Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute pulmonary embolism (PE) remains a global health problem and represents a life-threatening manifestation along the spectrum of venous thromboembolism (VTE). Although the true incidence of PE is unclear, it is recognized as a significant cause of morbidity and mortality in hospitalized patients. In the United States alone, it is estimated that there are 600,000 cases per year, and more than 300,000 people die every year from acute PE. Indeed, acute PE is believed to be the third most common cause of death among hospitalized patients.
Common risk factors for acute PE are related to underlying genetic conditions (e.g., factor V Leiden mutation, prothrombin 20210A mutation, and multiple other thrombophilias), acquired conditions (e.g., cancer, immobilization, trauma, surgery, prior deep vein thrombosis [DVT]), and acquired hypercoagulable states (oral contraceptive use, nephrotic syndrome, antiphospholipid syndrome, disseminated intravascular coagulation, hyperestrogenic states, obesity). There are three basic categories of acute PE: (1) simple PE: no associated heart strain and no hypotension, (2) submassive PE: associated right-sided heart strain, and (3) Massive PE: associated right-sided heart strain with hemodynamic shock. Patients with simple acute PE may be treated with therapeutic anticoagulation alone. Select patients with submassive PE may require treatment escalation beyond anticoagulation, and patients with massive PE require treatment escalation beyond anticoagulation alone. The immediate mortality rate related to simple PE is less than 8% when the condition is recognized early and treated with anticoagulation. However, patients with submassive PE have a higher cumulative mortality rate that, in real-world populations, may reach up to 20%. Finally, patients with massive PE have the highest mortality rate, which can exceed 58%, including a high risk of sudden death.
The pathophysiology of PE consists of direct physical obstruction of the pulmonary arteries, hypoxemic vasoconstriction, and release of potent pulmonary arterial vasoconstrictors that further increase pulmonary vascular resistance and right ventricular (RV) afterload. Acute RV pressure overload may result in RV hypokinesis and dilation, tricuspid regurgitation, and ultimately RV failure. RV pressure overload may also result in increased wall stress and ischemia by increasing myocardial oxygen demand, while simultaneously limiting its supply. Ultimately, cardiac failure from acute PE results from a combination of the increased wall stress and cardiac ischemia that compromise RV function and impair left ventricular (LV) output, resulting in life-threatening hemodynamic shock. Depending on underlying cardiopulmonary reserve, patients with acute massive PE may deteriorate over the course of several hours to days and develop systemic arterial hypotension, cardiogenic shock, and cardiac arrest. Because of the risk of sudden death, these critically ill patients with massive PE should be quickly identified as candidates for rapid treatment escalation with possible thrombolysis, surgical thrombectomy, or endovascular treatment as a lifesaving procedure.
Because of the high mortality associated with acute massive PE, successful management requires prompt risk stratification and decisive early intervention ( Table 55.1 ). Confirmation of hemodynamic shock attributed to central obstructing embolus should be present to justify treatment escalation beyond anticoagulation. The American College of Chest Physicians has recommended that percutaneous catheter-directed therapy (CDT) be considered in patients with acute PE associated with hypotension and who have (1) contraindications to thrombolysis, (2) failed thrombolysis, or (3) shock that is likely to cause death before systemic thrombolysis can take effect. In addition, global meta-analytic data has demonstrated that percutaneous CDT can be considered as a first-line treatment option in lieu of intravenous tissue plasminogen activator (tPA) for patients with massive PE.
| At least one of the following criteria must be present: |
|
|
|
Pulmonary angiography was once considered the gold standard for diagnosing PE, but it has largely been replaced by the wide availability of cross-sectional imaging. Historically many types of imaging studies have been used in the diagnosis of acute PE including ventilation-perfusion scanning, magnetic resonance angiography, and computed tomographic (CT) angiography (CTA). CTA is the preferred modality and has proven to be advantageous because of its wide availability, superior speed, characterization of nonvascular structures, and detection of venous thrombosis. CTA has the greatest sensitivity and specificity for detecting emboli in the main, lobar, or segmental pulmonary arteries. Systematic reviews and randomized trials suggest that outpatients with suspected PE and negative CTA studies have excellent outcomes without therapy.
If a patient has either acute or chronic renal insufficiency and contrast administration is undesirable, echocardiography may be used to evaluate for right-sided heart dysfunction as an indication for underlying acute PE. The echocardiogram can be performed at the bedside, and the study may reveal findings that strongly support hemodynamically significant PE, offering the potential to guide treatment escalation to thrombolytic and/or endovascular therapy. Large emboli moving from the heart to the lungs are occasionally confirmed with this technique. In addition, intravascular ultrasonography has also been used at the bedside to visualize central pulmonary emboli.
