Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Persistent pulmonary hypertension of the newborn (PPHN) is a syndrome of failed circulatory adaptation at birth, which is seen in about 2 in 1000 live-born infants; the incidence has not changed significantly over the last 20 years. , The syndrome is characterized by a sustained elevation of pulmonary vascular resistance (PVR) and is often associated with normal or low systemic vascular resistance (SVR). Consequently these infants exhibit extrapulmonary right-to-left shunting across persistent fetal channels (patent ductus arteriosus and patent foramen ovale), leading to labile hypoxemia. PPHN was previously referred to as persistent fetal circulation. Although it is seen mostly in term and late-preterm infants, it is recognized in up to 2% of premature infants with respiratory distress syndrome (RDS). , Most commonly, PPHN is secondary to delayed or impaired relaxation of the pulmonary vasculature and associated with a diverse group of pulmonary pathologies (e.g., meconium aspiration syndrome [MAS], congenital diaphragmatic hernia [CDH], pneumonia, and RDS). This chapter reviews the applied pathophysiology of fetal pulmonary circulation, pulmonary vascular transition at birth, hemodynamic and biochemical changes associated with PPHN, and the physiologic basis of various therapeutic interventions.
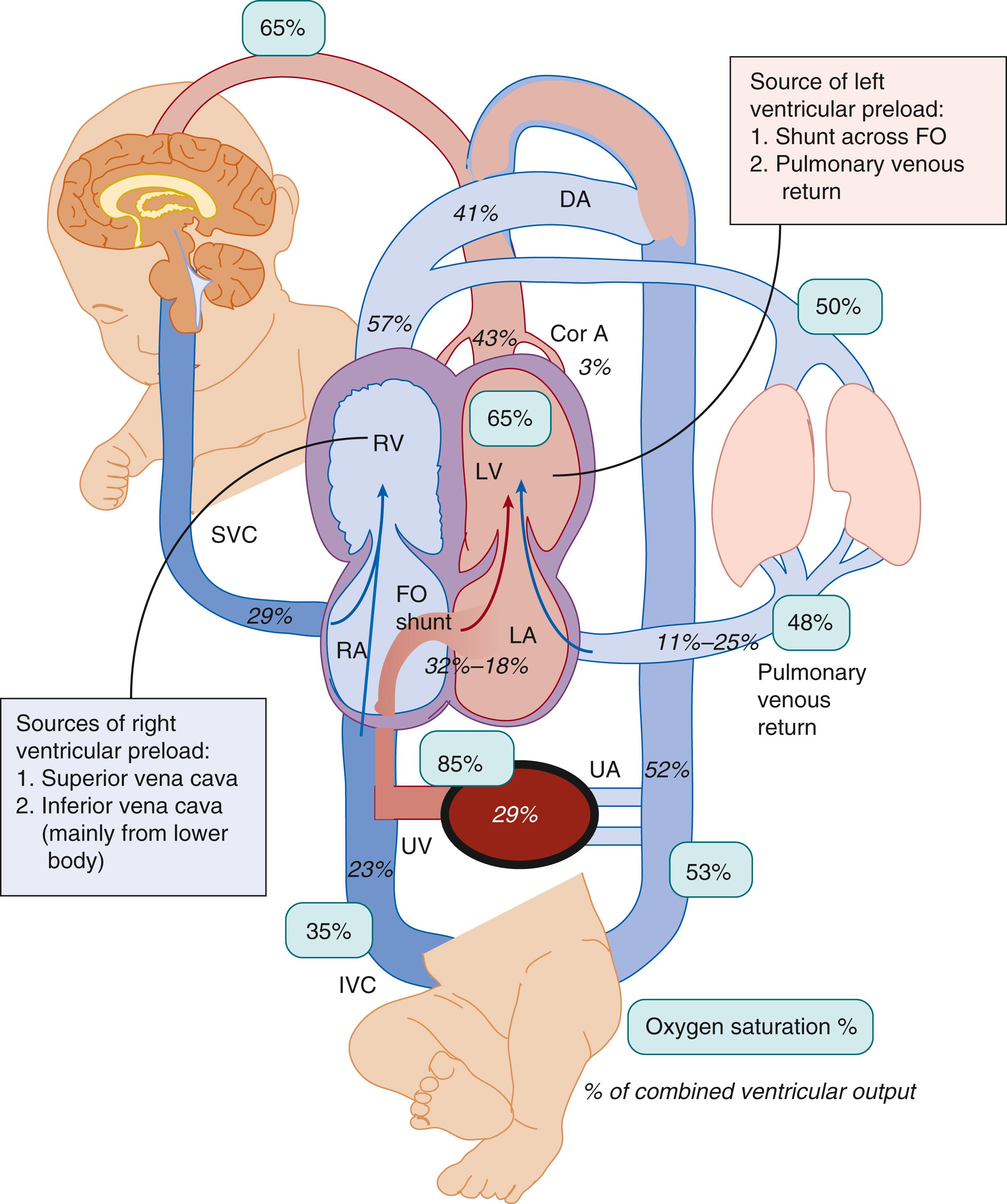
The fetus is in a state of physiologic pulmonary hypertension. In the fetal lamb, the pulmonary arterial blood has a partial pressure of oxygen (P o 2 ) of approximately 18 mm Hg and oxygen saturation (S o 2 ) of 50%. The PVR is high partly secondary to hypoxic pulmonary vasoconstriction. The pattern of blood flow in a normal fetus is shown in Fig. 154.1 . Our understanding of human fetal hemodynamics has improved secondary to cine cardiovascular magnetic resonance imaging (MRI) techniques. The opposing magnetic properties of oxy- and deoxyhemoglobin can quantify blood oxygenation. Human fetal studies demonstrate higher cerebral and pulmonary blood flow (PBF) and lower umbilical flow compared with sheep studies. The blood is oxygenated in the placenta and returns to the body through the umbilical vein (S o 2 ∼ 82% to 