Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The diagnostic pathology of bones and joints is not significantly more complicated than the pathology of other body sites, but tissues that are essentially invisible to an examining physician and that then must be sawed and decalcified can be technically and diagnostically challenging. In addition, the imperative for radiographic correlation with bone specimens may intimidate pathologists who are not familiar with clinical imaging studies. Until the advent of computed tomography (CT) and magnetic resonance imaging (MRI), most soft tissue sites were not as accessible on imaging studies as the bones, which have always been readily appreciable through routine radiographs due to their radiodensity. With the common use of CT and MRI, correlation of imaging studies with histology has become a valuable diagnostic adjunct in every area of pathology.
Dr. Henry L. Jaffe was one of the first to point out that specific lesions of bone occur in statistically reproducible clinical settings. Lesions are often age specific, favor particular bones or particular portions of a bone, and have imaging findings that allow them to be sorted into various categories of differential diagnoses simply by considering their radiographic appearances. Although, even in isolation, histopathology can often yield the correct diagnosis, microscopic findings provide much greater information in the appropriate clinical and radiologic background. The correct diagnosis is made with the greatest frequency when the clinical and imaging features are correlated with the histology. This chapter will summarize some of the parameters bone pathologists use to formulate their diagnostic evaluations.
The most important of the clinical parameters are the patient's age and the nature of the clinical complaints. Although the pathologist can usually obtain the patient's age from the pathology request, clinical complaints and the surgeon's interpretation are often woefully abbreviated or even frankly inadequate in the requisition. Often a person who is unfamiliar with the complete patient hurriedly jots down the history, and the result is the usual pathology laboratory scenario of receipt of a small amount of tissue with a request slip containing a history line that says simply “rule out tumor.” The word tumor can certainly induce anxiety in a pathologist unfamiliar with orthopedic diagnoses, but it is worthwhile remembering that bone neoplasms are among the least common of orthopedic diseases.
Assuming that there are 15,000 pathologists in the United States and that there are about 2500 new malignant bone tumors per year in the U.S. population, a pathologist can reasonably expect to see one bone tumor every 6 years if all malignant bone tumors in the country in a single year are seen in a randomly and evenly distributed fashion. This is equivalent to perhaps five or six tumors in a lifetime of practice, certainly too few to offer any meaningful working diagnostic experience. However, it is an ideal amount to induce the fear that the current request might be that one primary bone tumor that was not identified in the last one thousand “rule out tumor” pathology requests. In these circumstances, it is best to use the telephone to confirm the surgeon's thoughts. Even better, this can be done at a later time during which the patient is no longer under general anesthesia on an operating table. It often turns out that the surgeon was not expecting a diagnosis of anything serious, but just wrote the word tumor as a distant reminder to the pathologist. When the surgeon is on the telephone, some important parameters may be discovered. What is the radiologic differential diagnosis? How long has the patient had the complaint? Does the complaint cause pain or functional loss? If there is pain, is it present at rest? Does the pain wake the patient from sleep, and is there anything that relieves the pain? What are the indications that this patient could have a bone tumor? Perhaps most importantly, the diagnosis of bone lesions requires confidence, and confidence is not only acquired with experience but by constant communication with other physicians in the diagnostic and treatment chain.
Laboratory data are of some value in a tissue evaluation, although they are seldom specific. Often a surgical pathologist evaluates laboratory parameters after having seen the tissue sections, and then only to decide whether the presumptive diagnosis makes sense in the framework of laboratory evaluations. For instance, if a pathologist is considering a diagnosis of myeloma, a serum protein electrophoresis that demonstrates a monoclonal gammopathy reinforces the strength of diagnosis. A lesion that is histologically consistent with giant cell tumor of bone should be differentiated from the so-called “brown tumor” of hyperparathyroidism, a non-neoplastic process that may contain many multinucleated giant cells. Although the distinction is usually possible with pathoradiographic correlation alone, occasionally it is difficult. In these cases, serum calcium, phosphate, blood urea nitrogen, and creatinine values are useful discriminators. Other laboratory parameters, such as serum alkaline phosphatase activity, may reflect overall bone synthesis but are not specific in and of themselves.
