Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Vascular disease affecting the renal arteries presents complex challenges to clinicians. Partly due to advances in vascular imaging, more patients than ever before are being identified with some degree of atherosclerotic or fibromuscular renovascular disease. Many of these lesions are of minor hemodynamic importance at the time of detection. Some reach a degree at which perfusion pressures and intrarenal hemodynamics are altered, leading to changes in blood pressure regulation and renal function. Such hemodynamic changes can produce a variety of recognizable clinical syndromes illustrated in Fig. 22.1 . These range from modest changes in systemic arterial pressure to impaired volume control associated with congestive cardiac failure to threatened viability of the kidney, sometimes designated “ischemic nephropathy.” Understanding the pathways by which renovascular disease affects cardiovascular and renal disease is important both for diagnosis and for defining optimal management using tools to control blood pressure, to block the renin-angiotensin system, and to restore the circulation.
Most renovascular lesions are the result of atherosclerosis. With the aging of the US and other Western populations and reduced mortality from stroke and coronary disease, the prevalence of vascular disease in other vascular beds reaching clinically “critical” levels appears to be increasing. Understanding the variety of clinical manifestations of these lesions, the potential for disease progression, and the benefits and limitations of vascular repair are essential for vascular medicine specialists. This chapter will examine the pathophysiology of renovascular lesions regarding blood pressure control, ischemic nephropathy, and clinical syndromes such as “flash” pulmonary edema. Specific aspects regarding diagnostic evaluation and management will be addressed elsewhere (see Chapters 23 and 24 ).
A wide range of vascular lesions can affect the renal blood supply, some of which are summarized in Box 22.1 . Historically, recognition of renovascular disease resulted from searching for underlying causes of hypertension. This followed the seminal observations of Goldblatt more than 80 years ago that renal artery constriction produced an increase in arterial pressure in the dog. These studies were among the first to establish a primary role of the kidney in overall blood pressure regulation. Renovascular hypertension produced by a “clipped” or constricted renal artery remains among the most widely studied experimental forms of angiotensin-dependent hypertension.
Unilateral atherosclerotic renal artery stenosis
Unilateral fibromuscular dysplasia (FMD)
Medial fibroplasia
Perimedial fibroplasia
Intimal fibroplasia
Medial hyperplasia
Renal artery aneurysm
Arterial embolus
Arteriovenous fistula (congenital/traumatic)
Segmental arterial occlusion (posttraumatic)
Extrinsic compression of renal artery (e.g., pheochromocytoma)
Renal compression (e.g., metastatic tumor)
Stenosis to a solitary functioning kidney
Bilateral renal arterial stenosis
Aortic coarctation
Systemic vasculitis (e.g., Takayasu arteritis, polyarteritis)
Atheroembolic disease
Vascular occlusion due to endovascular aortic stent graft
Fibromuscular dysplasia may be identified in 1% to 3% of normal kidney donors subjected to angiography before donor nephrectomy. Of those developing clinical hypertension and referred for revascularization, more than 85% are females with a predilection for disease in the right renal artery. The location of these lesions is most commonly in the midportion and distal segments of the renal artery. A variety of fibromuscular lesions have been described, but the most common is medial fibroplasia (also see Chapter 58 ). Occasionally, such lesions may be found in the carotid and other vascular beds, but most commonly they are limited to the renal arteries. Most do not progress to impair renal function, although some lead to arterial dissection and/or thrombosis with loss of the kidney.
Atherosclerosis is the most common cause of renal artery disease. Its presence and severity are related to age and the presence of other atherosclerotic disease of the abdominal aorta and arteries of the lower extremities. In population-based studies in the United States, up to 6.8% of healthy people older than 65 years were found to have significant renal artery stenosis (defined as Doppler peak systolic velocity greater than 1.8 m/s). Renal arterial occlusive disease commonly represents an incidental finding in patients with widespread atherosclerosis undergoing carotid, coronary, or peripheral angiography. Peripheral artery and aortic disease is associated with higher prevalence (25% to 33%). As expected, risk factors predicting the presence of renal artery stenosis include smoking, hyperlipidemia, hypertension, and diabetes. A corollary observation is that renovascular hypertension resulting from these lesions is now most commonly superimposed gradually upon preexisting “essential” hypertension. Hence the blood pressure response and “cure” rates after successful restoration of blood flows to the kidney are limited by preexisting conditions.