Although the diagnosis of submassive PE follows a similar workup to evaluating massive PE, these patients do not present with systemic arterial hypotension, and particular attention must be made to detecting the presence of right-sided heart strain, which clinches the diagnosis of submassive PE. The identification of right-sided heart strain allows risk stratification for possible treatment escalation beyond anticoagulation in normotensive PE patients. Echocardiography is a useful imaging modality to detect RV dysfunction in the setting of acute PE. Characteristic echocardiographic findings in patients with submassive PE include RV hypokinesis and dilatation, interventricular septal flattening and paradoxical motion toward the LV, abnormal transmitral Doppler flow profile, tricuspid regurgitation, pulmonary hypertension as identified by a peak tricuspid regurgitant jet velocity >2.6 m/s, and loss of inspiratory collapse of the inferior vena cava (IVC). An RV-to-LV end-diastolic diameter ratio of 0.9 or greater, assessed in the left parasternal long-axis view or the subcostal view, is an independent predictor of hospital mortality. Detection of RV enlargement by chest CTA is especially convenient for the diagnosis of submassive PE because it uses data acquired during the initial diagnostic scan. Submassive PE can be diagnosed when RV enlargement on chest computed tomography, defined by an RV-to-LV diameter ratio > 0.9, is observed, and RV enlargement on chest CTA also predicts increased 30-day mortality in patients with acute PE. Furthermore, even if shock and death do not ensue, survivors of acute submassive PE remain at risk for developing chronic PE and thromboembolic pulmonary hypertension.
If significant right-sided heart strain is identified, then anesthesia and fluid resuscitation in these patients must be used with caution. Although small amounts of fluid resuscitation may improve RV filling, large amounts (1–2 liters) may exacerbate RV dysfunction and actually lead to a decrease in RV cardiac output (and therefore LV cardiac output), and this can precipitate systemic hypotension and death. Similarly, both the sedatives used for intubation and the positive pressure ventilation provided by the ventilator may decrease preload and cause hypotension, which can similarly worsen RV and LV cardiac output, which may further precipitate clinical deterioration.
