Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors’ research on neurulation and NTDs is supported by grants from the Medical Research Council, Wellcome, NC3Rs, Sparks, Newlife, Action Medical Research, the Bo Hjelt Spina Bifida Foundation, Great Ormond Street Hospital Children’s Charity, and the National Institute for Health Research, Biomedical Research Centre, at Great Ormond Street Hospital for Children, NHS Foundation Trust, and University College London.
Neural tube defects (NTDs) are among the commonest and most severe birth anomalies, second only in frequency to congenital heart defects. Worldwide, around 300,000 to 500,000 new cases of NTD are thought to arise each year, representing not only a significant challenge for the affected individuals and their families, but also a major financial burden for health services. For example, 166,000 people are estimated to live with spina bifida in the United States alone, with average lifetime medical and nonmedical costs for each affected person of around $560,000, not counting their “lost” earnings.
NTDs affect the central nervous system and axial skeleton and can vary from fatal to asymptomatic. The severe end of the spectrum is represented by defects of the cranial region: anencephaly and craniorachischisis (CRN), which are both incompatible with survival beyond birth, and encephalocele, which can be lethal depending on the extent of brain damage. Somewhat less severe NTDs affect the spine, of which open spina bifida (often called myelomeningocele) is usually not lethal but can be a cause of multiple disabilities throughout life. At the mild end of the NTD spectrum is closed spinal dysraphism (or lipomyelomeningocele), which can cause lower limb weakness and urologic disorders but is often clinically undetectable.
NTDs exhibit an average worldwide prevalence of 0.5 to 2 per 1000 births, but this varies markedly with geographic location and ethnic origin. For example, more abundant NTDs were observed in previous decades in Ireland, South Wales, and Northern China, and an especially high prevalence (6 to 13 per 1000 births) was reported recently in Ethiopia. , Differences in NTD prevalence between different ethnic groupings have been described: for example, people of Hispanic origin in the United States exhibit higher rates than those of white ancestry, with African Americans showing the lowest frequencies. Rates of NTDs are higher among miscarried fetuses, suggesting that the true incidence may be greater than is typically recorded from studies of late-stage pregnancies, planned abortions, and still/live births. Confirmed epidemiologic conditions associated with NTDs include low socioeconomic status, maternal obesity and/or diabetes, suboptimal folate status, and anticonvulsant medication usage. Female fetal sex is strongly associated with anencephaly.
Neurulation is the process by which the neural tube, the precursor of the brain and spinal cord, is formed during embryogenesis. Key events in this process include induction of neural identity in the embryonic epiblast, convergent extension (CE) cell rearrangements that produce an elongated, mediolaterally narrowed neural primordium, and conversion of this primordium into a neural tube. Two successive phases of neural tube formation occur, involving neural plate bending and fusion in the dorsal midline (primary neurulation) followed by canalization of a neural primordium in the caudal region (secondary neurulation).
Primary neurulation begins at several discrete points along the rostro-caudal axis, in a pattern that varies somewhat between mammals. In rodents ( Fig. 169.1A ), where neurulation has been most intensively studied, closure begins at the boundary between the future hindbrain and cervical spine (termed Closure 1). Twelve hours later, closure initiates independently at the forebrain/midbrain boundary (Closure 2) and, soon after, at a third initiation site at the rostral extremity of the forebrain (Closure 3). Recently, a further caudal initiation site (Closure 5) was described during the final stages of spinal closure. In human embryos (see Fig. 169.1B ), only two closure initiation sites have been described, termed α and β, which are equivalent to Closures 1 and 3 respectively in rodents. Closure 2 does not appear to occur in humans, and it is not yet clear whether an equivalent of Closure 5 may exist.
