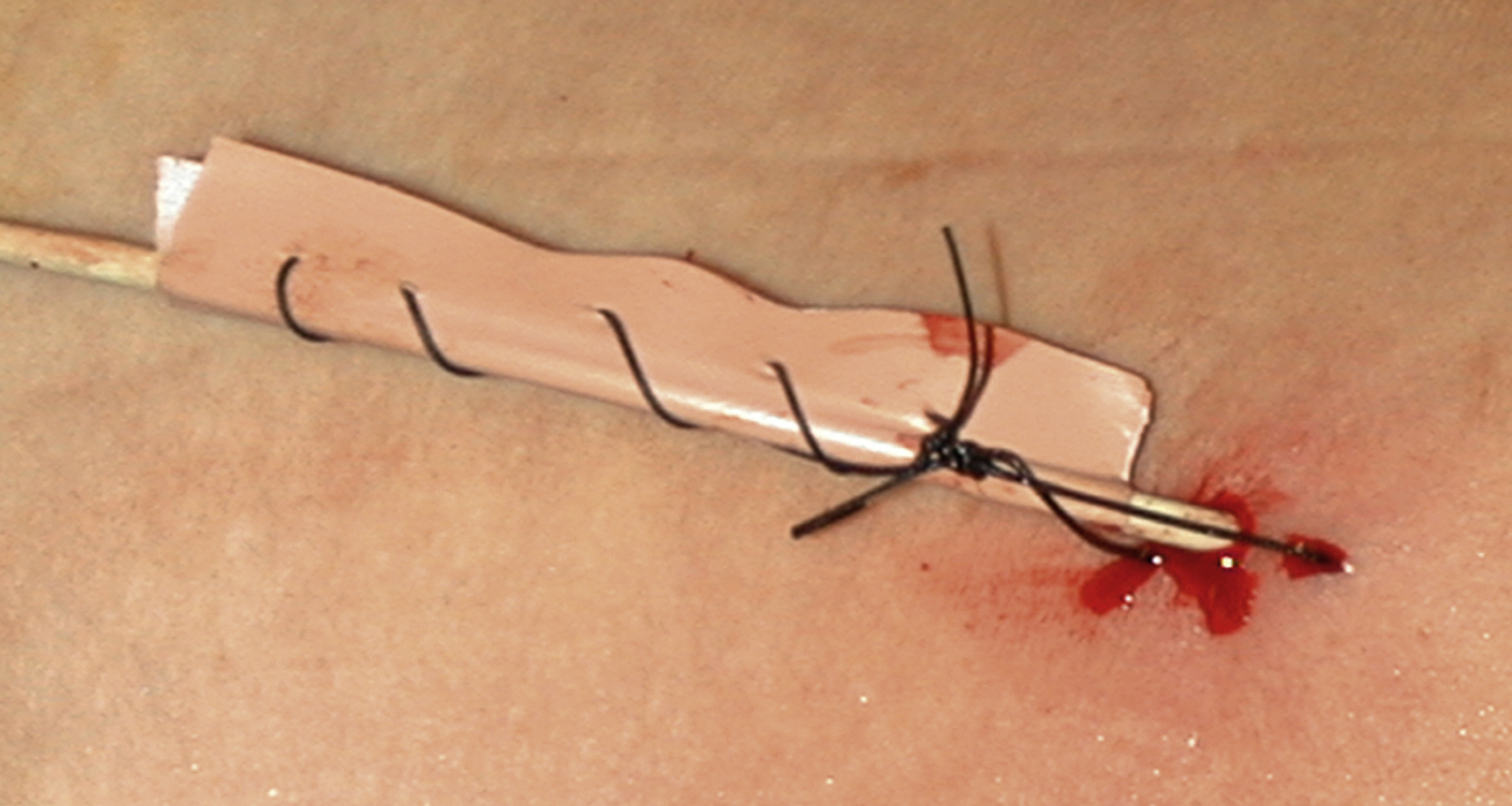
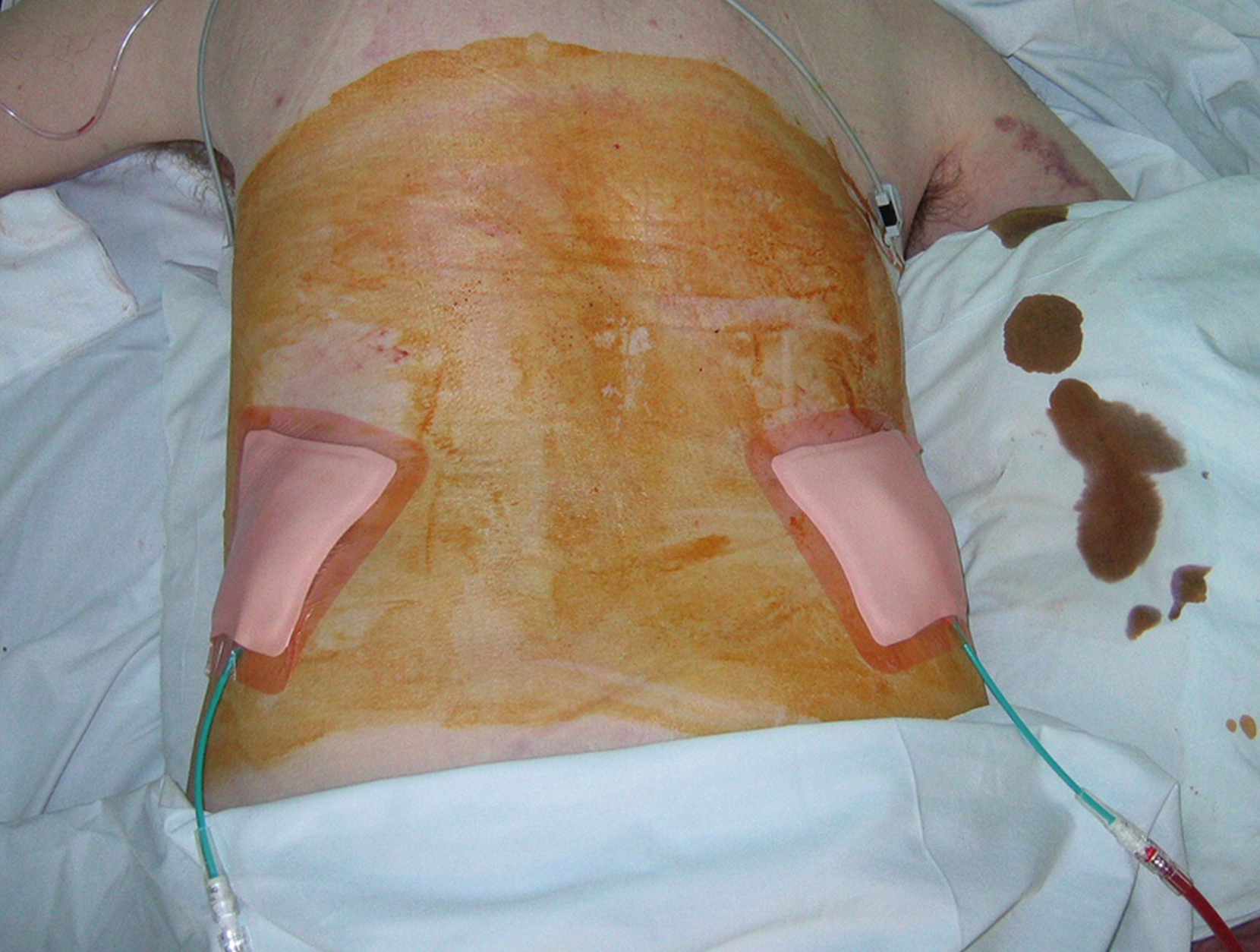
We insert a nonlocking pigtail PCN if a stent has been inserted. The nonlocking type avoids the problem of the locking mechanism sometimes getting caught in the stent and causing displacement when the tube is removed. Locking pigtails should be removed under screening in the presence of a stent to help avoid this problem. You could argue not to leave any PCN in situ if you have stented, but occasionally the stent does not function as well as expected and the PCN is needed for a check nephrostogram. For this reason, I almost always leave a “covering PCN” in situ clamped when stenting. I stitch this into place by making a “flag” of sticky tape around the PCN and stitching this to the skin. This works far better than most adhesive fixation devices ( Figs. 100.7, 100.8 ).