88% and P o 2 32 to 35 mm Hg in lambs). The umbilical venous return is preferentially directed through the ductus venosus and left lobe of the liver to form a high-velocity stream (with a velocity three to four times higher velocity than that of the flow in the inferior vena cava [IVC]; 59 to 71 cm/s vs. 16 cm/s) in the leftward posterior aspect of the IVC, which is directed toward the foramen ovale and the left ventricle (see Fig. 154.1 ). , This mechanism maintains a higher oxygen content of the blood in the left heart (∼65% with higher glucose content—which supplies cerebral and coronary circulation, and approximately 15% higher saturation than pulmonary arterial and umbilical arterial saturation ∼50% to 52%). The same streaming mechanism results in deoxygenated blood shunted to the placenta via the ductus arteriosus. This umbilical venous oxygenated blood shunting through the foramen ovale is an important component of left ventricular preload in the fetus. Because of high PVR, only about 16% of the combined ventricular output (11% to 25% in various studies) is directed to the lungs; the remainder passes through the ductus arteriosus to the descending aorta. The difference in S o 2 (a measure of oxygen uptake from the organ of gas exchange) between the umbilical vein (85%) and umbilical artery (52%) during fetal life (see Fig. 154.1 ) is similar to the difference between the pulmonary vein/aorta (95% to 100%) and pulmonary artery (60% to 70%) in an adult. The fetus achieves normal oxygen delivery at low P o 2 levels (<35 mm Hg) compared with the postnatal period, thus limiting the risk of oxygen toxicity.
Rasanen and colleagues used direct measurements of PBF in the right and left pulmonary arteries and found that it is approximately 21% of combined ventricular output by 38 weeks in human fetuses. Phase-contrast cine-MRI in late-gestation human fetuses demonstrated that PBF is 74 ± 43 mL/kg/min or approximately 16% ± 9% of combined ventricular output. There was a 10-fold difference in PBF between subjects (2% to 30% of combined ventricular output). It is possible that PBF is dynamic and varies between different fetal subjects, at different times within the same subject, and with advancing gestation.
