Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Pediatric cardiovascular catheterization laboratories today involve a variety of complex diagnostic and interventional procedures for congenital abnormalities using multiple imaging tools to improve procedural outcome and patient safety. Preprocedure imaging to map the complex anatomy is more routinely performed, including modalities such as computed tomography (CT), cardiac magnetic resonance imaging (cardiac MRI), and three-dimensional (3D) model creation to plan the interventional procedure. Many centers are also using 3D rotational angiography to outline anatomy and important stenoses in the laboratory. In addition, intracardiac echocardiography remains a frequently used modality to guide septal closures, assess paravalvar leaks during transcatheter valve implantation, and assess mitral valve anatomy or appendage anatomy during structural interventions. Consequently, the pediatric cardiovascular catheterization laboratory has evolved to diagnostic procedures using an assortment of imaging modalities to assess complex congenital abnormalities before surgery and interventional procedures that permit therapeutic options that often preclude surgery.
Catheterizations are performed with the Seldinger technique in the femoral vein and/or artery. Alternative venous access includes the internal jugular, subclavian, or transhepatic approaches. Arterial access also may be obtained via the arteries of the upper extremities or the carotid artery, but these approaches typically are either emergent conditions, secondary to occluded standard vessels, or anatomy deemed inadequate due to a small vessel diameter relative to the sheath required for the procedure. Anticoagulation is managed with heparin, 80 to 100 U/kg or 5000 U in patients greater than 50 kg, for an activated clotting time goal of 200 to 280 seconds, depending on the procedure to be performed. Antibiotics are administered to patients who receive an implanted device or are at risk for endocarditis based on the American Heart Association guidelines. Baseline hemodynamics (including pressures and oxygen saturations) and angiography are obtained in room air. Subsequently, relative blood flows and vascular resistances are calculated. The ratio of pulmonary blood flow (Qp) to systemic blood flow (Qs) is calculated according to the following formula: Qp/Qs = [(A o 2 − M vo 2 )/(P vo 2 − Pa o 2 )], where A o 2 is the aortic oxygen saturation, M vo 2 is the mixed venous oxygen saturation, Pa o 2 is the pulmonary arterial saturation, and P vo 2 is the pulmonary venous saturation. These data then determine whether additional information or interventions are necessary, as well as whether the patient is a future surgical or transplant candidate.
Valvar pulmonary stenosis is found in 8% to 10% of patients with congenital heart disease (see Chapter 74 ). Balloon pulmonary valvuloplasty has supplanted surgical valvotomy as the treatment of choice. The recommended balloon-to-annulus ratio is between 100% and 140%. The reported success rate is greater than 90% with complications occurring in less than 1%. Hemodynamic measurements include the right ventricular (RV) pressure compared with systemic arterial pressure and the peak-to-peak systolic pressure gradient across the pulmonary valve. The guideline recommendations for balloon pulmonary valvuloplasty is the presence of at least moderate pulmonary valve stenosis defined as peak instantaneous pressure gradient of ≥36 mm Hg by echocardiography. We use as a guideline the “rule of 50,” which is defined as a peak RV systolic pressure of more than 50 mm Hg, a peak RV systolic pressure more than 50% of the systemic systolic pressure, or a peak-to-peak systolic gradient across the pulmonary valve of more than 50 mm Hg. An end-hole catheter is used to obtain a pressure from the pulmonary artery to the body of the right ventricle to assess the severity and location of any stenoses.
RV angiography in the anteroposterior view angled cephalad and in the lateral view is performed to provide information about location and severity of pulmonary valve stenosis. The valve annulus is measured digitally. The angiographic diagnosis of dysplastic pulmonary valve stenosis is confirmed when the valve leaflets are markedly thickened and severely immobile. For patients who meet the hemodynamic criteria, balloon pulmonary valvuloplasty is performed using a standard technique and deemed successful if the estimated peak RV pressure is less than 50 mm Hg, less than 50% of the peak systemic pressure, and less than 50 mm Hg peak-to-peak gradient across the pulmonary valve (guidelines state less than 40 mm Hg). Also, procedures are deemed successful only if there is a stable saturation with a closed ductus arteriosus and no immediate operative intervention is necessary before discharge from the hospital.