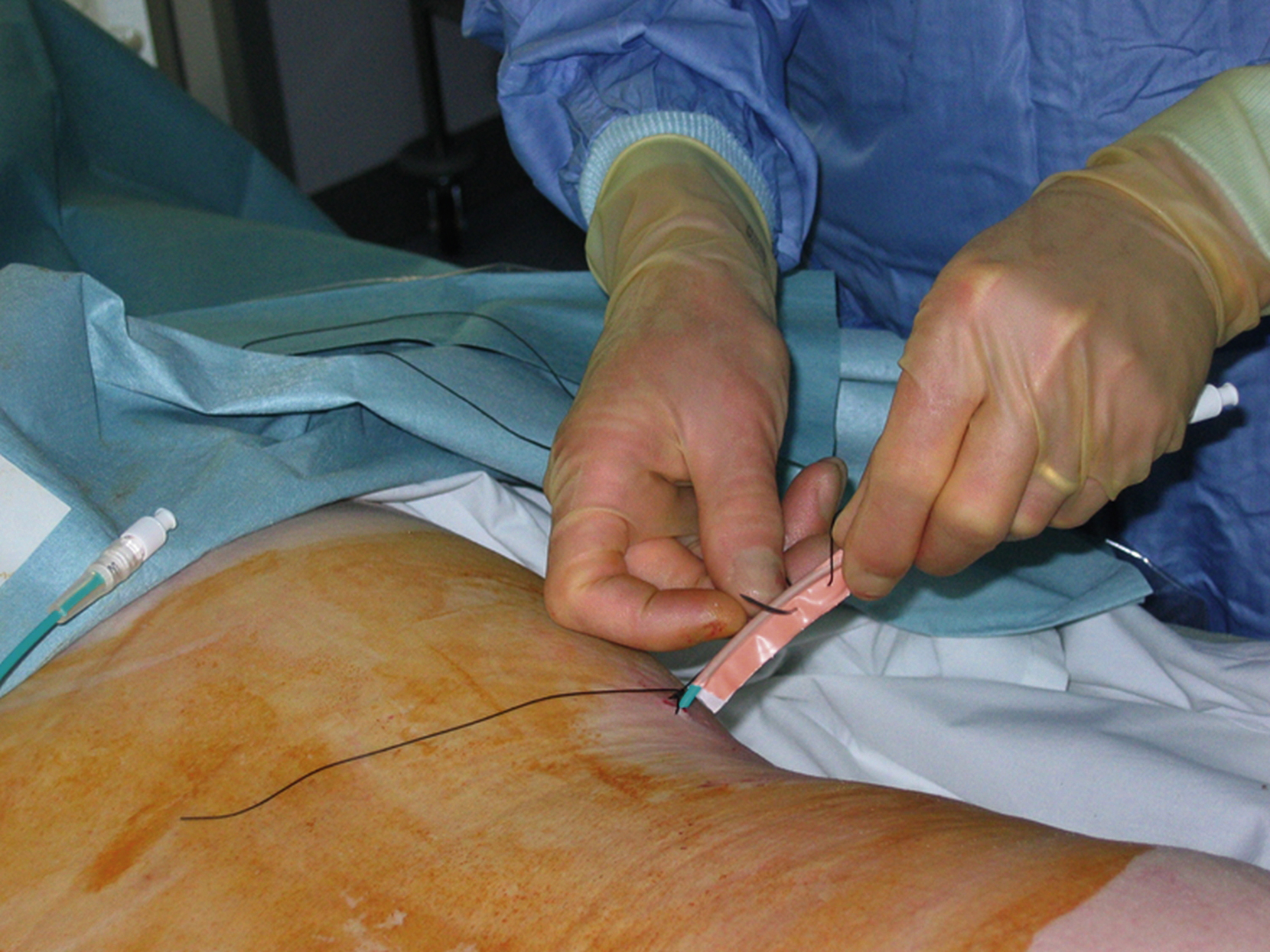
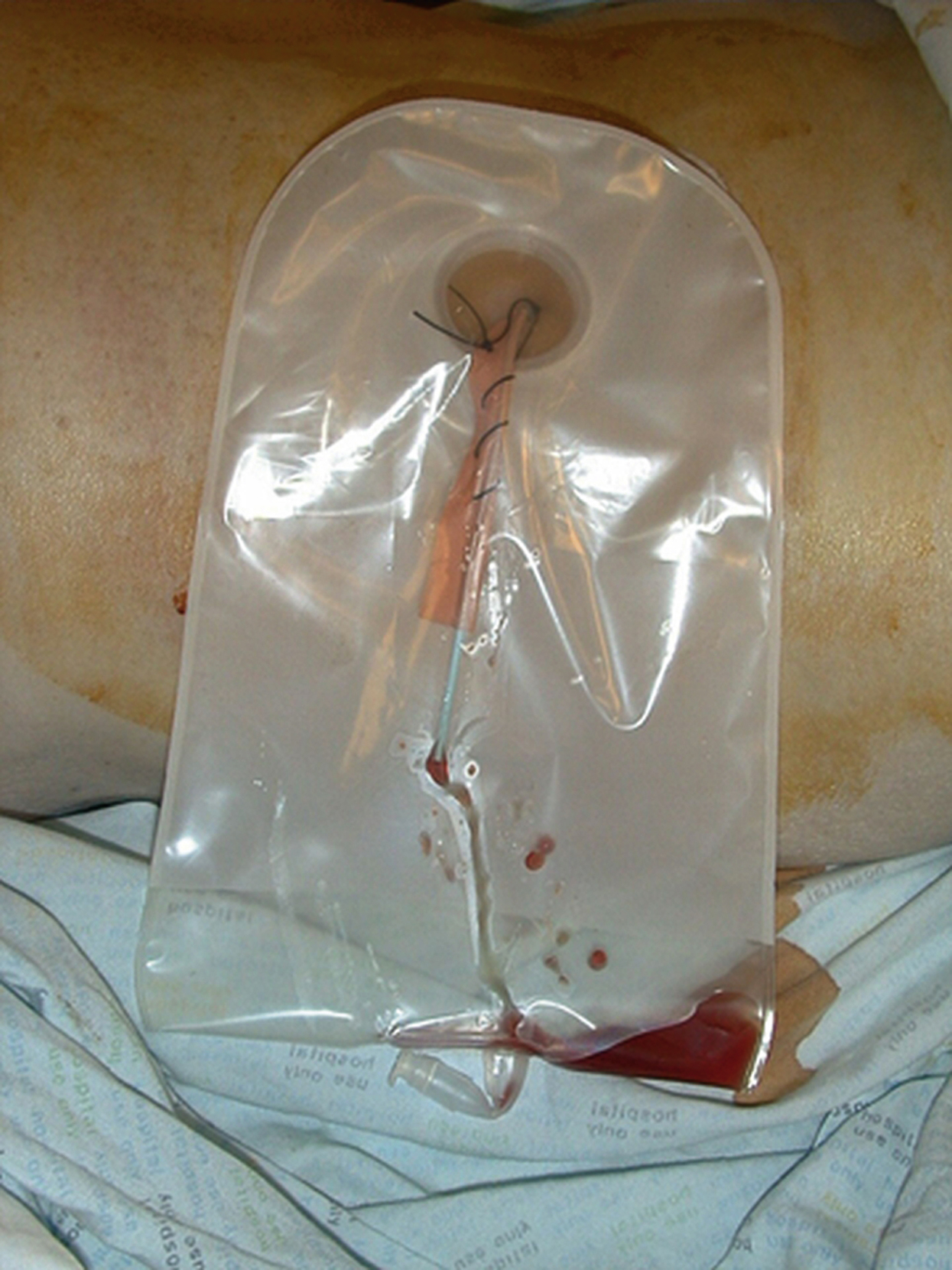
The end of the PCN is usually left inside a urine drainage bag on a stoma flange around the PCN site. This ensures that the end of the tube cannot be accidentally caught and the tube pulled out ( Fig. 100.9 ; ). A bag connect to the PCN by a tube is sometimes left for patients in intensive care, making bag change easier ( Fig. 100.10 ).
This is another technique for PCN that is sometimes used if the calyces are very difficult to see on ultrasound. The renal pelvis is directly punctured using a 22G needle and ultrasound guidance. The system is decompressed, and then contrast medium is injected to opacify the system and allow selection of a calyx for nephrostomy insertion ( ).
Nondilated systems are usually the result of ureteric transection or perforation, postoperative urine leaks, and fistulae related to surgery or radiotherapy. These nephrostomy procedures can be the most challenging. There are several techniques that can help in this situation.
PTC contrast technique. Gentle injection of contrast medium while withdrawing the needle slowly, having placed the tip in the region of an expected calyx under ultrasound (US) guidance.
US-guided puncture of the renal pelvis with a fine 22G needle to opacify the system (double puncture technique).
Intravenous contrast medium. This may provide sufficient opacification to allow fluoroscopy-guided needle insertion.
Intravenous frusemide to dilate the system. This is rarely of benefit.
CT guidance.
I find the first technique to be the most successful. Ultrasound is used in the long axis of the kidney (as usual for PCN). The location of the collapsed lower pole calyx can be estimated by looking at the renal parenchyma and more central fat. An 18G or 22G needle can then be inserted into this region and hopefully through the lower pole calyx. A syringe of dilute contrast is then connected, and gentle pulses of pressure are applied to the plunger as the needle is very slowly withdrawn back through the kidney, taking care not to stain the area with contrast. As soon as the needle traverses an area of lowered resistance, contrast medium will flow, i.e., into the collecting system or possibly a renal vein ( Fig. 100.11 ).