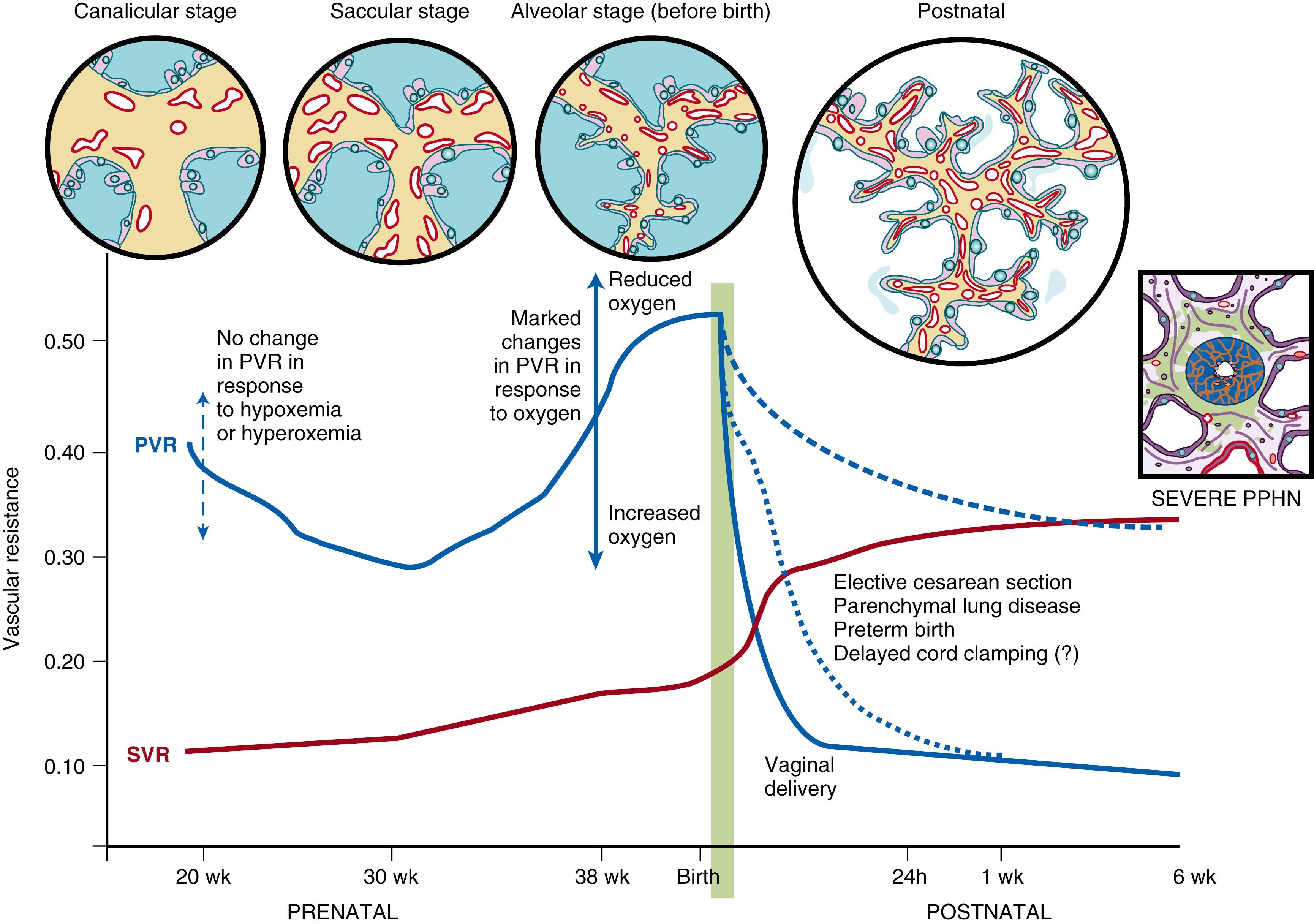
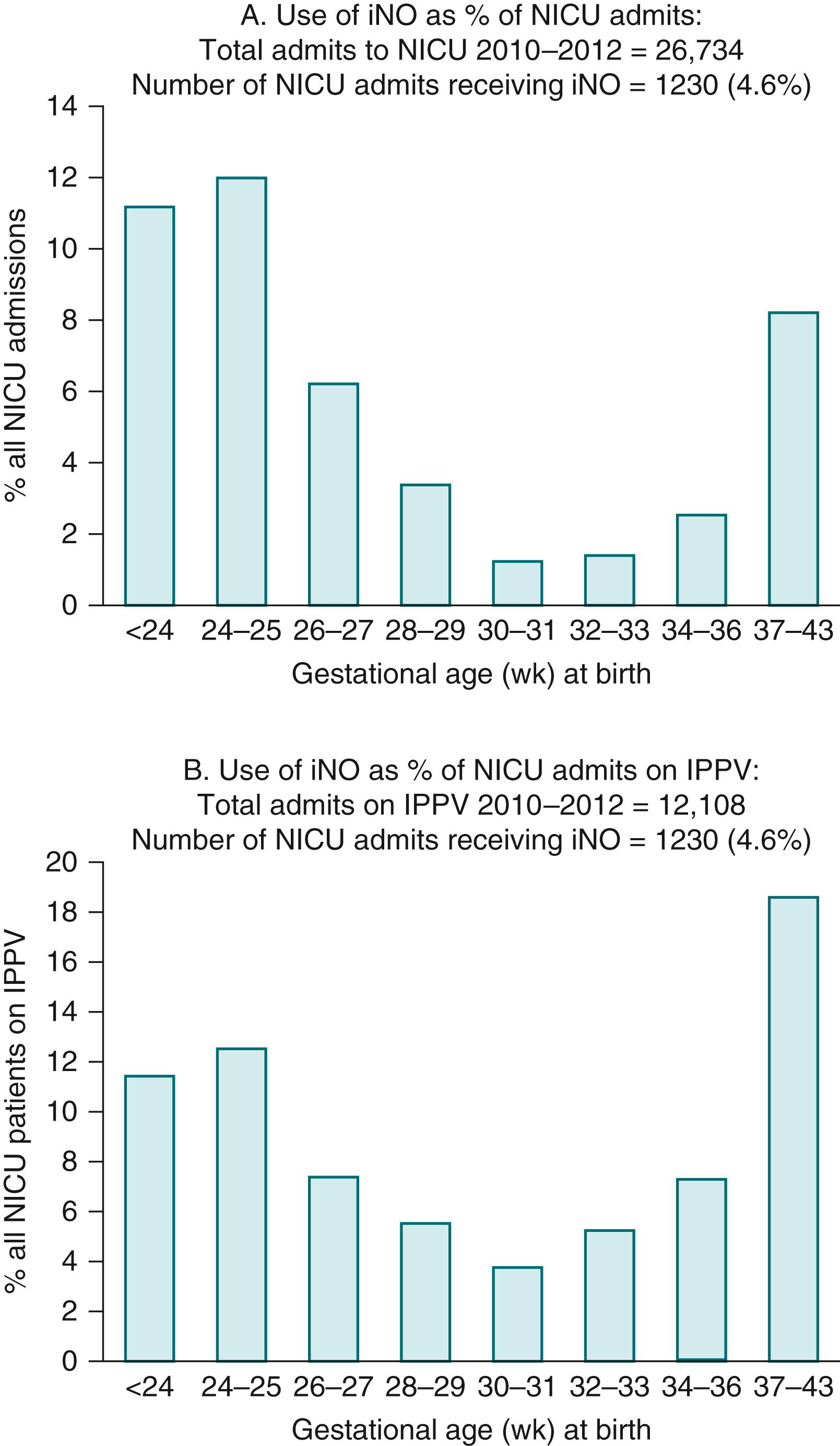
Doppler flow studies in human fetuses have demonstrated that flow into the left and right pulmonary arteries is 13% of combined ventricular output at 20 weeks of gestation (canalicular stage); it increases to 25% at 30 weeks (saccular stage) and decreases to 21% at 38 weeks (alveolar stage) ( Fig. 154.2 ). The fetal PVR is high during the canalicular stage secondary to the paucity of a pulmonary vascular network, leading to a reduced cross-sectional area of the immature pulmonary vascular bed. Experiments in fetal lambs have shown that the PBF does not increase in response to hyperoxia at 94 to 101 days’ gestation (term ∼147 days). At this gestation, fetal hypoxia results in very minimal change in PVR. Similarly, Rasanen and colleagues demonstrated that, between 20 and 26 weeks of gestation, maternal hyperoxygenation using 60% oxygen by face mask does not result in pulmonary vasodilation in the human fetus. These findings suggest a lack of sensitivity to oxygen and high PVR in early gestation. Interestingly, birth at this gestational age (23 to 26 weeks in humans) is associated with a much higher risk (2%) of pulmonary hypertension