Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A developmental, social, and family history should be obtained for every pediatric patient seen as an emergency. The guardians’ understanding of any preexisting diseases and of the cause of the current events also should be elicited. At first glance, determine the degree of neurologic compromise by estimating the level of alertness of the child and the need for rapid intervention. Then, direct the interview toward assessing the child’s baseline neurologic performance and how the current event departs from it. Infants become more cooperative when they are spoken to in a pleasant voice, and children are less intimidated when the examiner appears to ignore them at first. When possible, children should be examined while resting on their caretaker’s lap and should be engaged in conversation and play. Careful observation and holding of normal-appearing children may reveal unsuspected tone anomalies ( Fig. 27.1 ) but should be reserved for the end of the examination. Both intellectual and motor milestones should be documented ( Table 27.1 ). The basic anthropometric measures (weight, length and, most importantly, head circumference) also should be charted. Normative growth charts are available from the National Center for Health Statistics, Hyattsville, MD 20782 or at http://www.cdc.gov/growthcharts/ .
| Age (Months) | Milestones |
|---|---|
| 1 to 1½ | Head control; identification of familiar persons |
| 4 | Smiling; attempts at lifting up the head briefly |
| 6 | Reaching for objects; rolling from prone to supine |
| 8 | Transfer between hands; sitting with support; combination of syllables |
| 10 | Standing held; fine grasp |
| 12 | Walking supported; two-word or three-word vocabulary |
| 15 | Walking unsupported |
| 18 | Command following |
| 24 | Phrases |
| 36 | Handedness develops |
Large (over 4500-g) infants, use of instrumentation during delivery, uncommon fetal presentations, augmented delivery, and first vaginal delivery are all associated with neurologic birth trauma. Clavicular fracture, the most common form of trauma, is diagnosed by palpation and, if needed, by radiographs. Spontaneous recovery is the rule.
presents with flaccid paralysis as a result of injury to one or several brachial plexus roots. It occurs rarely and affects less than 1% of live births, with an incidence of 0.04% to 0.4%. Obstetrical history, prolonged labor, or use of instrumentation are potential risk factors to explain the nature of brachial plexus injury. Shoulder dystocia and microsomia are the most common causes leading to brachial plexus injury.
The clinical examination is essential to make the diagnosis of brachial plexus injury. Inspection of the newborn may reveal an asymmetry in spontaneous movements. During the examination mobilization of the affected extremity might be painful, particularly within the first week of life. However, asymmetry in the Moro reflex can elicit clinical signs of flaccid arm paralysis during the examination. Involvement of the upper roots, C5-C7, is seen in nearly 75% of the newborns presenting with brachial plexus injury. The affected limb presents with a characteristic position such as shoulder in adduction and internal rotation, elbow in extension, forearm in pronation, and wrist in extension. Paralysis of the hemidiaphragm can occur in the presence of phrenic nerve damage. Injury of the upper roots or proximal portion of the brachial plexus occurs in 50% to 80% of cases. Distal paralysis (Klumpke palsy) accounts for only 2% of the cases presenting with brachial plexus injury. In the presence of distal brachial plexus injury, flaccid paralysis involves the wrist and the hand, whereas the functions of elbow and shoulder are spared ( Fig. 27.2 ).
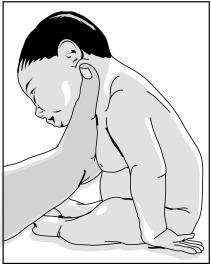
Brachial plexus injury resulting secondary to birth trauma has a favorable outcome with the chance of complete recovery in 75% to 95% of the cases. The presence of total paralysis and Horner syndrome suggest poor prognosis.
Spinal cord injury, sometimes caused by rotation of the head during forceps or forceful extraction, may be difficult to appreciate in infants with a low Apgar score. Long-term sequelae include hydromyelia and myelomalacia. C1-C2 subluxation, however, is more common and follows a benign course. Atlantoaxial rotatory subluxation may cause torticollis. When caudal dysraphism is suspected, lumbosacral ultrasounds may be obtained until the sixth month of life. Magnetic resonance imaging (MRI) is the preferred diagnostic test for an accurate diagnosis.