Additionally, monitoring of cardiac troponin T identifies the high-risk group of normotensive patients with submassive PE at risk of rapid cardiovascular collapse. A persistent increased cardiac troponin T level (>0.01 ng/mL) in PE patients with right-sided heart strain predicts a significant risk of a complicated clinical course and fatal outcome, and these patients therefore may require more aggressive treatment The identification of submassive PE for treatment escalation is important because these normotensive PE patients demonstrate increased short-term mortality and high risk of adverse outcomes when the degree of heart strain results in elevations in levels of cardiac troponins and brain-type natriuretic peptide. The optimal protocol for the treatment of acute submassive PE is still in evolution, but a proposed algorithm for managing submassive PE has been published describing treatment escalation beyond anticoagulation ( Fig. 55.1 ).
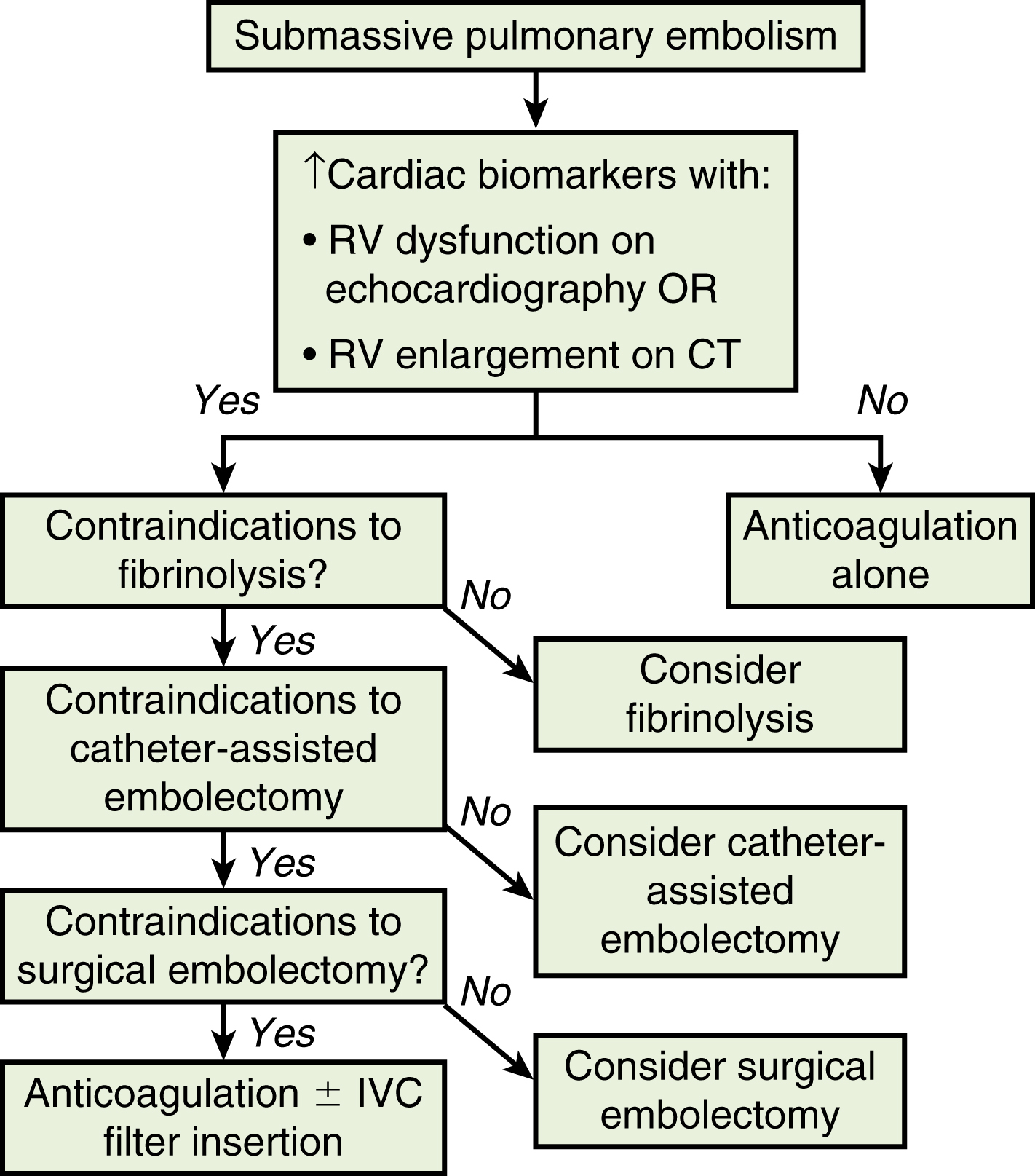
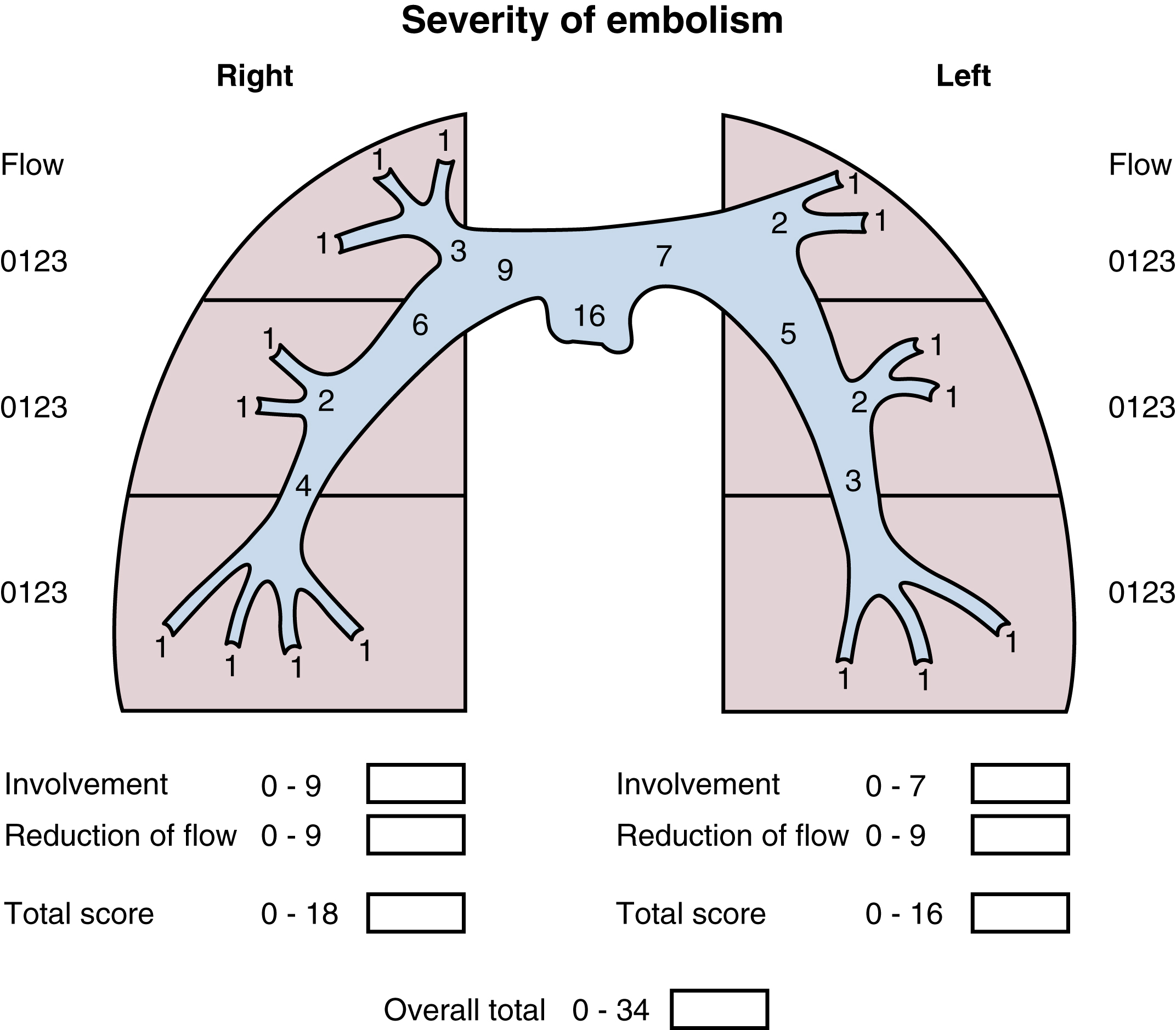
Systemic thrombolysis or local catheter-directed pharmacologic thrombolysis may be contraindicated in some patients (e.g., intracranial lesion, recent major general or intracranial surgery) because of the risk of major hemorrhage. In such cases, mechanical methods of percutaneous catheter-directed thrombectomy, fragmentation, and/or aspiration may be considered without the use of pharmacologic agents. Because pulmonary hypertension is a relative contraindication to conventional pulmonary angiography, the degree of pulmonary hypertension and underlying cardiopulmonary reserve are important considerations before performing contrast-injection pulmonary angiography. These factors must be used to determine a safe rate and volume of contrast injection into the pulmonary circulation. For instance, when systolic pulmonary artery pressure (PAP) exceeds 55 mm Hg or RV end-diastolic pressure is greater than 20 mm Hg, the mortality associated with pulmonary angiography using large-volume power injection may reach up to 3%. Therefore, such patients with massive PE should receive only a limited-rate contrast injection controlled by hand. In the submassive PE patient, a selective contrast injection into the main left or right pulmonary artery (PA) should not exceed a 20-cc volume at a rate of 10 cc/s. Lower injection parameters by hand may be considered depending on degree of heart failure as determined by the operator’s judgment, to achieve adequate vessel opacification without endangering the patient. Pulmonary angiography can be used to calculate the degree of pulmonary obstruction before and after treatment using the calculated Miller index ( Fig. 55.2 ). Finally, in PE patients with contraindications to anticoagulation and/or thrombolytic agents, or in patients who have suffered life-threatening massive PE, placement of a retrievable IVC filter should be considered for prophylaxis of recurrent PE.