In recent years, diagnostic evaluations have been based on ever-smaller biopsies. In orthopedic pathology, where lesions are invisible without imaging studies, small biopsies must always be placed into the context of the abnormalities seen in imaging studies. This is the only way to be certain that the actual abnormalities are even represented in the biopsies. In addition, because bone is a hard tissue, there may be squeeze artifacts in closed needle biopsies that are not seen in open biopsies ( Fig. 4-1 ). In general, however, the smaller the biopsy, the greater is the need for correlative imaging studies.

Orthopedic surgeons occasionally use frozen section analysis. In many institutions, the most common current intraoperative consultation scenario is the evaluation of synovium or periprosthetic tissues in failed total joint revisions for possible infection. This type of evaluation is becoming increasingly common, given the number of total joint replacements performed; moreover, it has a large and growing literature documenting its value. The less common scenario is for the intraoperative evaluation of a bone or soft tissue lesion. Because endosteal lesions are invisible to the orthopedic surgeon, a frozen section of this type is often not so much for definitive diagnosis but to decide if there is enough representative tissue to examine on permanent section for definitive diagnosis.
Frozen sections of bone can be intimidating to many pathologists because of the potential technical difficulties in sectioning hard tissues without decalcification. The vast majority of space-occupying bone lesions, however, are sufficiently soft to have a technically acceptable frozen section performed on them. If possible, the relevant clinical imaging studies should be reviewed with the surgeon or radiologist, because this may help reinforce the pathologist's view that (1) there is a departure from normal in the bone and (2) what is in the section can be representative of that departure.
Tissue submitted for permanent sectioning from an orthopedic frozen section or from any small orthopedic biopsy should be adequately fixed. In addition, it should always undergo a period of decalcification and washing prior to processing, unless one can demonstrate by specimen radiography that there is no bone present in the specimen. Because bone cannot be adequately sectioned in paraffin blocks, any biopsy from the inside of a bone, regardless of how soft it feels at the grossing station, should receive some decalcification to avoid artifacts and potential technical difficulties induced by a small fragment of hard tissue in the paraffin block ( Fig. 4-2 ).

Gross examination of tiny biopsy fragments is not apt to be diagnostically rewarding. Larger biopsies may contain some macroscopically discernible diagnostic information that may be relevant for microscopic correlation. Resection specimens usually contain very meaningful information, particularly in the case of total joint replacements, in which loss of articular cartilage and production of marginal osteophytes can be detected.
In the case of large bone resections for tumors, much of the important information may only become available after manipulation of the specimen, usually by sawing. Laboratory saws come in several varieties. Oscillating saws that preferentially cut hard tissue such as those used for skulls and ribs in the autopsy suite are to be avoided for histology specimens because these saws both destroy and compact bone tissue, causing undue amounts of tissue artifact ( Fig. 4-3 ). Handsaws are useful tools for cutting bones, particularly if the specimens are relatively small in size and quantity. A tabletop vise is a handy adjunct for holding specimens in a fixed position for using a handsaw. In addition, commercially available saws invented for bone cutting have built-in specimen holding capabilities, although because they are small they have limited usefulness.
Power saws, particularly band-type butcher saws, are useful in hospital laboratories that process many specimens because of time efficiency; however, operator safety is a major issue. It is imperative that face and hand protection be used with these instruments and that there is no operator distraction, to prevent finger and hand injuries. It is also important to keep saws clean after use, which results in minimal cross contamination of cases.
When a radical resection specimen containing an entire bone lesion is received, there is less need for radiographic correlation for diagnosis than there is with a biopsy, because when small biopsies are performed, the imaging essentially substitutes for the gross findings. On the other hand, prior to making a decision regarding the plane in which to saw a gross specimen, it is useful to have clinical radiographs to best judge the ideal anatomic plane of section. The context of clinical imaging and how these images correlate with pathologic interpretation is even better understood when specimen radiographs are prepared in the laboratory.