A tentorial subdural hematoma that resolves within the first weeks of life occurs predominantly in neonates who are extracted with a vacuum. Subgaleal hemorrhage is palpable as a soft collection that crosses skull sutures and may cause progressive anemia and consumption coagulopathy. Cephalohematoma is confined to the subperiosteum and therefore respects suture lines; it is firm to palpation and self-limited. Both should be differentiated from caput succedaneum caused by subcutaneous edema involving the presenting part during delivery.
Extreme prematurity and sepsis are the leading causes of newborn hypotonia. When encephalopathy is caused by ischemia, and Down syndrome, Prader-Willi syndrome, and neurotransmitter disorders have been excluded, attention must be turned to the spinal cord, nerve, or muscle as the cause of hypotonia. Infantile spinal muscular atrophy (SMA) may occasionally present in the newborn period and is sometimes accompanied by arthrogryposis or respiratory failure. Lower motor neuron signs and tongue fasciculation are present. The first diagnostic step for SMA is the genotyping of the SMN gene for deletions. Congenital myotonic dystrophy can be associated with a diaphragmatic hernia and is usually maternally inherited in an autosomal dominant fashion; therefore mothers should be examined for myotonia. Neonatal myasthenia gravis is caused by placental antibody transfer from a mother afflicted by myasthenia gravis or inflammatory bowel disease. Congenital myopathies are characterized by their histologic appearance (with nemaline, central cores, or myotubules), whereas the metabolic myopathies mitochondrial DNA depletion syndrome, Pompe disease (with cardiomegaly, macroglossia, and anterior horn cell dysfunction), and cytochrome C oxidase deficiency exhibit specific metabolic abnormalities useful for diagnosis. Congenital muscular dystrophies may be associated with cerebral dysgenesis and signs of severe encephalopathy including seizures and hydrocephalus. The congenital myasthenic syndromes are caused by mutations in the neuromuscular junction apparatus, and some can manifest with diminished pupillary reactivity or recurrent apnea (the latter sometimes becoming prominent later in childhood), in addition to fatigability and weakness. Often, an “unexplained” elevation of serum aspartate transaminase (AST) and alanine transaminase (ALT) (originating from muscle instead of the liver) in a weak infant (or a child) is the first clue to a myopathy until creatine kinase (CK) is eventually measured.
Premature infants are susceptible to intraventricular hemorrhage, periventricular hemorrhagic infarction, and periventricular leukomalacia. Diagnosis by ultrasonography can be performed for as long as the anterior fontanelle remains open. Intraventricular hemorrhage is associated with extreme prematurity (or birth weight below 1500 g) and occurs within the first few days of life. It is divided into grades I (germinal matrix), II (intraventricular blood that does not distort the ventricular system), III (blood that causes ventricular enlargement), and IV (parenchymal infiltration). Higher-grade hemorrhages cause hydrocephalus, which is manifested as an abrupt increase in head circumference and a bulging fontanelle. Decreased tone or spontaneous movements, loss of pupillary reactivity, apnea, hypotension, and anemia may be associated features. Serial lumbar punctures relieve the hydrocephalus in some cases; the remaining hydrocephaluss may require ventriculoperitoneal or ventriculosubgaleal shunt. Long-term outcome correlates with the degree of parenchymal damage.
Periventricular hemorrhagic infarction , which must be distinguished from intraventricular hemorrhage type IV, is a venous infarct probably caused by compression of terminal veins located under the germinal matrix of the lateral ventricles. The infarct involves the dorsal and lateral aspect of the lateral ventricle and is usually asymmetric, evolving into a cavity that communicates with the ventricle. It is associated with a significant mortality rate and with spastic hemiparesis in survivors.
Periventricular leukomalacia affects the white matter of the centrum semiovale. It is caused by perfusion failure at the border zone between the long penetrator vessels branching off the middle cerebral artery that enter the brain from its surface and the basal lenticulostriate arteries (short penetrators). It may cause spastic quadriparesis with predominant lower extremity involvement or paraplegia (see Fig. 27.1 ). The lesions tend to cavitate, causing a typical Swiss cheese ultrasound appearance of the white matter.
The etiology, clinical, and electroencephalography (EEG) features of neonatal seizures are different from the seizures reported in the infants and older children. Neonatal seizures are considered “acute seizures” as a result of a specific etiology such as hypoxia, ischemia, and other metabolic derangements. Hypoxic ischemic encephalopathy remains the most common cause of neonatal seizures followed by ischemic stroke, intracerebral hemorrhage, infection, and metabolic abnormalities.