Under basal conditions, renal blood flow is among the highest of all organs. This feature reflects the kidney’s filtration function, and less than 10% of delivered oxygen is sufficient to maintain renal metabolic needs. The renal cortex receives more blood flow than the medullary segments, but the latter consume more oxygen as a result of active tubular solute transport. Hence the kidney normally functions with a gradient of tissue oxygenation that decreases to overtly hypoxic ranges in deeper medullary areas. Reductions in renal blood flow are accompanied initially by decreased glomerular filtration and oxygen consumption, partly due to reduced metabolic demands of filtration and tubular solute reabsorption. As a result, moderately reduced renal blood flow can be sustained without measurable change in total kidney oxygen levels (as assessed by renal vein oxygen tension) or reduced medullary and cortical tissue oxygenation as measured in human subjects using blood oxygen level–dependent (BOLD) magnetic resonance ( Fig. 22.2 ). These observations argue against an overall lack of oxygen as an initial stimulus for either hypertension or renal tissue injury in moderate renal artery stenosis and underscore the tolerance of normal kidneys to tolerate moderately reduced perfusion pressures beyond the stenotic lesions. Nonetheless, increasingly severe vascular stenosis leading to diminished renal perfusion eventually does lead to renal hypoxia, tissue injury, and interstitial fibrosis (see Fig. 22.2 , left).
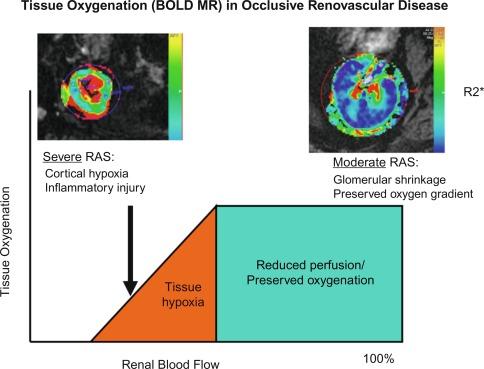
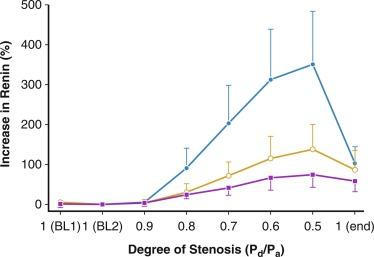
The majority of renal arterial lesions that compromise renal function are caused by gradually developing atherosclerosis of the renal vascular bed. As noted previously, some patients undergoing cardiac catheterization have “incidental” renal lesions producing more than 50% cross-sectional stenosis for whom the presence of renal artery stenosis is a strong independent predictor of mortality. Moreover, nonobstructive renal artery stenosis (20% to 50% decrease in renal arterial luminal diameter) can be found in almost one-third of patients undergoing cardiac catheterization and half of those undergoing aortography for peripheral vascular disease. Although lesions producing less than 50% arterial luminal diameter are not considered hemodynamically significant, the relationships between resting pressure gradients and angiographic degree of stenosis are curvilinear and only approximate at best. As a predictor of mortality, even low-grade atherosclerotic lesions denote a hazard nearly equal to more advanced disease. Estimating severity of vascular occlusion from angiographic images is notoriously unreliable. It should be emphasized that activation of pressor mechanisms depends upon the presence of a pressure gradient between the aorta and distal renal vasculature ( Fig. 22.3 ). Although there is a general relationship between estimated diameter stenosis and peak translesional pressure gradients, the relationship is not linear. In some instances, an abrupt decrease in poststenotic pressure develops beyond a subcritical range of stenosis. Moderate stenosis, especially when superimposed on intrarenal microvascular disease, may contribute to adverse renal outcomes. Even relatively minor stenosis in the renal artery might have long-term functional implications, including renal artery disease progression and atrophy (defined radiologically as a loss of kidney size), especially in the presence of additional risk factors or coexisting renal disease.