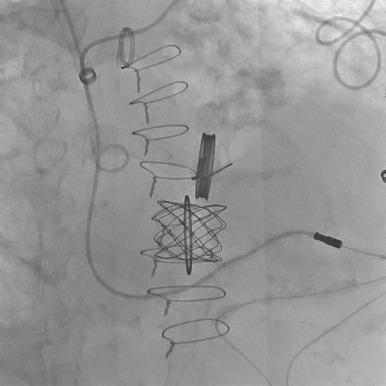
Bioprosthetic valves in the tricuspid position are amenable to transcatheter valve implantation. Data are limited, but limited experience using the internal jugular approach has shown promising results ( e-Fig. 68.1 ). Further data and clinical studies are necessary.
Nearly 5,000 babies are born each year in the United States with some form of RV outflow tract derangement, and many of these patients require surgery early in life. As these patients return with either hemodynamically significant RV outflow stenosis, regurgitation, or a combination, they are now candidates for a transcatheter pulmonary valve replacement.
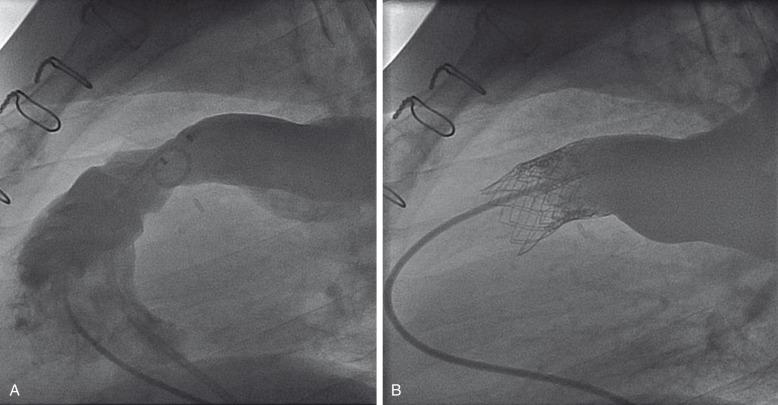
Currently there are two Food and Drug Administration (FDA)–approved transcatheter pulmonary valves available for patients with hemodynamically abnormal RV outflow tracts, including either free pulmonary insufficiency and RV volume overload or RV outflow obstruction with RV hypertension and RV systolic or diastolic failure. The two valves are the Sapien XT (Edwards Lifesciences, Irvine, CA) and the Melody valve (Medtronic, Minneapolis, MN). The transcatheter pulmonary valve has replaced many patients' need for a reoperation. Consequently, patients who often required two to four surgeries before an adult age can be treated with a nonsurgical valve technique ( e-Fig. 68.2 ).