Modern CDT for massive PE has been defined according to the following general criteria: use of low-profile catheters and devices (≤ 10Fr), catheter-directed mechanical fragmentation and/or aspiration of emboli, and intraclot thrombolytic injection if a local drug is infused. Therefore, a variety of devices can be used to safely and successfully treat acute PE based on the criteria for modern CDT ( Table 55.2 ).
From Kuo WT, Gould MK, Louie JD, et al., Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J Vasc Intervent Radiol 2009;20:1431-1440.
Schmitz-Rode et al., 1998 , Schmitz-Rode et al., 2000 , Muller-Hulsbeck et al., 2001 , Prokubovsky et al., 2003 , Tajima et al., 2004 , Barbosa et al., 2008 , Brady et al., 1991 , Rafique et al., 1992 , Uflacker et al., 1996 , Fava et al., 1997 , Stock et al., 1997 , Basche et al., 1997 , Hiramatsu et al., 1999 , Wong et al., 1999 , Murphy et al., 1999 , Voigtlander 1999 , Fava et al., 2000 , Egge et al., 2002 , De Gregorio et al., 2002 , Zeni et al., 2003 , Reekers et al., 2003 , Tajima et al., 2004 , Fava et al., 2005 , Siablis et al., 2005 , Yoshida et al., 2006 , Li J-J et al., 2006 , Pieri and Agresti. 2007 , Chauhan et al., 2007 , Krajina 2007 , Yang 2007 , Margheri 2008 , Vecchio et al., 2008 , Chen et al., 2008 , Eid-Lidt et al., 2008 Kuo et al., 2008
| Author, Year (Reference) | Country | Patients ( n ) | Sex( n ) | Age (y) Mean (Range) | Technique | Local Intraclot Lytic During CDT | Local Intraclot Lytic, Extended Infusion | Minor Cxs | Major Cxs | Clinical Success (%) a |
|---|---|---|---|---|---|---|---|---|---|---|
| Prospective Studies | ||||||||||
| Schmitz-Rode et al., 1998 | Germany | 10 | M-6, F-4 | 54 (36–70) | PF-10 | 8 | 1 | 0 | 0 | 8/10 (80) |
| Schmitz-Rode et al., 2000 | Germany | 20 | M-10, F-10 | 59 (48–60) | PF-20 | 0 | 0 | 1 | 0 | 16/20 (80) |
| Muller-Hulsbeck et al., 2001 | Germany | 9 | M-4, F-5 | 55 (27–85) | ATD-9 | 0 | 5 | 0 | 0 | 9/9 (100) |
| Prokubovsky et al., 2003 | Russia | 20 | na | 51 (32–75) | PF-20 | 0 | 16 | 0 | 0 | 14/20 (70) |
| Tajima et al., 2004 | Japan | 25 | M-8, F-17 | 61 (35–77) | PF & AT-25 | 25 | 21 | 0 | 0 | 25/25 (100) |
| Barbosa et al., 2008 | Brazil | 10 | M-7, F-3 | 57 (39–75) | PF-10, (ATD-na) | 0 | 0 | 0 | 0 | 9/10 (90) |
| Retrospective Studies | ||||||||||
| Brady et al., 1991 | England | 3 | M-0, F-3 | 36 (18–71) | PF-1, MC-2 | 2 | 2 | 0 | 0 | 3/3 (100) |
| Rafique et al., 1992 | South Africa | 5 | M-1, F-4 | 35 (21–47) | MC-5 | 5 | 5 | 1 | 0 | 5/5 (100) |
| Uflacker et al., 1996 | United States | 5 | M-4, F-1 | 45 (25–64) | ATD-5 | 1 | 1 | 0 | 1 | 3/5 (60) |
| Fava et al., 1997 | Chile | 16 | M-8, F-8 | 49 (20–68) | PF-16, (BA-na) | 16 | 16 | 3 | 0 | 14/16 (88) |
| Stock et al., 1997 | Switzerland | 5 | M-3, F-2 | 50 (21–80) | PF & BA-5 | 5 | 5 | 0 | 2 | 5/5 (100) |
| Basche et al., 1997 | Germany | 15 | na | na (21–73) | PF & BA-2, BA-13 | na | na | 0 | 0 | 12/15 (80) |
| Hiramatsu et al., 1999 | Japan | 8 | M-4, F-4 | 58 (42–87) | AT & WD-8 | 0 | 8 | 0 | 0 | 7/8 (88) |
| Wong et al., 1999 | England | 4 | M-2, F-2 | 33 (18–46) | PF-1,PF & G-1, G-2 | 0 | 4 | 0 | 1 | 3/4 (75) |
| Murphy et al., 1999 | Ireland | 4 | M-2, F-2 | 60 (46-66) | MC & WD-4 | 4 | 4 | 0 | 0 | 4/4 (100) |
| Voigtlander 1999 | Germany | 5 | M-4, F-1 | 57 (25–72) | RT-5 | 0 | 0 | 4 | 0 | 3/5 (60) |
| Fava et al., 2000 | Chile | 11 | M-3, F-8 | 61 (37–79) | Hy-11 | 0 | 4 | 0 | 0 | 10/11 (91) |
| Egge et al., 2002 | Norway | 3 | M-2, F-1 | 49 (40–54) | PF-3 | 3 | 3 | 0 | 0 | 3/3 (100) |