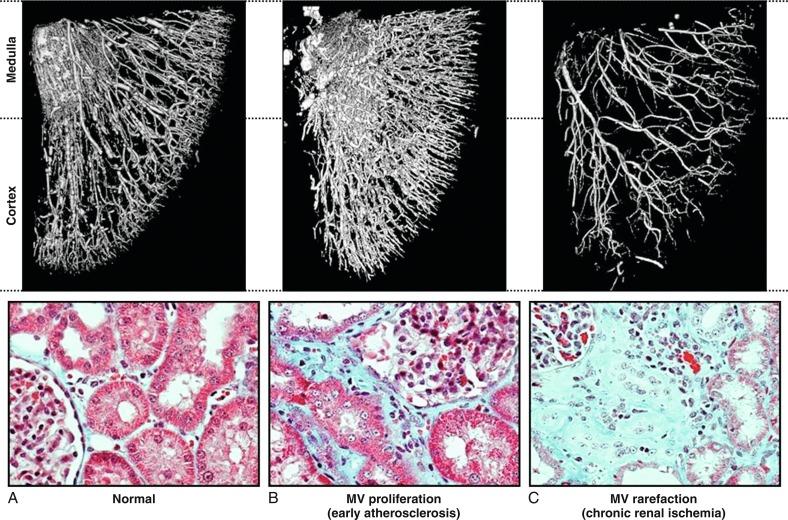
Lesions in the main renal artery may be superimposed upon or confused with other causes for ischemic renal injury. Intrarenal vascular lesions are commonly observed in the course of various nephropathies, many of which have an ischemic component. Risk factors including diabetes, hypertension, atherosclerosis, and aging elicit vasoconstriction and/or structural changes leading to intrarenal small vessel disease and ischemic injury similar to that observed in large vessel disease. The loss of microvessels and impaired capillary repair correlate with the development of glomerular and tubulointerstitial scarring and may lead to end-stage renal failure. Renal microvascular disease distal to a stenosis in the renal artery may perpetuate and exacerbate renal parenchymal injury and may blunt renal recovery. The presence of small microvessel injury is difficult to verify in human subjects but may account for changes in diastolic blood flow, such as that producing changes in renal resistance index. Elevations of renal resistance index tend to predict poor outcomes in many renal diseases, including renovascular disease.
High-grade vascular stenosis eventually leads to a decrease in renal perfusion pressure. “Critical” stenosis is identified when it produces a decrease in renal blood flow and glomerular filtration rate. During experimental renal artery occlusion, the kidney sustains autoregulation of blood flow through a range of perfusion pressures from 200 mm Hg to approximately 80 mm Hg. Mechanisms underlying autoregulation include myogenic responses to changes in wall tension, release of vasoactive substances, and the tubuloglomerular feedback. The latter responds to decreased renal perfusion pressure and salt delivery by decreasing vascular resistance distal to the obstruction. In addition, during a decrease in renal perfusion pressure, the kidney activates multiple pathways that elevate systemic blood pressure, an effect which tends to restore renal perfusion pressure and sustain renal blood flow at the expense of systemic arterial hypertension ( Fig. 22.4 ). Consequently, as long as systemic arterial pressure is allowed to increase, a decrease in renal blood flow does not occur until renal arterial diameter is reduced by 65% to 75%.
Noninvasive radiologic imaging commonly overstates the degree of stenosis. Measurement of physiologic stimuli, such as the release of renin, indicates that a translesion gradient of at least 10% to 20% reduction is necessary for biologic responses to occur in humans. To achieve such a gradient, luminal occlusion may need to exceed 80% stenosis. Under some conditions, gradients ≥ 20 mm Hg that develop during intrarenal hyperemic challenge with dopamine may disclose hemodynamic significance of lesions under 60% in severity.
When the renal perfusion pressure decreases gradually, additional mechanisms are recruited that protect the kidney from the functional and morphologic consequences observed after acute ischemic injury. These include development of collateral vessels and redistribution of intrarenal blood flow from the cortex to the medulla. Renal cortical blood flow autoregulates more efficiently than the outer medulla, which is continuously at the verge of hypoxia. During chronic reduction of renal blood flow, medullary perfusion and oxygenation are relatively maintained by adaptive mechanisms at the expense of cortical blood flow. When poststenotic renal artery pressures eventually decrease further, either due to progressive vascular occlusion or to reduction of systemic blood pressures by drug therapy, renal volume decreases.