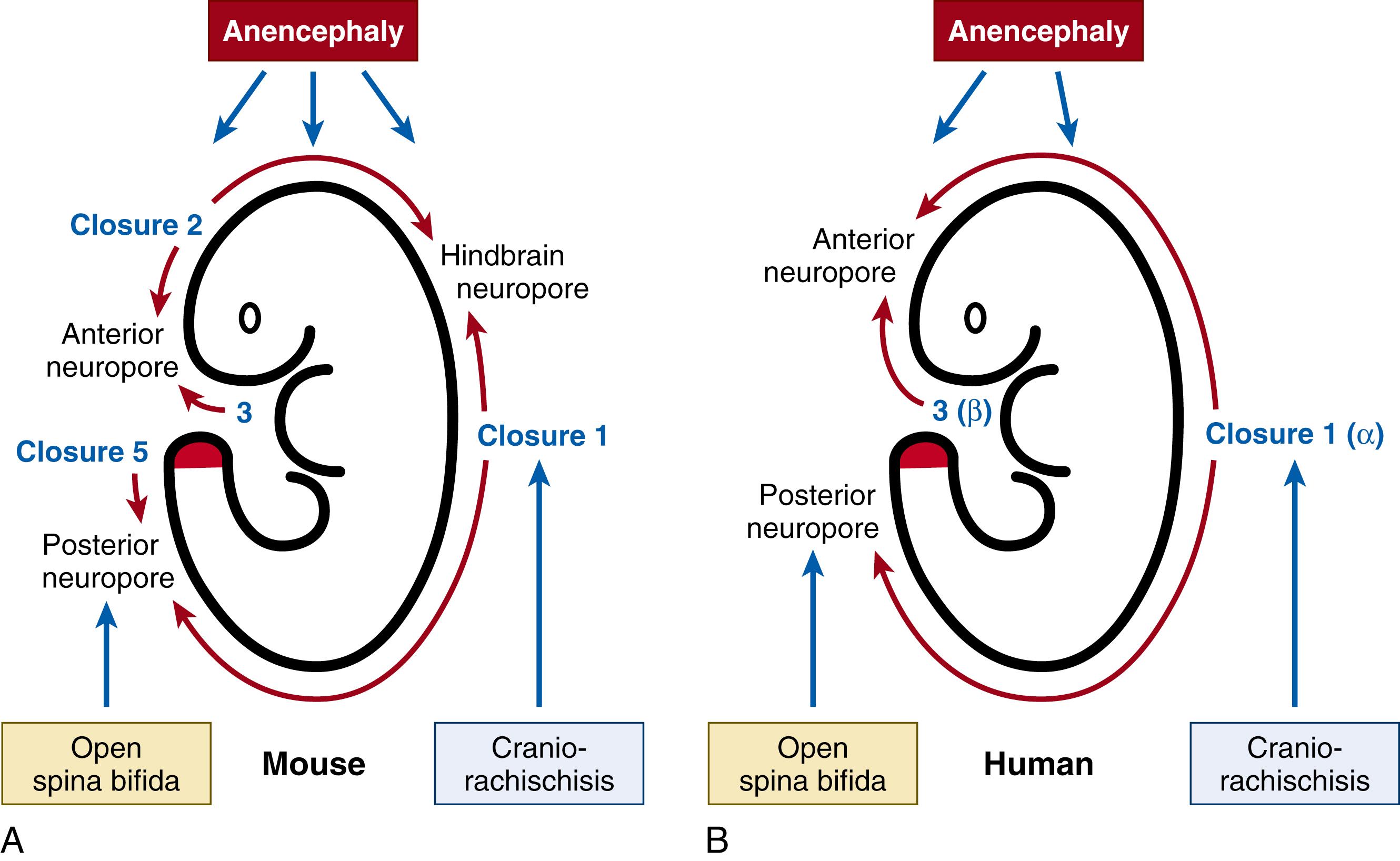
The open regions of neural folds between the different closure initiation sites are termed neuropores; they close progressively by “zippering” along the body axis (see Fig. 169.1 ). Mouse embryos have three neuropores: the anterior and hindbrain neuropores complete closure within a few hours of Closures 2 and 3, whereas spinal neurulation continues zippering over a much longer period, along the growing spinal region, until the posterior or caudal neuropore (PNP) completes its closure in the upper sacral region. This marks the end of mouse primary neurulation, a process that takes ∼40 hours, between embryonic days (E) 8.5 and 10. Because human embryos lack Closure 2, they exhibit only single anterior (rostral) and posterior (caudal) neuropores. Human neural tube closure begins around 22 days post-fertilization and is completed by 26 days (i.e., between Carnegie Stages 10 and 12).