| De Gregorio et al., 2002 | Spain | 59 | M-25, F-34 | 56 (22–85) | PF-52, PF & BA-4, PF & DB -3 | 59 | 57 | 8 | 0 | 56/59 (95) |
| Zeni et al., 2003 | United States | 16 | M-9, F-8 | 52 (30–86) | RT-16 | 0 | 10 | 2 | 1 | 14/16 (88) |
| Reekers et al., 2003 | Netherlands | 7 | M-2, F-6 | 46 (28–76) | Hy-6, Oa-1 | 7 | 0 | 0 | 0 | 6/7 (86) |
| Tajima et al., 2004 | Japan | 15 | M-4, F-11 | 60 (27–79) | AT-15 | 9 | 0 | 0 | 0 | 15/15 (100) |
| Fava et al., 2005 | Chile | 7 | M-3, F-4 | 56 (30–79) | Hy-4, Oa-3 | 3 | 3 | 1 | 1 | 6/7/ (86) |
| Siablis et al., 2005 | Greece | 6 | M-4, F-2 | 59 (42–76) | RT-6 | 4 | 0 | 2 | 0 | 5/6 (83) |
| Yoshida et al., 2006 | Japan | 8 | M-4, F-4 | 61 (47–75) | PF & AT-8 | na | na | 0 | 1 | 7/8 (88) |
| Li J-J et al., 2006 | China | 15 | M-11, F-4 | 56 (19–73) | PF & ATD-13, PF & Hy-1, PF & Oa-1 | 6 | 0 | 0 | 0 | 15/15 (100) |
| Pieri and Agresti, 2007 | Italy | 164 | na | 68 (35–78) | PF-164 | 164 | 164 | 0 | 0 | 138/164 (84) |
| Chauhan et al., 2007 | United States | 6 | M-2 F-4 | 64 (49–78) | RT-6 | 2 | 0 | 5 | 2 | 4/6 (67) |
| Krajina 2007 | Czech Republic | 5 | M-1, F-4 | 67 (52–80) | PF-3, PF & AT-2 | 3 | 0 | 0 | 0 | 2/5 (40) |
| Yang 2007 | China | 19 | M-13, F-6 | 62 (22–87) | PF-10, PF & AT-5, PF & SR-4 | 19 | na | 0 | 0 | 18/19 (95) |
| Margheri 2008 | Italy | 20 | M-12, F-8 | 66 (32–85) | RT-20 | na | 0 | 8 | 8 | 17/20 (85) |
| Vecchio et al., 2008 | Italy | 13 | na | 68 (54–80) | RT-13 | na | 0 | 6 | 8 | 8/13 (62) |
| Chen et al., 2008 | China | 26 | M-15, F-11 | 53 (36–71) | ATD-17, SR-9 | 21 | 0 | 1 | 0 | 26/26 (100) |
| Eid-Lidt et al., 2008 | Mexico | 18 | M-6, F-12 | 51 (47–55) | PF-5, PF & SR-13 | 2 | 0 | 0 | 0 | 16/18 (90) |
| Kuo et al., 2008 | United States | 12 | M-7, F-5 | 56 (21–80) | PF & AT-6, PF & AT & BA-2, RT & AT-2, AT & IC-2 | 8 | na | 1 | 0 | 10/12 (83) |
| Total = 35 | 594 | 53 (18–87) | 356/535 67% |
329/552 60% |
(7.9%) b [5.0%–11.3%] | (2.4%) b [1.9%–4.3%] | (86.5%) b [82.2%–90.2%] | |||
Depending on anticipated bleeding risk, CDT may be performed with either no or low-dose local thrombolytic injection. The initial goal of all these techniques is rapid debulking of central thrombus to relieve life-threatening heart strain, immediately improve pulmonary perfusion, and improve oxygenation ( Fig. 55.3 ).
In contrast to CDT for massive PE, which often requires mechanical methods for urgent clot evacuation, the most common protocol for submassive PE consists of gentle image-guided infusion catheter placement and overnight pharmacologic thrombolysis without aggressive mechanical intervention given that mechanical CDT for acute PE remains experimental and data are pending. This treatment may be offered to patients who present primarily with submassive PE or as adjunctive treatment in patients with prior massive PE who have been “downstaged” via central clot debulking to submassive PE. For at least one access site, it is preferable that the sheath be sized at least 2Fr larger than the infusion catheter to allow subsequent central PA pressure measurements through the sheath. For example, a 7Fr or 8Fr vascular sheath (e.g., Flexor sheath, Cook, Bloomington, IN) may be used in conjunction with a 5Fr multi-sidehole infusion catheter, and the sheath should be long enough to reach from the access point to the main pulmonary trunk. If a second sheath is placed for dual catheter infusions, the operator can decide on the specific diameter and length, based on vessel tortuosity, sufficient to provide stability for the second infusion catheter.