Specimen radiographs may be obtained with the cooperation of a helpful and interested radiologist or radiology department, but ideally the laboratory should produce them with a specimen radiography apparatus. Older versions of these devices are present in many pathology laboratories because historically they have been used to detect microcalcifications in breast specimens. However, most versions of these machines in pathology laboratories use actual x-ray films, rendering them increasingly obsolete in an age in which developing facilities for analog films are disappearing from digitally based radiology departments. On the other hand, digital cassettes such as those now used in radiology departments can often be purchased and substituted for film in the older specimen radiography machines. The newer digital versions of specimen radiography machines are much more expensive than their predecessors, but they have the advantage that results are instantly available and can be sent directly to a computer for electronic storage and subsequent retrieval.
Specimen radiography often yields much more sensitive detail than clinical radiographs ( Fig. 4-4 ). In theory, CT scanning provides the ability to slice an object into thin sections and make specimen radiographs of these slices. When a surgical specimen is cut into thin slices axially, and radiographs are made of the slices, the information corresponds almost exactly to what can be obtained in a very sensitive CT image but with much greater detail ( Fig. 4-5 ). By comparing these images with the gross specimen slices, a deeper understanding of the relationship between normal bone and the processes affecting it can be obtained. This, in turn, increases the effectiveness with which a diagnostic pathologist perceives and understands clinical images.

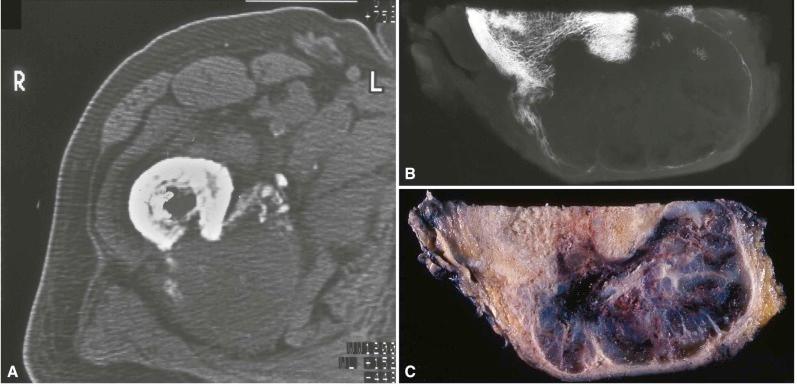
Specimen radiography can also be used as a tool in preparing section maps for very large osseous specimens, especially when trying to evaluate specimens for tumor necrosis following neoadjuvant chemotherapy regimens. In addition, the initial specimen radiograph can be used as a control to monitor decalcification prior to the submission of tissue for embedding. In this way, decalcification can be carried to completion but tissues do not remain in contact with chemicals that are potentially destructive or inhibitory to staining.
Once a large specimen has been opened, its surfaces can be examined for the relevant pathology. The location, size, and extent of space-occupying lesions, as well as their relationship to the adjacent bone, should be noted and documented. Also, the bone compartment or compartments containing the lesion should be noted, and the relationship of the lesion to the cortex, periosteum, and soft tissues should be recorded. If a bone lesion is neoplastic, the resection margins should be examined and sectioned, and if there has been a prior biopsy, the tissue at the margins of the biopsy tract should be permanently marked and sectioned, as well as the biopsy tract itself.
If at all possible the specimen should be photographed for documentation. Prior to photographing, the specimen should be cleaned so that (1) artifacts created by manipulations are removed and (2) bony alterations are more visible. This usually consists of removing the bone dust created in the sawing process. This can be accomplished with a bristle brush under a stream of cool water, or even using a scalpel blade to scrape the bone surface under running water. It is essential to perform this step prior to fixation, because formalin fixation makes the bone dust more adherent to the bone surface. When photographing a specimen, it should be remembered that rulers and accessioning numbers should be present only if it is necessary to preserve both identification and scale in a specimen photograph. Thus, it is seldom necessary to place a measuring device next to a specimen of an entire extremity or a large bone.