compared with term gestation (0.2%) , and high rates of clinical use of inhaled nitric oxide (iNO). In an Australia–New Zealand database of neonatal intensive care unit admissions requiring invasive ventilation, the U-shaped curve of PVR during fetal life (see Fig. 154.2 ) resembled the U-shaped curve of iNO ( Fig. 154.3 ) use: 15.4% at 24 to 25 weeks, 3.8% at 30 to 31 weeks, and 19% at 37 to 44 weeks of gestational age at birth. , Similar trends in iNO use were observed among preterm infants in the Ohio Perinatal Network (17.5% at 22 to 24 weeks and 3.7% at 31 to 33 weeks’ gestational age at birth) and California Perinatal Quality Care (4.9% at 22 to 24 weeks, 1.1% at 31 to 33 weeks, and 6.2% at 38 to 43 weeks). During the early saccular stage, the rapid proliferation of pulmonary vessels decreases fetal PVR; during late gestation, there is a marked increase in cross-sectional area of the pulmonary vascular bed. However, pulmonary vessels become more sensitive to vasoconstrictive mediators such as endothelin and hypoxia during this period, resulting in active pulmonary vasoconstriction (see Fig. 154.2 ) , and dilation in response to increased oxygen. , In late-gestation lambs (136 to 146 days of gestation), maternal hyperbaric hyperoxia increased pulmonary arterial P o 2 from 19 ± 1.5 to 48 ± 9 mm Hg, leading to an increase in PBF from 34 ± 3.3 to 298 ± 35mL/kg per minute. Similarly, maternal hyperoxygenation increased PBF in late-gestation (31 to 36 weeks) human fetuses. The maternal hyperoxygenation test (administered with 60% oxygen by face mask) has been proposed to measure the ability of fetal pulmonary arteries to vasodilate in response to oxygen in late gestation and predict pulmonary vascular reactivity and survival in cases of CDH.
In fetal lambs, pulmonary vasodilation in response to endothelium-independent mediators such as NO precedes response to endothelium-dependent mediators such as acetylcholine and oxygen. Response to NO is dependent on activity of its target enzyme, soluble guanylate cyclase (sGC), in the smooth muscle cell ( Fig. 154.4 ). In the ovine fetus, sGC mRNA levels are low during early preterm (126 days) gestation and increase markedly during late preterm and early term gestation (137 days). Low levels of pulmonary arterial sGC activity during the late canalicular and early saccular stages of lung development may partly explain the poor response to iNO observed in some extremely preterm infants.