This is the process of neural tube formation in the lower sacral and coccygeal regions, and is part of “secondary body” development that also forms caudal notochord, somites and postcloacal gut. It begins at Carnegie stage 12 and follows on seamlessly from primary neurulation, so that ultimately the primary and secondary neural tubes have no obvious boundary between them. The caudal end of the embryo comprises the tail bud (also called the caudal eminence), which contains self-renewing, multipotent progenitor cells whose derivatives condense into longitudinal cell masses. The dorsal mass then undergoes canalization to form the hollow secondary neural tube, while the ventral mass canalizes to form the tail gut. The longitudinal structure lying between neural tube and gut forms the solid notochord. A multipotent stem cell population, the “neuro-mesodermal progenitors” (NMPs), is present in the tail bud at this stage, and NMPs give rise to all nonepidermal tissues of the lower body, including neural tube and vertebrae. Possibly for this reason, malformations and tumors (e.g., sacrococcygeal teratoma) of the lower body often embrace several tissue types.
In the mouse and rat, the entire tail region (∼50% of the total body axis) is originally occupied by secondary neural tube. Following completion of tail formation at E13.5 (i.e., three days after the end of primary neurulation), the neural tube and tail gut regress with cells dying by apoptosis. In contrast, the notochord (around which the vertebral centra develop) and somitic derivatives (the future vertebrae and tendons) persist in the tail. Human secondary body formation ceases much earlier than in embryos of long-tailed rodents; the tail structure subsequently regresses completely, so that all tissues, not just the neural tube and tail gut, are “reabsorbed” into the upper body. The human secondary neural tube contributes to the lowest part of the spinal cord: the conus medullaris, cauda equina, and filum terminale.
While the neural tube and somitic (prevertebral) segments are in register at the time of their formation during neurulation, the growth of the somitic system outstrips the neural tube postneurulation, so that the caudal end of the neural tube appears to “ascend”; by birth, the conus lies at the level of the L3 vertebra and in the adult at the L1 level. This is important in NTDs, as “tethering” of the cord, which happens in both open and closed low-spinal defects, prevents ascent of the cord and causes symptomatic stretching of the spinal nerves.
The clinical classification of NTDs is inconsistent and confusing, with variation in terminology even between medical specialties. For example, public health studies typically refer to “NTDs” or “ASB” (anencephaly and spina bifida), whereas pediatric neurosurgeons use “dysraphism” to denote both open and closed spinal lesions. In this chapter, NTDs are classified based on their developmental (embryonic and fetal) origins. While this system can be applied with some degree of confidence to NTDs where human defects are modeled faithfully in an animal model (thereby allowing longitudinal and experimental studies), it is more hazardous for NTDs that have no animal models. Speculation on the “embryological” origin of NTDs without experimental evidence is rife in the neurosurgical literature but can be highly misleading and is best avoided.
Failure of any aspect of the complex primary neurulation process can yield an open NTD.