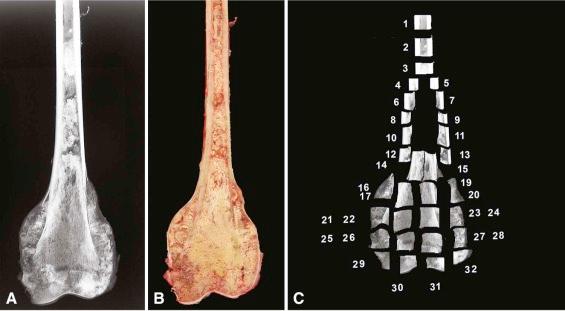
Specimen blocks can be prepared immediately for fixation from soft lesions, but sections to demonstrate the relationship of lesional tissue with residual host bone or those of a lesion producing calcified matrix must be trimmed and decalcified prior to submission. The gross photograph or a specimen radiograph may then serve as a sectioning map if necessary ( Fig. 4-6 ).
Two major methods are commonly used to remove calcium from tissues: acid decalcification and chelation. In each, the calcium ions in the hydroxyapatite crystals of bone matrix must pass into solution, leaving behind a collagenous matrix soft enough to be sectioned.
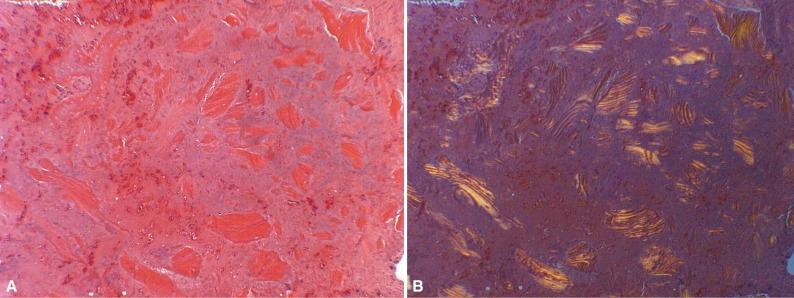
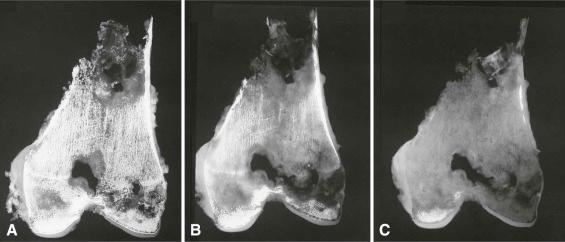
Acid decalcification is more commonly used in histology laboratories. In this method, hydrogen ions in the specimen bath change the ionic activity of calcium, rendering it soluble and allowing it to pass into solution. The acid solutions used are typically dilute solutions of hydrochloric or nitric acids. Strong acids dissociate into hydrogen ion and its corresponding anion in aqueous solution, allowing fairly rapid formation of an ionic solution. These acid solutions can be prepared in the histology laboratory by simple dilution of commercially available concentrated acids, but if safety and storage considerations outweigh expenses, many laboratories purchase premixed commercially available decalcification solutions. Acid decalcification methods are relatively fast, but they can be applied only to tissues that have been thoroughly fixed, which itself increases processing time. Their low pH induces chemical changes in the tissues that affect staining, hydrolyze nucleic acids, and may alter tissue antigens, which will secondarily alter the potential effectiveness of immunohistochemistry. Tissues must be well rinsed and even chemically neutralized prior to embedding; otherwise, the chemical reaction may proceed slowly not only in the paraffin-embedded tissue but also in the sections already stained. Consequently, inadequately washed tissues may produce stained sections that degrade rather rapidly over time. Moreover, if a specimen remains in an acid decalcification solution for too extended a period of time, its staining capacity, particularly with hematoxylin, may be permanently impaired ( Fig. 4-7 ).