Newborns do not display generalized seizures, possibly because of immature myelination; however, focal cortical excitation in newborns affects the brain function diffusely. Causes of neonatal and early infantile seizures are listed in Table 27.2 . In neonates, clinical diagnosis of seizures may not be straightforward, and EEG monitoring is required to assess baseline EEG findings and characterize the clinical events of interest. Approximately 50% of seizures in neonates present without clinical manifestations or with signs. Therefore recognition of nonconvulsive seizures by EEG is crucial to optimize the medical treatment.
| Sepsis and meningitis Intrauterine infection (TORCH) Drug effect or withdrawal Cerebral dysgenesis Ischemic encephalopathy Intraventricular hemorrhage of prematurity Other intracranial hemorrhages Biotinidase deficiency Folinic-acid responsive seizures Pyridoxine dependency Glycine encephalopathy Neonatal maple syrup urine disease Hypoparathyroidism and hypocalcemia Menkes disease Cerebral venous thrombosis Tuberous sclerosis Fukuyama muscular dystrophy Muscle-eye-brain disease Infantile neuronal ceroid lipofucsinosis Incontinentia pigmenti Urea cycle defects Familial benign neonatal seizures Organic acidemia Ketotic hyperglycinemia Neonatal adrenoleukodystrophy and other leukodystrophies Gaucher disease type 2 GM 1 gangliosidosis Herpes simplex encephalitis Sulfite oxidase deficiency Glucose transporter type 1 deficiency Pyruvate dehydrogenase deficiency Pyruvate carboxylase deficiency |
Except in rare cases, neonatal convulsions are not a benign phenomenon. Newborns and infants younger than 3 months of age with unexplained new-onset seizures should be evaluated and treated for infection until blood, urine, and cerebrospinal fluid (CSF) cultures are negative for at least 2 days, even without fever. In the absence of infection or of cerebral structural abnormality detectable by imaging, the single most important diagnostic procedure is the lumbar puncture. CSF protein levels can be as high as 150 mg/dL in normal newborns, but glucose should never fall below 40 mg/dL. Several polymorphonuclear cells also may be found in the CSF after delivery. A small volume of extra CSF may be stored in ice for specialized analyses for up to 16 hours. Continuous video EEG monitoring may reveal unsuspected ictal events and background rhythm abnormalities in neonates. Management also should include immediate evaluation of electrolytes with correction if needed.
Currently, there is no consensus for the optimal treatment of neonatal seizures. Phenobarbital is given as a 20 mg/kg intravenously (IV) load, followed by 5 mg/kg daily orally or IV. Two repeat loading doses of 10 mg/kg may be administered for refractory seizures. To avoid respiratory depression, care must be taken not to add a benzodiazepine while administering phenobarbital. Fosphenytoin at a loading dose of 20 mg/kg IV may substitute or be added to phenobarbital. Levetiracetam is an alternative, although the efficacy of levetiracetam as a first-line treatment remains to be determined. In the case of refractory neonatal seizures, consider underlying metabolic encephalopathy, for which specific treatment may be warranted.
A number of metabolic disorders including mitochondrial disorders may be responsive to vitamin therapy. In pyridoxine-dependent epilepsy, pyridoxine administration is diagnostic for this epileptic encephalopathy Low EEG demonstrates the burst suppression pattern, which reverses with IV pyridoxine (50–100 mg). In suspicious cases, oral administration at 30 mg/kg/day divided into 2 to 3 doses may be given as a trial for 3 consecutive days. Diagnosis can be established by elevated alpha amino adipic acid and pipecolic acid, and by mutation in the ALDH7A1 by genetic test. Clinical management should include oral administration of pyridoxine with the daily dosage of 15 to 30 mg/kg/day divided into two or three doses.
Other vitamin-responsive epilepsies include pyridoxine-5 phosphate (PLP5)–dependent epilepsy treated with PLP 30 to 60 mg/kg/day divided into 4 to 6 daily doses, folinic acid–responsive seizures treated with folinic acid 3 to 5 mg/kg/day plus pyridoxine, and biotinidase deficiency, which responds to biotin supplement. Children diagnosed with biotinidase deficiency often present with infantile-onset seizures including infantile spasms.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here