This prenatally lethal NTD, whose name means “brain and spine open,” arises when Closure 1 fails, so that the neural tube remains open from mid/hindbrain level to the end of the spine. The open neural tube degenerates due to prolonged exposure to amniotic fluid, with loss of neural tissue, absence of dorsal skull and vertebral structures, and angioma-like formations. The independent closure initiation site at the rostral end of the neural tube (site β or Closure 3), generally does not fail in CRN, so forebrain closure occurs relatively normally and there are distinct optic vesicles. This contrasts with another dramatic brain defect, holoprosencephaly, where the most severe manifestation is univentricular forebrain and fused optic vesicles (cyclopia). A high prevalence of CRN has been seen in some geographical locations: for example on the Texas-Mexico border, where CRN comprised 3.8% of NTDs, in contrast to the usual 1% of NTDs. In a north Chinese region 18.8% of NTDs were CRN, with an overall NTD frequency that was six times the world average (5.7/1000 births in N. China, vs. 1/1000 worldwide). The origin of CRN is closely linked to faulty function of the planar cell polarity (PCP) signaling pathway, and harmful PCP genetic variants are associated with CRN in humans and mice.
This is the most common cranial NTD, characterized by congenital absence of a major portion of the brain, skull, and scalp. It accounts for around 40% of all NTDs and shows a strong female preponderance, with an average sex ratio of 2 to 3:1. It is uniformly fatal, and is invariably detected by prenatal ultrasound examination, in countries where this test is available. Most affected fetuses are spontaneously aborted, are stillborn, or die within the first week after birth. Anencephaly arises when some aspect of cranial closure fails, most commonly faulty completion of anterior (rostral) neuropore closure in humans. Holoanencephaly is a severe form that extends through the level of the foramen magnum whereas meroanencephaly is a less extensive form. In anencephaly, the calvarium is hypoplastic or absent, the base of the skull is thick and flattened, and there is a constant anomaly of the sphenoid bone resembling “a bat with folded wings.” The orbits are well formed, as in CRN, but are shallow, causing protrusion of the eyes. Attached to the skull base is a dark reddish irregular mass of vascular tissue with