Solid catheter-based skills along with knowledge of the cardiopulmonary anatomy are important for safe intravascular placement and manipulation of catheters and thrombectomy devices. Catheterization of the PA can be performed using a variety of methods depending on the operator’s preference and experience. An example via the transfemoral approach is using a 5Fr or 6Fr pigtail catheter in conjunction with a hydrophilic Glidewire (Terumo, Somerset, NJ) and torque control device. An example via the right transjugular approach is using a C2 catheter (Cook) in conjunction with a Glidewire and torque control device. Regardless of technique used, the cardiac electrocardiogram should be continuously monitored by trained nursing staff. PA catheterization should be meticulously performed to minimize guidewire contact with cardiac structures to reduce the risk of ventricular tachycardia, damage to valvular structures, and vascular perforation while passing through the right side of the heart and into the pulmonary trunk. Once the pulmonary outflow trunk has been successfully catheterized depending on operator preference, the hydrophilic wire can be exchanged for a nonhydrophilic wire such as a 0.035-inch Rosen wire (Boston Scientific, Natick, MA) to provide stability for subsequent vascular sheath placement. Selective catheterization of the main right and left pulmonary arteries is routinely performed, but selective and subselective catheterization of pulmonary segments may be necessary for further treatment.
Although the femoral approach may be used in patients who are candidates for catheter-directed pulmonary thrombolysis or thrombectomy, the right internal jugular approach may be preferred in the presence of IVC or iliofemoral thrombus. Most low-profile thrombectomy devices may be easily passed through a vascular sheath from the right internal jugular vein into the RV outflow and PA.
Initial intravenous administration of heparin is the therapy of choice to treat all forms of acute PE. Heparin binds to and accelerates the activity of antithrombin III, prevents additional thrombus formation, and permits endogenous fibrinolytic mechanisms to thrombolyse clot that has already formed. During CDT for massive PE, full heparin anticoagulation may be continued during the thrombus debulking procedure. However, once the massive PE has been converted to submassive PE, and overnight catheter-directed thrombolytic infusion has been initiated, full heparin anticoagulation should be down-titrated to minimize the risk of bleeding complications. During concomitant low-dose tPA infusion, a subtherapeutic heparin dose is desirable (partial thromboplastin time <60 s) to minimize the risk of perisheath thrombus formation. To achieve this, the heparin infusion rate is typically between 300 and 500 units/h through a peripheral intravenous (IV) site. When the patient has completed a course of catheter-directed thrombolytic therapy, full therapeutic anticoagulation can be resumed with full-dose IV heparin or low-molecular-weight heparin. Full heparin anticoagulation should be maintained for 7–10 days as a bridge to subsequent oral anticoagulation.
Current approved medical therapy for acute massive PE consists of systemic thrombolysis with 100 mg of alteplase (tPA; Genentech, South San Francisco, CA) infused intravenously over 2 hours, and the most widely accepted indication for thrombolytic therapy in these patients is cardiogenic shock from acute PE. However, many patients cannot receive systemic thrombolysis because of contraindications, and even when patients with acute PE are prescreened for absolute contraindications, the rate of major hemorrhage from systemic thrombolytic administration can reach up to 20%, including a 3%–5% risk of hemorrhagic stroke. Furthermore, there may be insufficient time in the acute setting to infuse full-dose intravenous thrombolytic therapy.
In some instances it may be desirable to initiate IV tPA in appropriate candidates while simultaneously activating the interventional team to perform CDT. For example, in select patients who are in extremis from PE and are deemed candidates for any thrombolytic treatment, some clinicians may wish to initiate urgent “medical” treatment in the form of intravenous thrombolysis as a bridge to escalation “surgical” treatment with CDT. When used in this fashion, intravenous tPA could also be less risky. For instance, the amount of intravenous thrombolytic could be reduced by at least 50% (from the standard 100 mg tPA dose infused over 2 hours) if catheter intervention is initiated promptly, allowing discontinuation of IV tPA within 30–60 minutes.