![Fig. 154.4, Endothelium-derived vasodilators. Prostacyclin (PGI 2 ), nitric oxide (NO), and vasoconstrictor (endothelin [ET-1] ) pathways. Therapeutic interventions are shown in black boxes. AC, Adenylate cyclase; ANP, atrial natriuretic peptide; BNP, brain type natriuretic peptide; CNP, C-type natriuretic peptide; COX, cyclooxygenase; eNOS, endothelial nitric oxide synthase; PDE, phosphodiesterase; pGC, particulate guanylate cyclase; PGIS, prostacyclin synthase; sGC, soluble guanylate cyclase. 119 , 166 , 167 Fig. 154.4, Endothelium-derived vasodilators. Prostacyclin (PGI 2 ), nitric oxide (NO), and vasoconstrictor (endothelin [ET-1] ) pathways. Therapeutic interventions are shown in black boxes. AC, Adenylate cyclase; ANP, atrial natriuretic peptide; BNP, brain type natriuretic peptide; CNP, C-type natriuretic peptide; COX, cyclooxygenase; eNOS, endothelial nitric oxide synthase; PDE, phosphodiesterase; pGC, particulate guanylate cyclase; PGIS, prostacyclin synthase; sGC, soluble guanylate cyclase. 119 , 166 , 167](https://storage.googleapis.com/dl.dentistrykey.com/clinical/PathophysiologyofPersistentPulmonaryHypertensionoftheNewborn/3_3s20B9780323712842001543.jpg)
Fetal growth restriction is associated with chronic hypoxia and reductions in umbilical venous S o 2 and flow. Lower S o 2 in the ascending aorta is associated with higher superior vena caval flow (“brain-sparing physiology”). In contrast, lower S o 2 in pulmonary arteries is associated with decreased PBF. Pulmonary hypertension with bronchopulmonary dysplasia (BPD) is associated with fetal growth restriction in preterm infants and with a trend toward higher morbidity and mortality during longitudinal follow-up. In the SUPPORT trial, randomization to a lower S o 2 target was associated with increased mortality in growth-restricted infants with RDS and BPD as leading causes. However, individual patient data meta-analysis of all the oxygen saturation trials in preterm infants did not show a similar association. Other mechanisms in addition to hypoxemia may also contribute to a higher incidence of PPHN in growth-restricted infants.
The shunt across the foramen ovale and PBF are the two sources of left ventricular filling during fetal life (see Fig. 154.1 ). Left ventricular preload is an important determinant of blood flow to the heart and brain and of left ventricular development. Prsa et al. found a strong inverse correlation between foramen ovale shunt and PBF in human fetuses. , The physiologic mechanism behind this finding is not clear. One potential explanation is that fetal PVR is very sensitive to small changes in P o 2 . Arraut et al., working with nonhuman primate fetuses, evaluated the effect of maternal hypoxemia (by the administration of 12% oxygen) and hyperoxemia (by the administration of 100% oxygen) on the fetal right pulmonary arterial pulsatility index (PI) (PI = [peak systolic velocity − end-diastolic velocity] ÷ time-averaged maximum velocity over the cardiac cycle). An increase in right pulmonary arterial PI suggests an increase in peripheral pulmonary vasoconstriction with decreased flow. Maternal hypoxemia increased the fetal right pulmonary arterial PI fivefold, suggesting fetal pulmonary vasoconstriction. Maternal hyperoxemia decreased the right pulmonary arterial PI fourfold and also decreased blood flow through the ductus arteriosus. Maternal oxygenation status did not affect the umbilical arterial or ductus venosus PI, suggesting that umbilical flow is neither influenced by nor regulates fetal oxygenation. Similarly, Konduri et al. showed that an increase in pulmonary arterial P o 2 of 7 mm Hg resulted in a threefold increase in PBF in fetal lambs. However, the variability of PI changes in response to maternal hyperoxygenation have limited the utility of PI as a diagnostic tool.