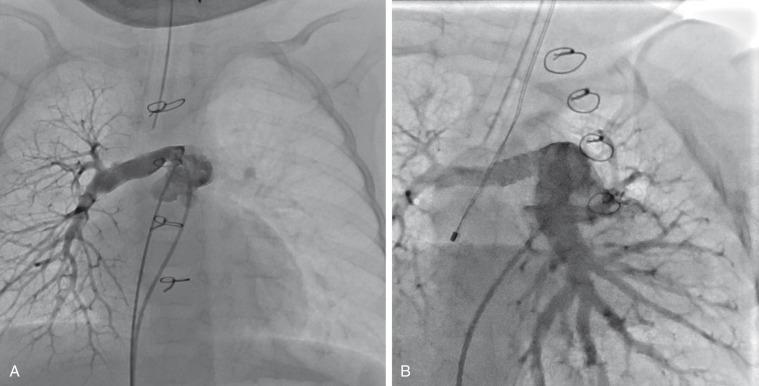
Since the introduction of high-pressure balloons (>30 atmospheres), pulmonary artery angioplasty has a success rate of more than 70%. Patients with severely hypoplastic pulmonary arteries with multiple stenoses that are refractory to high-pressure angioplasty may be amenable to cutting balloon angioplasty. Cutting balloons are now FDA approved for pulmonary artery angioplasty with balloons 4 to 8 mm in diameter going to 7 to 8 atmospheres. Cutting balloon angioplasty often is followed by standard angioplasty (8–12 atm) for the best final result. Balloon-expandable stents have improved results in proximal vessels, eliminating the need for surgery in most patients ( e-Fig. 68.3 ), but they are of limited value in distal pulmonary vessels. Furthermore, stents are contraindicated in noncompliant vessels that cannot be expanded using high-pressure balloons. Although balloon catheters, as well as stents, have not specifically been approved by the FDA as a treatment for pulmonary artery stenoses, these techniques have become the mainstay of treatment for distal vessel obstruction.
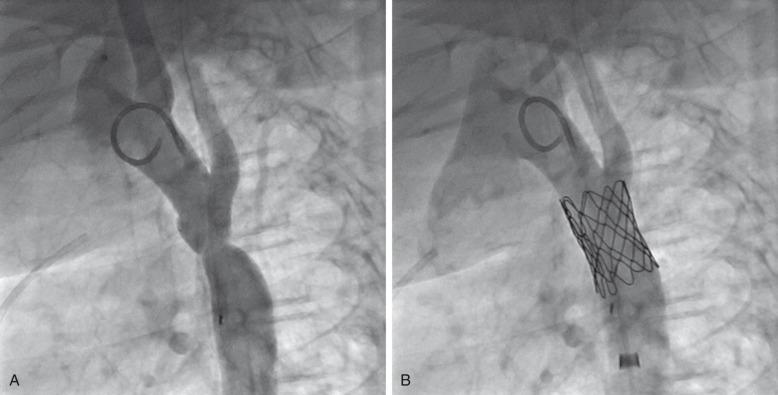
Valvar aortic stenosis results from fusion of the aortic valve leaflets, creating either a unicuspid or bicuspid apparatus with functionally a one- or two-leaflet aortic valve. This is the most common congenital heart lesion, occurring in 1% to 2% of the population (see Chapter 73 ). Valvar aortic stenosis can be classified into two groups: group 1 has disease that is severe enough that it presents at birth or within 1 to 2 years of age (10%–15%), and group 2 has disease that is not diagnosed until after age 2 years and will progress much more slowly. Morbidity, mortality, and the need for intervention are significantly skewed toward group 1. As with pulmonary stenosis, noninvasive imaging techniques have advanced to the point that nearly all anatomic and functional information about the valve may be obtained without catheterization. Fusion of the right and left coronary cusps is more significantly associated with coarctation of the aorta compared with fusion of the right and noncoronary cusp. All bicuspid aortic valve patients are at risk for aortic root enlargement, and 8% to 10% may have a cerebral aneurysm. Catheterization is performed for valves that clearly merit intervention or when symptoms and imaging findings are incomplete or confounding.
Aortic valve stenosis is classified into the following categories: trivial, mild, moderate, severe, and critical. Critical aortic stenosis is not defined by a specific pressure gradient or valve orifice size but on the basis of physiologic manifestations. If the stenosis is such that the patient is unable to produce and maintain an adequate cardiac output, the stenosis is critical. Patients in this group may have a low valve gradient due to decreased cardiac function and low cardiac output. Although controversy may exist with regard to the most beneficial treatment method for this patient population, most centers have adopted balloon valvuloplasty as the initial treatment of choice. Balloon valvuloplasty has demonstrated a reduction in gradient and resultant valve regurgitation with a shorter course in the cardiac intensive care unit and a shorter hospital stay compared with surgical valvotomy.