In clinical terms, renal “atrophy” can be defined as a loss of renal length by at least 1 cm, and a difference in size between the two kidneys is suggestive of unilateral renal artery stenosis (or a higher grade of stenosis in one of the kidneys). A decrease in renal volume results from a decrease in filling pressure, filtrate, and blood content of the kidney, as well as structural atrophy of the renal tubules due to apoptosis and necrosis. Apoptosis is an active, preprogrammed form of cell death, which is intricately regulated and distinct from cellular necrosis and likely serves as a protective mechanism to allow renal “hibernation.” These changes may be reversible, because tubular cells show vigorous potential for regeneration. Loss of intrarenal microvessels that accompanies the ongoing scarring process may also contribute to renal shrinkage ( Fig. 22.5 ) but might be partly reversible upon enhancement of angiogenic signaling. However, if a blood flow deficit persists, permanent damage to the kidney may occur. As mentioned previously, decreased renal blood flow is often accompanied by a decline in GFR and inhibition of tubular epithelial transport that limit renal oxygen consumption and maintain oxygen saturation. Hence the kidney does not actually develop tissue hypoxia until an extreme decrease in renal blood flow develops.
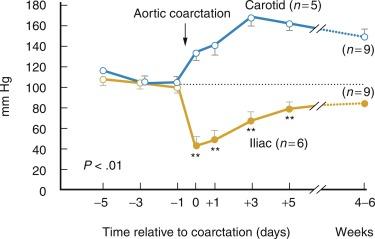
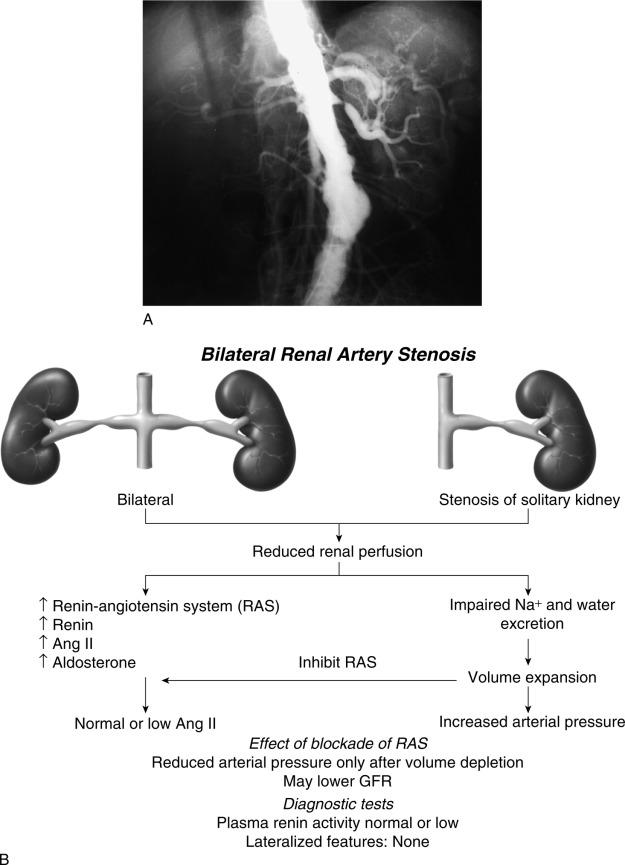
Goldblatt et al. and Loesch showed in the 1930s that obstruction of the renal artery is followed by an increase in systemic blood pressure. The characteristics of renovascular hypertension depend to a large extent on the status of the kidneys. Unilateral renal artery stenosis may be present with an intact contralateral renal artery (the experimental form is termed two-kidney, one-clip [2K1C]). This model is characterized by counteregulatory processes in the contralateral kidney leading to sodium excretion in response to elevated arterial pressure (“pressure natriuresis”) ( Fig. 22.6 ). Alternatively, renal artery stenosis may affect a solitary kidney (one-kidney, one-clip [1K1C]) ( Fig. 22.7 ). Bilateral renal artery stenosis and 1K1C lead to more severe renovascular hypertension, although bilateral renal artery stenosis may behave similar to 2K1C if one kidney is significantly less ischemic than the other. Patients with this constellation of findings have higher mortality, are more prone to circulatory congestion, and are more likely to experience deterioration of kidney function during administration of antihypertensive agents, including angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs).