These studies indicate that fetal PVR is dynamic and an important regulator of the distribution of cardiac output and the relative contributions of the pulmonary circulation and foramen ovale shunt to left ventricular filling. The S o 2 of blood in the left atrium (and left ventricle) is dependent on the relative contributions of oxygenated blood from the foramen ovale and deoxygenated blood from the pulmonary veins. The mean value is usually around 65%. During periods of hypoxemia, fetal PVR increases markedly , and the left ventricle is filled with better oxygenated umbilical venous blood shunted across the foramen ovale (see Fig. 154.1 ). Conversely, during maternal hyperoxemia, PBF increases and flow through the ductus arteriosus and foramen ovale decreases. , These changes in PVR will determine the distribution of fetal cardiac output and oxygen delivery to the brain and heart. Furthermore, reduced pulmonary venous return and left ventricular filling in fetuses with CDH may contribute to left ventricular hypoplasia, emphasizing the role of PBF during fetal life. ,
Numerous factors—such as mechanical factors (compression of the small pulmonary arterioles by the fluid-filled alveoli and a lack of rhythmic distension) and a relative lack of vasodilators— contribute to the high PVR in utero. Low oxygen tension and elevated levels of vasoconstictor mediators, such as endothelin-1 (ET-1) and thromboxane, play a crucial role in maintaining the elevated fetal PVR.
Conditions such as CDH, antenatal closure of the ductus arteriosus, and idiopathic PPHN are often associated with vascular remodeling and elevated PVR during fetal life. Studies in animal models and human populations have suggested that maternal therapy can alter fetal PVR. Two classes of medications, selective serotonin receptor inhibitors (SSRIs) and nonsteroidal antiinflammatory agents (NSAIDs), have been relatively well studied.
Serotonin infusions increase PVR in fetal lambs, , and exposure of pregnant rats to the SSRI fluoxetine produced pulmonary hypertension, hypoxia, and increased mortality in rat pups. , The use of SSRIs during the last half of pregnancy has been associated with an increased incidence of PPHN in at least three human studies. Although the mechanism by which SSRIs induce pulmonary hypertension in the newborn is not known, it is speculated that drug-induced elevation in serotonin levels results in pulmonary vasoconstriction. Some studies have questioned the association between maternal SSRI intake and PPHN. , Furthermore, the severity of PPHN has not been well described; a report observed no differences in right pulmonary artery Doppler PI in fetuses of mothers exposed to SSRI antidepressants. In addition, the risk appears to be more with exposure beyond 20 weeks of gestation. Alternate antidepressants, such as selective serotonin norepinephrine reuptake inhibitors (SNRIs), are not well studied, and their association with PPHN in offspring is not known at this time. At present, maternal physical and psychological well-being should be the primary factor guiding antidepressant therapy during pregnancy and the postpartum period.
Ingestion of NSAIDs such as aspirin during late gestation may be associated with closure of the fetal ductus arteriosus. Experimental ligation or constriction of the ductus arteriosus in lambs during fetal life is associated with the rapid development of pulmonary vascular remodeling and PPHN. Prostaglandins maintain ductal patency in utero and are important mediators of pulmonary vasodilation in response to ventilation at birth. Pharmacologic blockade of prostaglandin production by NSAIDs can result in PPHN. Analysis of meconium from newborn infants with PPHN revealed the presence of a NSAID in approximately half of the samples, linking antenatal NSAID exposure to PPHN. This association has been questioned, although aspirin use during late pregnancy remains a risk factor for PPHN.
Maternal medications also have the potential to correct pathologic fetal vascular development. Maternal betamethasone reduced oxidative stress and improved the relaxation response to adenosine triphosphate and NO donors in fetal lambs with PPHN induced by ductal ligation. In fetal rats with nitrofen-induced CDH, antenatal administration of sildenafil to the dam improved lung structure (decreased mean linear intercept) and reduced pulmonary hypertension (decreased right ventricle/left ventricle + septum ratio). However, the use of sildenafil during pregnancy may be associated with adverse effects on the fetus. The Sildenafil TheRapy in dismal prognosis early onset fetal growth restriction (STRIDER), an international consortium of randomized placebo-controlled trials, explored the effects of sildenafil for treatment of fetal growth restriction in four trials. The Dutch STRIDER trial was suspended evoking significant global interest in media reports due to a signal of potential harm relating to increased PPHN and a nonsignificant trend towards an increase in neonatal death. In contrast, the UK and New Zealand/Australia STRIDER trials found no evidence of PPHN or neonatal death but did not find any beneficial effect of sildenafil therapy on fetal growth restriction. This consortium strongly recommends not to prescribe sildenafil for fetal growth restriction outside clinical trials until more data are available.