However, balloon valvuloplasty has been associated with an increased rate of reintervention compared with surgical valvotomy as a result of recurrent stenosis or worsening regurgitation. Given that residual aortic valve disease, especially regurgitation, may progress over time, the recommendation for the valvuloplasty technique is more conservative, with a smaller maximal balloon diameter (80% to <100% of the annulus) than that recommended for the pulmonary valve (100%–140%). The valve may be approached retrograde from the aorta, with arterial access usually from the femoral or carotid via a surgical cutdown. Our preference is to approach the aortic valve prograde by crossing an existing atrial communication or by performing a transseptal puncture to access the left side of the heart. Once in the left ventricle, angiography is performed to measure the valve annulus and identify landmarks for valvuloplasty. Many centers have adopted rapid right or left ventricular pacing at the time of balloon inflation. This rapid pacing transiently reduces cardiac output and the shearing force transmitted to the balloon as it is inflated across the valve annulus. The goal is to reduce the balloon shear force motion on the fragile valve leaflets and prevent excessive damage and subsequent regurgitation. Repeat angiography and echocardiography after inflation are essential to evaluate the success of the valvuloplasty and monitor for regurgitation or other complications. The differentiation between noncritical stenosis categories is made by noninvasive echocardiographic measurements of valve area and Doppler gradient. A normal valve area is 2 cm 2 /m 2 . Mild obstruction is consistent with valve areas less than 2 cm 2 /m 2 but greater than 0.7 cm 2 /m 2 , and severe obstruction is consistent with valve areas less than 0.5 cm 2 /m 2 . Mean echocardiographic Doppler gradients are good predictors of the peak-to-peak pressure gradient measured at catheterization. Gradients less than 25 mm Hg are considered trivial, those 25 to 50 mm Hg are mild, those 50 to 75 mm Hg are moderate, and those greater than 75 mm Hg are severe. These measurements are made with the understanding that the cardiac function and cardiac output are normal. Catheterization is not recommended for trivial or mild stenosis. Moderate and severe stenoses are approached with primary balloon valvuloplasty using the techniques previously described.
Often, aortic obstruction after an end-to-end surgical repair at the isthmus is a result of aortic narrowing within the transverse arch. These obstructive lesions are further defined as either the proximal transverse arch between the innominate artery and left carotid artery or the distal transverse arch, defined as the region between the left carotid artery and the left subclavian artery. Although few data exist regarding stent angioplasty within the distal transverse aortic arch, general experience has been that this procedure is safe and effective. A sheath is placed in the femoral artery and an arterial monitoring catheter is placed in the right upper extremity. A 4-Fr sheath is used to advance a 4-Fr pigtail catheter from the right radial artery to the proximal transverse aortic arch. This catheter is used to monitor pressures during stent angioplasty of the distal transverse arch from the femoral artery and to perform cine angiograms to determine appropriate stent placement distal to the takeoff of the left carotid artery.
Transcatheter stent angioplasty for postoperative recoarctation of the aorta at the isthmus has been demonstrated to be safe and effective. The technique usually involves a femoral arterial approach. Recently, the coarctation of the aorta stent trial (COAST) resulted in an FDA approval for a bare metal stent (CP stent, NuMED, Hopkinton, NY) for coarctation of the aorta and COAST II resulted in FDA approval of a Gore-Tex–covered stent (CP Covered Stent, NuMED, Hopkinton, NY) for coarctation of the aorta ( Fig. 68.4 ).
An exchange length wire is placed in the ascending aorta or right subclavian artery. An angioplasty balloon is used with a maximum diameter that is equal to or less than the diameter of the normal aortic segments adjacent to the stenotic region and/or the diameter of the descending thoracic aorta at the diaphragm. The stent is mounted on the angioplasty balloon and passed through a sheath at least 1 to 2 Fr larger than that required by the balloon. The stent length is dependent on the lesion length but is usually 28 to 45 mm. The stent is fully dilated in most cases, but at times it is deemed safer to serially dilate the lesion over two procedures.