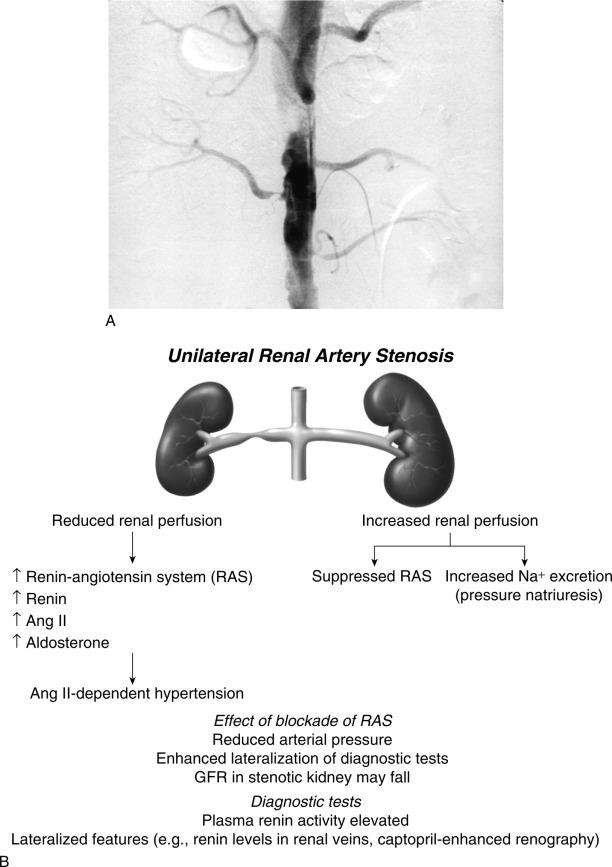
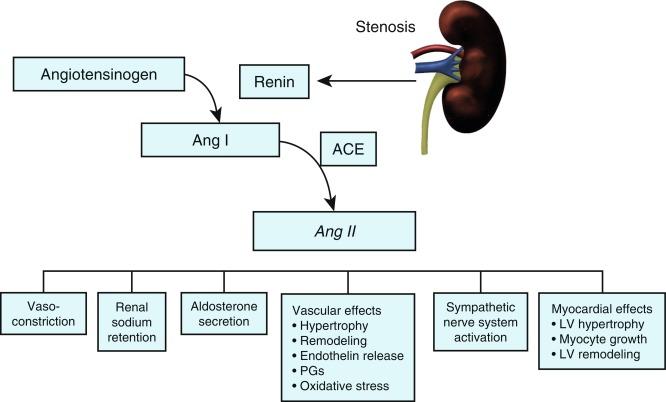
The exact mechanisms responsible for renovascular hypertension have long been debated. The immediate increase in blood pressure in renal artery stenosis results from release of renin from the stenotic kidney. This leads to increased formation of angiotensin II, which increases peripheral vascular resistance, plasma aldosterone, sodium retention, extracellular volume, and cardiac output ( Fig. 22.8 ). Blockade of angiotensin action in experimental models prevents the initial series of events and delays the development of renovascular hypertension indefinitely. Activation of the sympathetic nervous system also plays an important role in the pathogenesis of renovascular hypertension primarily via the renal afferent nerves. Both the peripheral and central aspects of the autonomic system are also under the influence of angiotensin II. If the increase in pressure restores renal perfusion pressure distal to the stenosis, most of these alterations return to baseline levels, with the exception of peripheral vascular resistance.
After the initial increase in activity from the renin-angiotensin system, maintenance of renovascular hypertension in 1K1C models depends mainly on volume expansion. In 2K1C models the interplay between plasma renin activity and extracellular volume is more complex. The contralateral kidney responds to the elevated systemic pressure by increasing sodium excretion (pressure natriuresis), an effect that tends to drive the blood pressure down and decrease perfusion pressure of the stenotic kidney. This effect again leads to an increase in renin release, which in turn elevates systemic blood pressure, and so forth. In high-grade renal artery stenosis, this cycle of events may induce extracellular volume depletion and renal failure. Although these features are consistently demonstrated in experimental models, human renovascular hypertension frequently has elements of both 1K and 2K pathophysiology, particularly when the function of the contralateral kidney is compromised.
It is important to recognize that activation of the systemic renin-angiotensin system is transient in renovascular hypertension. Circulating levels of plasma renin activity and angiotensin decrease after some time, despite sustained elevation of peripheral vascular resistance. This may be the result of both a “slow-response” to angiotensin II through which low levels of angiotensin have pressor actions and recruitment of additional mechanisms of vasoconstriction. The latter includes activation of vasoconstrictor lipoxygenase products, oxidative stress, and endothelin. Further increments in pressure result from an imbalance between vasoconstrictors and vasodilators, including decreased bioavailability of nitric oxide (NO). An important role is ascribed to dissociation between systemic blood pressure, extracellular volume, and inappropriate levels of angiotensin II. The complexity of these relationships partly explains the failure of measuring any single clinical pathway to predict blood pressure responses to renal revascularization.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here