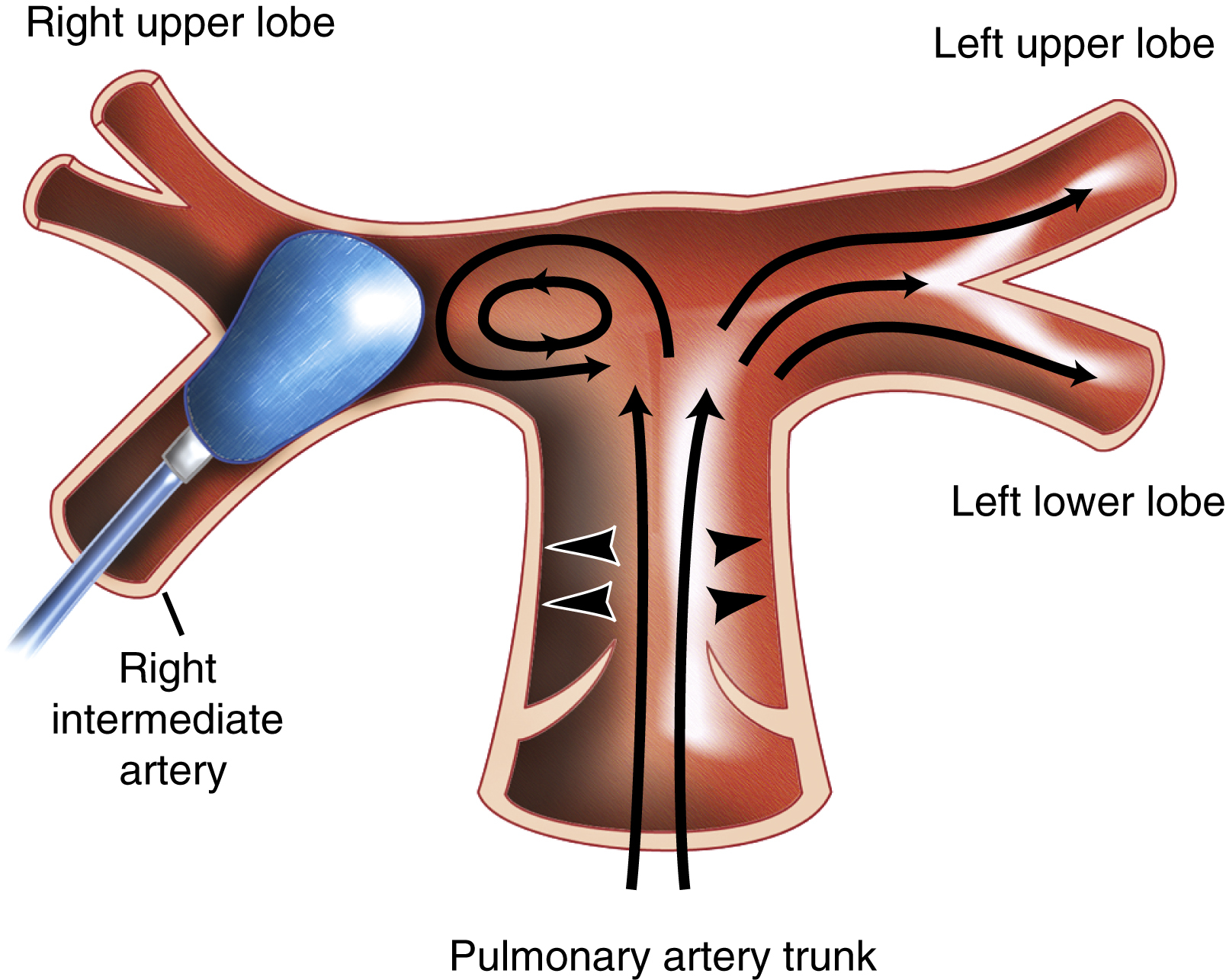
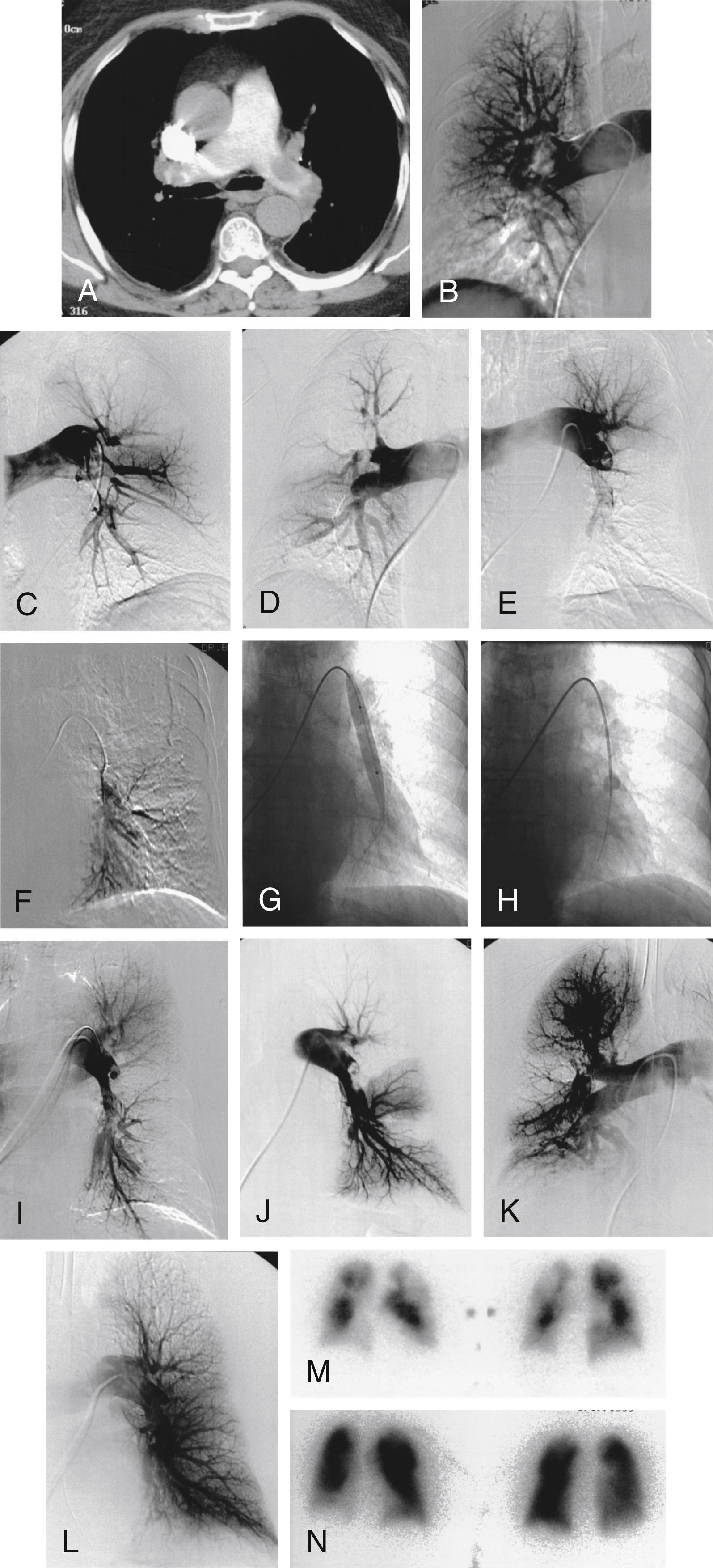
Under the definition of modern CDT, protocols of catheter-directed thrombolysis have emerged for the treatment of massive and submassive PE, respectively. For massive PE, a catheter-directed bolus of thrombolytic drug is used in conjunction with mechanical clot fragmentation and/or aspiration to achieve central clot debulking. Depending on the anticipated bleeding risk, CDT may be performed with either no or low-dose local thrombolytic injection. The goal of these techniques is rapid central clot removal to relieve life-threatening heart strain and immediately improve pulmonary perfusion. Catheter intervention is important not only for creating an immediate flow channel through the obstruction, but also for exposing a greater surface area of thrombus to the effects of locally infused thrombolytic drug. If thrombolysis is performed without intraclot drug injection, and if the thrombolytic is instead infused proximal to the target embolus, as performed in older studies, there is little added benefit compared with systemic intravenous infusion. Schmitz-Rode et al. demonstrated with in vitro and in vivo flow studies that an obstructing embolus causes proximal vortex formation that prevents a drug infused upstream from making rapid contact with the downstream embolus, and the eddy currents instead cause washout of thrombolytic into the nonobstructed pulmonary arteries ( Fig. 55.4 ). These flow studies emphasize the importance of direct intrathrombus injection as an adjunct to embolus fragmentation to achieve rapid and effective catheter-directed thrombolysis.
Several devices meeting criteria for modern CDT have been used effectively, but the most common technique is rotating pigtail catheter fragmentation, which has been used either alone or in combination with other methods in 70% of patients worldwide receiving CDT. Although pigtail clot fragmentation appears to effectively debulk proximal emboli, in some instances it has resulted in distal embolization with PAP elevation requiring adjunctive aspiration thrombectomy to complete treatment. Aspiration can be performed with virtually any end-hole catheter such as an 8Fr JR 4 catheter (Cook). Additional clot fragmentation may also be achieved with insertion and inflation of an angioplasty balloon sized below the target arterial diameter ( Fig. 55.5 ). Thus it is important to have adjunctive methods available to use in conjunction with pigtail catheter rotation. The main advantage of the rotating pigtail is its wide availability and low cost relative to the mechanically driven thrombectomy devices.