Although atrial septal defect (ASD) diagnosis rarely requires cardiac catheterization, today many patients undergo cardiac catheterization for therapeutic device closure. These patients require assessment of associated anomalies such as abnormalities of pulmonary venous connections. A step-up in oxygen saturations in the right atrium and pulmonary arteries is characteristic for an ASD, and the degree of left-to-right shunting or the pulmonary to systemic blood flow ratio (Qp:Qs) can be determined. The ideal age or timing for elective ASD closure is 2 to 5 years of age or within 6 to 12 months of diagnosis. Rarely, a child with an ASD presents with severe congestive heart failure and requires intervention in the first year of life.
Percutaneous ASD closure has been established as a safe and effective alternative to operative repair. The technique involves transcatheter delivery of a device in its retracted state via the femoral vein under fluoroscopic and echocardiographic guidance. Echocardiographic guidance can be transthoracic in young children and transesophageal, intracardiac, or even 3D in older children and adults. The most commonly available devices today consist of a double-disc design made of nickel and titanium (Nitinol) with deployment of the first disc on the left atrial aspect of the septum followed by deployment of the second opposing disc on the right atrial wall. The expanded discs are tightly approximated, thus closing the defect. The device becomes endothelialized during the next 3 to 6 months while the patient is treated with antiplatelet medications. Relative contraindications include some defect diameters too large in relation to body weight; the absence of adequate septal tissue margins, especially the posterior inferior septum near the atrioventricular valves; and close proximity to vital cardiac structures.
The world experience of transcatheter ASD closure using the FDA-approved Amplatzer Septal Occluder (St. Jude Medical, St. Paul, MN) has been reported with a greater than 97% immediate success rate. The occlusion rate reached 100% by 3 years. An overall 2.8% adverse event rate was found, and no procedural deaths occurred. Several studies regarding comparisons of device occlusion versus surgical closure have been performed prospectively. These reports include the Amplatzer (St. Jude Medical, St. Paul, MN) and Helex (W. L. Gore & Associates, Flagstaff, AZ) devices. Findings include shorter hospital stays, less discomfort, and shorter convalescence in patients undergoing successful device closure. Hospital costs are similar. Regression of right ventricular dilation was similar for both groups of patients; however, it was dependent on the patient's age at the time of closure, with greater regression after earlier intervention and before puberty. Most recently, the Gore Septal Occluder (GSO) device (W. L. Gore & Associates, Flagstaff, AZ) reached FDA approval and is routinely use to close ASD with diameters up to 17 to 18 mm ( e-Fig. 68.5 ).
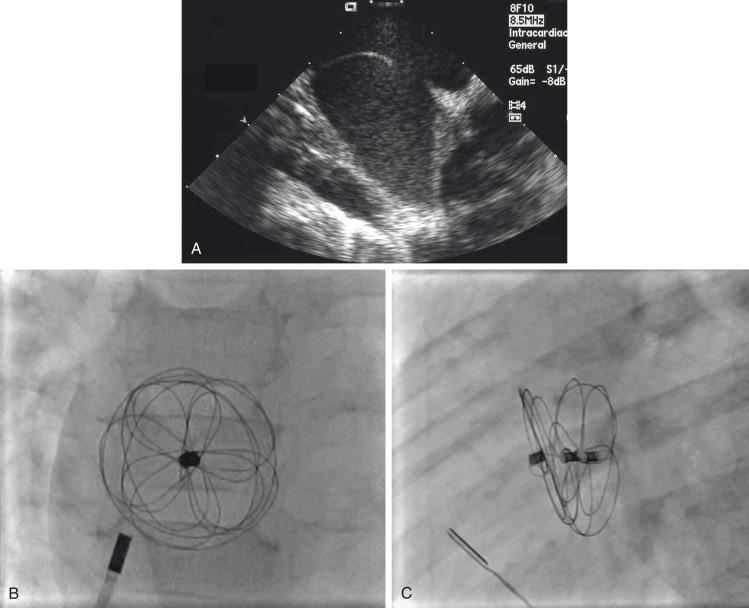
Procedural adverse events are uncommon but include embolization into the right or left atrium, pulmonary artery, left ventricle, and aorta; stroke as a result of a clot or air embolization; and bleeding complications. Both acute and late embolization have been reported, and thus it is critical for the radiologist to be familiar with the location of the interatrial septum on radiography to ascertain correct device position in the anteroposterior and lateral chest radiograph.