After birth and following initiation of air breathing, PBF markedly increases, , resolving fetal physiologic pulmonary hypertension. In some infants with adverse in utero events or with abnormalities of pulmonary transition at birth, pulmonary hypertension persists into the newborn period, resulting in PPHN and hypoxemic respiratory failure (HRF). ,
A series of circulatory events take place at birth to ensure a smooth transition from fetal to extrauterine life. Clamping of the umbilical cord removes the low resistance placental circulation, increasing SVR (see Fig. 154.2 ). Simultaneously, various mechanisms operate to rapidly reduce pulmonary arterial pressure and increase PBF. Of these, the most important stimuli appear to be ventilation of the lungs and an increase in oxygen tension. The vascular endothelium releases several vasoactive products that play a critical role in achieving rapid pulmonary vasodilation. Pulmonary endothelial NO production increases markedly at the time of birth. Oxygen is believed to be an important catalyst for this increased NO production, although the precise mechanism is not clear. NO exerts its action through sGC and cyclic guanosine monophosphate (cGMP) (see Fig. 154.4 ). Bloch et al. reported that expression of sGC peaks in late gestation in rats, which may explain the better response to NO at birth in neonates than in any other reported age group. Phosphodiesterase 5 (PDE5) catalyzes the breakdown of cGMP (see Fig. 154.4 ). Similar to sGC, expression of PDE5 in the lungs peaks in the immediate newborn period in sheep and rats. , The recognition of the role of NO in mediating pulmonary vascular transition at birth has led to the development of iNO as a therapeutic strategy in PPHN.
The arachidonic acid–prostacyclin pathway also plays an important role in the transition at birth. The cyclooxygenase enzyme acts on arachidonic acid to produce prostaglandin endoperoxides. Prostaglandins activate adenylate cyclase to increase cAMP concentrations in vascular smooth muscle cells. Phosphodiesterase 3A (PDE3A) catalyzes the breakdown of cAMP (see Fig. 154.4 ).
Vaginal delivery is associated with a reduction in fetal PVR at birth. In comparison, delivery by elective cesarean section delays the decrease in pulmonary arterial pressure (see Fig. 154.2 ), as evidenced by prolonged right-sided systolic time intervals and increases the risk of PPHN. Compared with matched control subjects, infants with PPHN are more likely to have been delivered by cesarean section. ,
Early clamping of the umbilical cord before the first breath results in a decrease in heart size during the first 3 or 4 cardiac cycles, presumably due to decreased left ventricular filling. In preterm lambs, delaying cord clamping for 3 to 4 minutes until after ventilation is established improves cardiovascular function by increasing PVR and left ventricular filling before the cessation of umbilical venous return. The precise effect of delayed cord clamping on pulmonary vascular transition is not clear. Interestingly, Arcilla et al. demonstrated increased pulmonary arterial pressure following late cord clamping in newborn infants nearly 50 years ago by catheterizing the pulmonary artery. There are no reports of increased incidence of PPHN associated with delayed cord clamping. In lambs with CDH, a condition commonly associated with PPHN, delayed cord clamping after onset of effective ventilation results in a threefold decrease in PVR. Two delivery room trials have demonstrated the feasibility of this technique without any negative consequences. , Larger trials are needed to confirm the benefits of delayed cord clamping in CDH. Similarly, it is possible that a slower decrease in PVR may offer circulatory stability in extremely preterm infants.
The decrease in pulmonary arterial pressure after birth is significantly slower in preterm infants (especially if associated with RDS) than in term infants. The pulmonary arterial pressure is increased relative to the systemic arterial pressure, and such elevation may persist for a few days in extremely preterm infants with respiratory disease. , Skimming et al. have questioned whether the naturally increased PVR affords benefit to the preterm infant by reducing the ductal steal and stabilizing systemic circulation. Such delay in decrease in PVR may contribute to the high incidence of PPHN in extremely preterm infants.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here