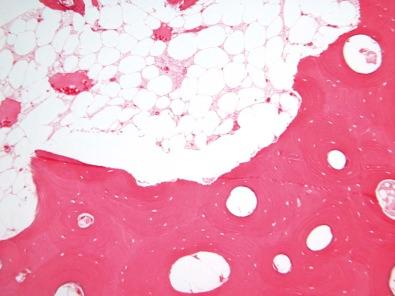
The second type of decalcification uses complexing (chelating) agents. These are chemicals which complex with the calcium in solution, thus reducing free calcium and increasing the tendency of calcium in hydroxyapatite to dissolve. The most common chelating agent used is ethylene diamine tetra-acetate (EDTA). Decalcification by this agent can be carried out in a neutral solution, avoiding the artifacts associated with low pH. This method also requires thorough tissue fixation, but the removal of calcium proceeds so slowly that it is not useful for tissue diagnosis in diagnostic laboratories. In addition, the binding of calcium to the complexing agent is a limiting factor in a finite amount of EDTA, so the initial solution must be frequently changed for a fresh solution until decalcification is complete.
The choice of decalcification method for bone depends on the type and volume of calcified specimens to be sectioned as well as the individual needs of the laboratory for processing speed. In very high volume laboratories, there is pressure to use methods that expedite tissue processing, because for optimal patient care pathology reports must be generated in as little time as possible. If excellent histologic preparation is required, as might be needed for critical interpretation of the volume of necrosis following neoadjuvant chemotherapy for osteosarcoma, decalcification should be performed with greater attention to tissue preservation. This means that if commercial reagents are used to decalcify specimens, the process must be carefully monitored, either by specimen radiography or with chemical monitoring of the decalcification solution in order to determine the precise time they can be sectioned. If time and personnel do not permit such careful monitoring, the decalcification method should be modified so as to minimize tissue damage.
When time and motivation permit, large specimens should be trimmed into smaller ones prior to decalcification so as to minimize the time softer tissues and cancellous bone are in acid solutions, and solutions that do less potential damage to bone should be considered (see Fig. 4-6C ). Trimming may be performed with a power or hand jigsaw or even a band saw. However, most saws remove a fair amount of bone tissue at their edges. Diamond-edged, slow-speed power saws with built-in water sprays to control heat generated by friction are commercially available for this purpose.
Some of the commercially available decalcifying agents are a compromise between acid decalcification and chelating agents. They have somewhat weaker acidity, which is compensated for by adding chelating agents to bind excess calcium and hasten the exchange of calcium into the solution.
A good practical compromise for laboratories preparing their own decalcification solutions is to use a formic acid–formalin mixture to which a cationic exchange resin has been added. In this method, the organic ion is the buffer ion for the organic anion of the acid and tends to keep the pH at constant levels, allowing uniform dissolution of the calcium ion from hydroxyapatite. In addition, the cationic exchange resin complexes with the free calcium, allowing lower calcium concentration in solution and easier dissolution of hydroxyapatite. Concentrations of formalin and formic acid can be as high as 20%, allowing fairly rapid decalcification. This method has the advantage of requiring little fixation prior to decalcification and little washing afterward to produce optimal staining of sections. Furthermore, if specimens are left in this solution past the point of complete decalcification, the destruction of tissues is less marked than with other acids. A slab of bone can be suspended in this solution and sections can be submitted during the monitoring of the decalcification procedure. Various ancillary techniques have also been used to hasten the decalcification procedure by acid or chelating methods. These include agitating the solution mechanically or with ultrasound, increasing the ambient temperature of the decalcification solution, decalcifying it while decreasing the ambient pressure, and adding an electrical current to the decalcification solution. A detailed description of the advantages or disadvantages of these modifications is of interest but is beyond the scope of this text. Suffice it to say that in high-volume histology laboratories, the fewest labor-intensive techniques for processing are generally used. Because many of these manipulations can be labor-intensive, their effectiveness is of decreasing marginal utility; the simple immersion of hard tissues in decalcifying solutions usually suffices.
The point of complete decalcification should be determined prior to specimen sectioning. In busy laboratories, this is usually performed mechanically, either by compressing the tissues with a needle or simply by bending them. These methods are not reliable, particularly if the specimen blocks are thick. Qualitative and quantitative chemical methods have also been used, but these are not suitable in a busy histopathology laboratory. The most practical method is to use specimen radiography, especially if specimen radiographs are digitally available ( Fig. 4-8 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here