The first transcatheter closure of a persistently patent ductus arteriosus (PDA) was performed by Portsmann in 1967. Since that time a number of different PDA closure devices have been studied, some of which are no longer available. The goal of the procedure has always been to achieve 100% closure of the PDA without obstruction of either adjoining blood vessel (i.e., the aorta and the left pulmonary artery) with minimal risk of complications. The difficulty is that the PDA exhibits extreme variability in size and shape. Krichenko and colleagues classified the PDA into five anatomic types based on the lateral aortic angiogram. The most common (type A) ductus is conical with a narrowed pulmonary arterial end and large aortic ampulla. Other types include those with a narrowed aortic end, narrowing at both ends, and a tubular configuration.
There has been great success since the first coil closure of a PDA in 1992. As expected, the recommended technique for coil embolization is variable, depending on the size and shape of the PDA. The vessel can be approached from the venous or arterial side, and the coils may be deployed “free hand” or secured with a bioptome or modified catheter. Smaller coils, such as the Flipper coil (Cook Medical, Bloomington, IN), are attached to a delivery system and are released once they are verified to be in the proper position.
A more recent addition to the interventional cardiologist's armamentarium for PDA closure is the Amplatzer Duct Occluder and the Amplatzer Duct Occluder II (St. Jude Medical, St. Paul, MN). Both devices are self-expanding wire mesh devices attached to a delivery cable and deployed through a long sheath from the venous system ( e-Fig. 68.6 ). The device has a retention skirt to occupy the aortic ampulla and tapers slightly to the pulmonary artery end. The device is filled with a polyester mesh that stimulates thrombus formation within the lumen of the PDA. The shape of the device and self-expanding properties exert radial force on the walls of the PDA, holding the device in place until endothelialization occurs. The device has excellent closure rates approaching 100% at 1 month after the procedure. The main limitation is the size and bulky nature of the device. The PDA must have an aortic ampulla adequate to accommodate the retention skirt without creating aortic obstruction. Furthermore, the device can create left pulmonary artery stenosis by compressing adjoining structures once it is released.
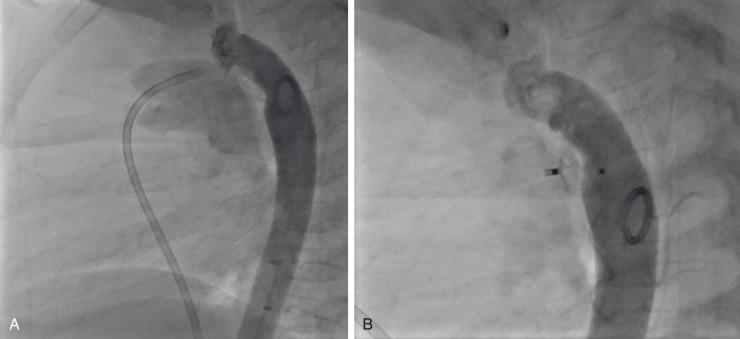
More recently, the Nit Occlude (pfm medical, Carlsbad, CA) reached FDA approval for PDA closure. This device is deployed through a 4-Fr system making it a preferred technique in smaller children ( e-Fig. 68.7 ).