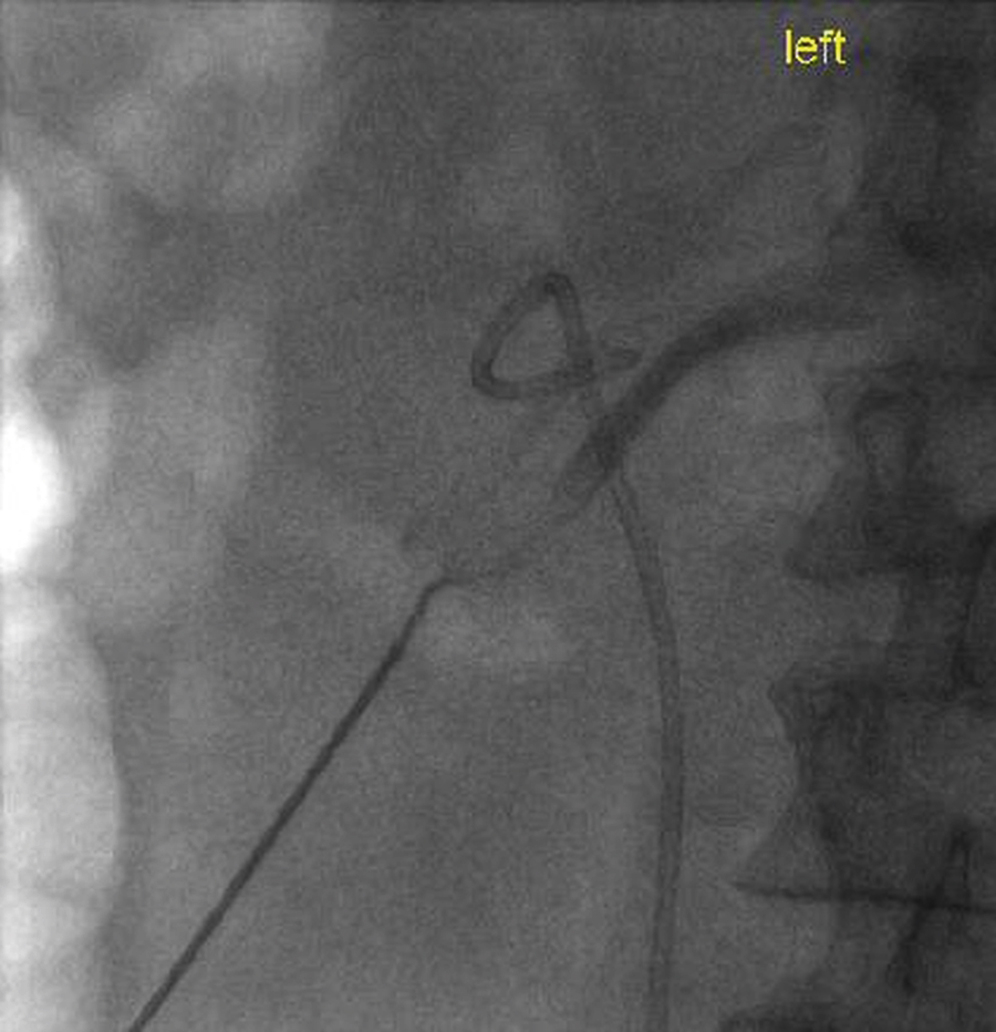
If contrast is seen flowing into a branch of the renal vein, the needle tip is almost certainly very close to the calyx. Further withdrawal will allow the tip to enter the calyx and opacify the system. An angled hydrophilic wire is then carefully passed through the needle outer sheath, attached to a torque device, to allow gentle manipulation of the wire tip. It is usually then possible to manipulate it into the calyx and subsequently down the ureter. Once the wire is seen to pass down the ureter, you can be confident of the correct location, otherwise it is possible that the wire has passed alongside the pelvicaliceal system in the fat or even along a renal vein branch ( , 100.7 ).
The most common indication for PCN in renal transplants is obstruction due to an anastomotic stricture where the ureter is anastomosed to the bladder. These strictures are like ureteroileal strictures in ileal conduits in that they are frequently due to ureteric ischemia. Occasionally calculi can cause obstruction. PCN is usually easier to perform than retrograde stent insertion because of the variable location of the anastomosis on the bladder wall. PCN may also be used as a trial to find out if failing function in the transplant is due to obstruction.
The technique for transplant PCN is almost identical to PCN in a native kidney. There are some differences, however.
The kidney orientation can be variable, and careful calyx selection is necessary to avoid bowel and aid antegrade stent placement.
The kidney is fixed and does not move with respiration, making PCN easier.
Scarring around the kidney can make tract dilatation difficult, and a stiffer wire is frequently needed.
The kidney is usually very superficial, which makes the procedure easier. ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here