For treatment of submassive PE, or when massive PE has been downstaged to submassive PE, further mechanical debulking is usually unnecessary and the current protocol consists of careful image-guided infusion catheter placement into thrombosed segments for overnight pharmacologic thrombolysis. Once the infusion catheters have been properly positioned and connected to IV pumps, catheter-directed thrombolysis may be initiated using alteplase for example, at a rate of 0.5 mg/h through the drug lumen of each catheter if bilateral catheters are used. If only one catheter has been placed for unilateral treatment, the rate may be increased, for example, up to 1.0 mg/h or a weight-based dose through the single infusion catheter. For tPA, the recommended infusion rate should typically not exceed 0.01 mg/kg/h or 1.0 mg/h. To achieve the infusion dose, the reconstituted drug can be diluted in normal saline solution to yield a concentration of 0.1 mg of tPA per milliliter of solution, and the pump can be set accordingly to deliver the prescribed dose. An alternative to catheter-directed tPA is urokinase infusion. The regimen consists of a bolus infusion of 200,000–500,000 IU followed by an infusion of 100,000 IU/h urokinase for 12 –36 hours. The utility of monitoring fibrinogen levels is debatable, but fibrinogen can be monitored if desired in those patients at greater risk of bleeding (e.g., an elderly patient) or if the infusion will be continued beyond 24 hours. When fibrinogen levels drop below 150–200 mg/dL, the thrombolytic infusion may be reduced, discontinued, or alternatively continued with transfusions of fresh-frozen plasma if further thrombolysis is desired.
Contrary to invasive open surgical thrombectomy, which has been associated with high perioperative morbidity and mortality, modern percutaneous CDT represents a less invasive and potentially safer option for treating patients with acute massive PE. As mentioned previously, the rationale for using percutaneous devices in the pulmonary circulation is rapid central clot debulking to relieve life-threatening heart strain and to immediately improve pulmonary perfusion, which can be verified by follow-up angiography and hemodynamic assessments. Percutaneous embolectomy, clot fragmentation, and mechanical thrombolysis also serve to expose a greater surface area of thrombus to the effects of locally infused thrombolytic drug. However, depending on the anticipated bleeding risk, CDT may be performed with either no or low-dose local tPA injection depending on operator preference and risk assessment. Regardless of the decision to use thrombolytic drugs, CDT can be used effectively when full-dose thrombolytic therapy is contraindicated or fails to resolve hemodynamic shock.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here