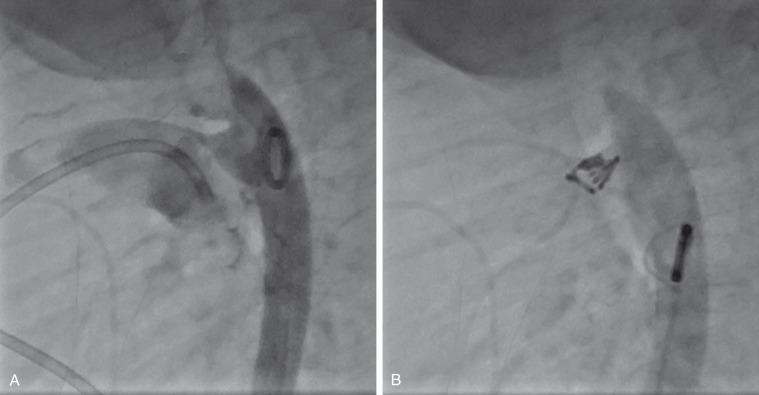
Accessory blood vessels are of concern for the interventional radiologist and interventional cardiologist. These accessory blood vessels may include aortopulmonary or venovenous collaterals, arteriovenous fistulae and malformations, surgically placed shunts, and transhepatic access tracts. Techniques for closure are similar to those described for the PDA, predominately involving Gianturco (Cook Medical, Bloomington, IN) and similar coils, the Amplatzer Duct Occluder (St. Jude Medical, St. Paul, MN), or the Amplatzer Vascular Plug (St. Jude Medical, St. Paul, MN). The vascular plug is similar to the duct occluder in that it is a self-expanding wire mesh design. It differs in that it is cylindrical in shape with no retention skirt, no tapering through its length, and no polyester mesh interior fabric. The device has excellent occlusion results but has been unsuccessful in short arterial vessels, such as aortopulmonary collaterals and the PDA. It is thought that the lack of occlusive material through the center of the device does not provide enough restriction to arterial blood flow to stimulate thrombosis and occlude the vessel.
With regard to imaging, the clinical practices of the pediatric radiologist and pediatric electrophysiologist intersect in two ways: Both subspecialists are interested in detailed internal anatomy, and both subspecialties have evolved toward performing therapeutic procedures. In particular, advances in imaging have helped improve outcomes of catheter ablation for nearly every tachyarrhythmia.
This section is divided into two parts: (1) items of interest to the radiologist pertaining to activities in the electrophysiology laboratory, especially newer imaging technologies to guide catheter ablation and radiation exposure risks during catheter ablation; and (2) cardiac rhythm device therapy and special concerns in the radiology department.
Curative therapies for pathologic tachyarrhythmias began with open-chest surgery for Wolff – Parkinson – White syndrome in 1968 followed by transvascular catheter delivery of alternating current in the radiofrequency range (about 550 kHz) in 1987 in adults and in 1994 in children for common forms of supraventricular tachycardia (SVT). The importance of complex electroanatomic correlation within the cardiac chamber walls was first applied to atrial and ventricular tachycardias post–congenital heart surgery in the late 1990s and in adults having paroxysmal atrial fibrillation starting with the seminal discovery by Haissaguerre in 1998 that repetitive pulmonary vein discharges are its source. Since 2000, technical advances in catheter design have enabled the creation of larger lesions—necessary for transmural arrhythmia substrates—and have brought about the use of cryoenergy, with its greater safety profile when substrates are proximate to vital structures such as the coronary arteries and atrioventricular node. Owing to improved outcomes and safety profiles, almost no tachyarrhythmia substrate is now considered exempt from catheter ablation. Accordingly, current indications to perform catheter ablation in children and after congenital heart surgery are more liberal ( e-Box 68.1 ) compared with those from 2002.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here