multiple cavities containing cerebrospinal fluid (CSF), the area cerebrovasculosa. The mass is cystic with a midline dorsal aperture opening to the exterior. No recognizable neural tissue can be found in the anterior and middle fossae except for the trigeminal ganglia and limited lengths of the 2nd to 5th cranial nerves. A hypoplastic anterior pituitary is present in a shallow sella, but the intermediate and posterior pituitary lobes are missing. Other terms, including acrania and cranioschisis, are sometimes used to describe anencephaly.
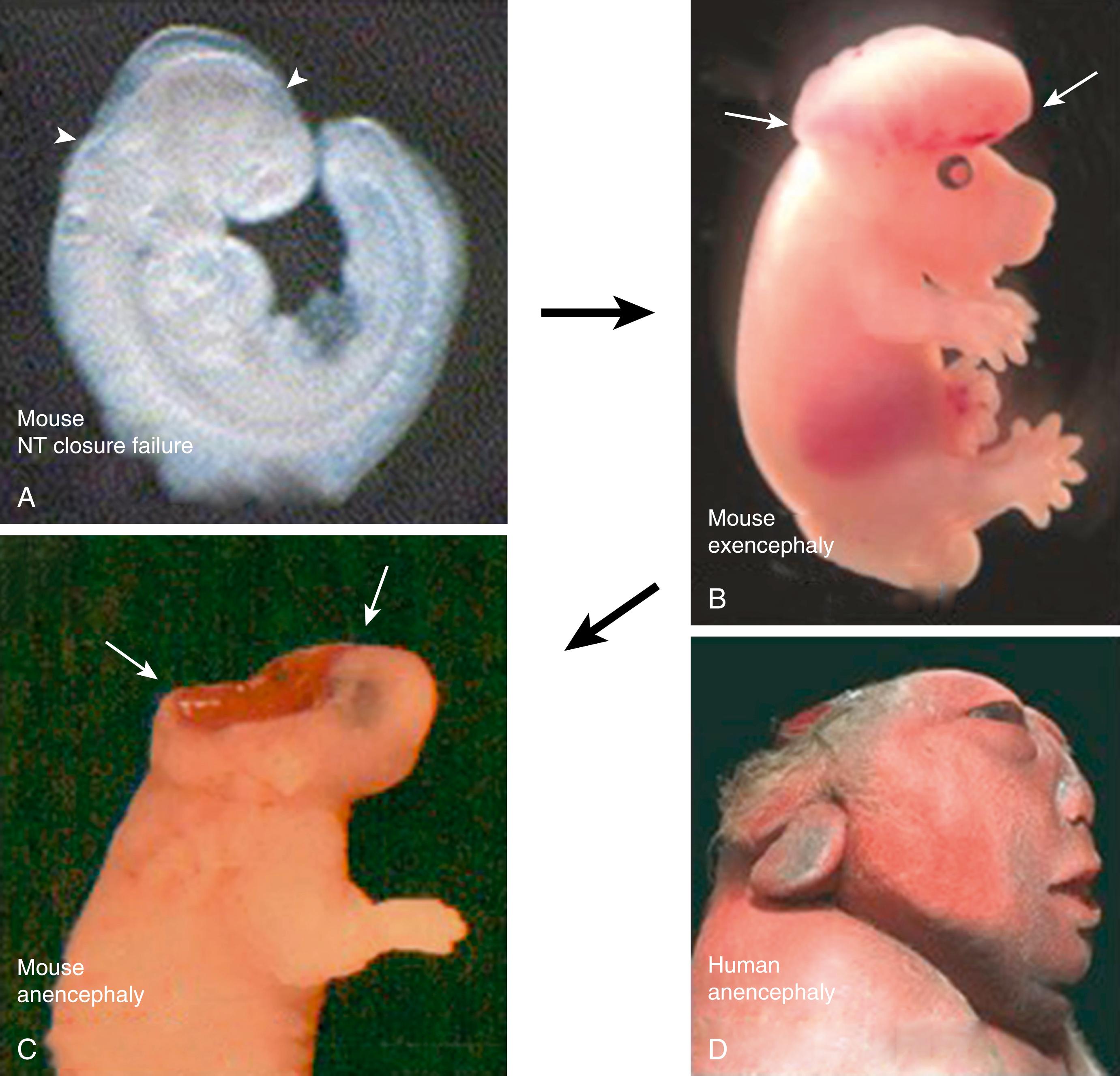
Confusion exists over this condition, in which the brain fails to close and neural tissue (often voluminous) projects from the top of the embryonic head. Some authors have suggested it to be a rare condition, distinct from anencephaly, whereas longitudinal studies of rodent NTD models , show clearly that exencephaly is the embryonic forerunner of anencephaly. Hence, the embryonic and fetal pathogenesis of anencephaly begins with failure of cranial closure ( Fig. 169.2A ), which is followed by eversion of the neural folds, giving the “overgrown” exencephalic appearance (see Fig. 169.2B ). Subsequently, the toxic effects of amniotic fluid exposure cause the protruding neural tissue to degenerate, leading ultimately to the absent brain phenotype, anencephaly (see Fig. 169.2C ). Exencephaly can be missed in humans as it occurs early and is usually converted to anencephaly (see Fig. 169.2D ) by the late fetal stages when detailed observations are made of the fetal head.
This is often used synonymously with myelomeningocele (also called myelodysplasia and “open spinal dysraphism”). It is a nonlethal NTD that has a similar prevalence to anencephaly and comprises around 40% of NTDs. It arises due to a primary neurulation defect in the spinal region, with failure of the posterior neuropore to complete its zippering closure. Open spina bifida has two typical clinical manifestations: (1) the common cystic myelomeningocele lesion (spina bifida cystica) in which the open portion of neural tube (often called the placode) is located on the surface of a meningeal sac that comprises CSF within the subarachnoid space ( Fig. 169.3A and B ), and (2) the less common myelocele or myeloschisis defect in which a CSF-containing sac is absent, and the open spinal cord is exposed on the back of the fetus or child (see Fig. 169.3C and D ).
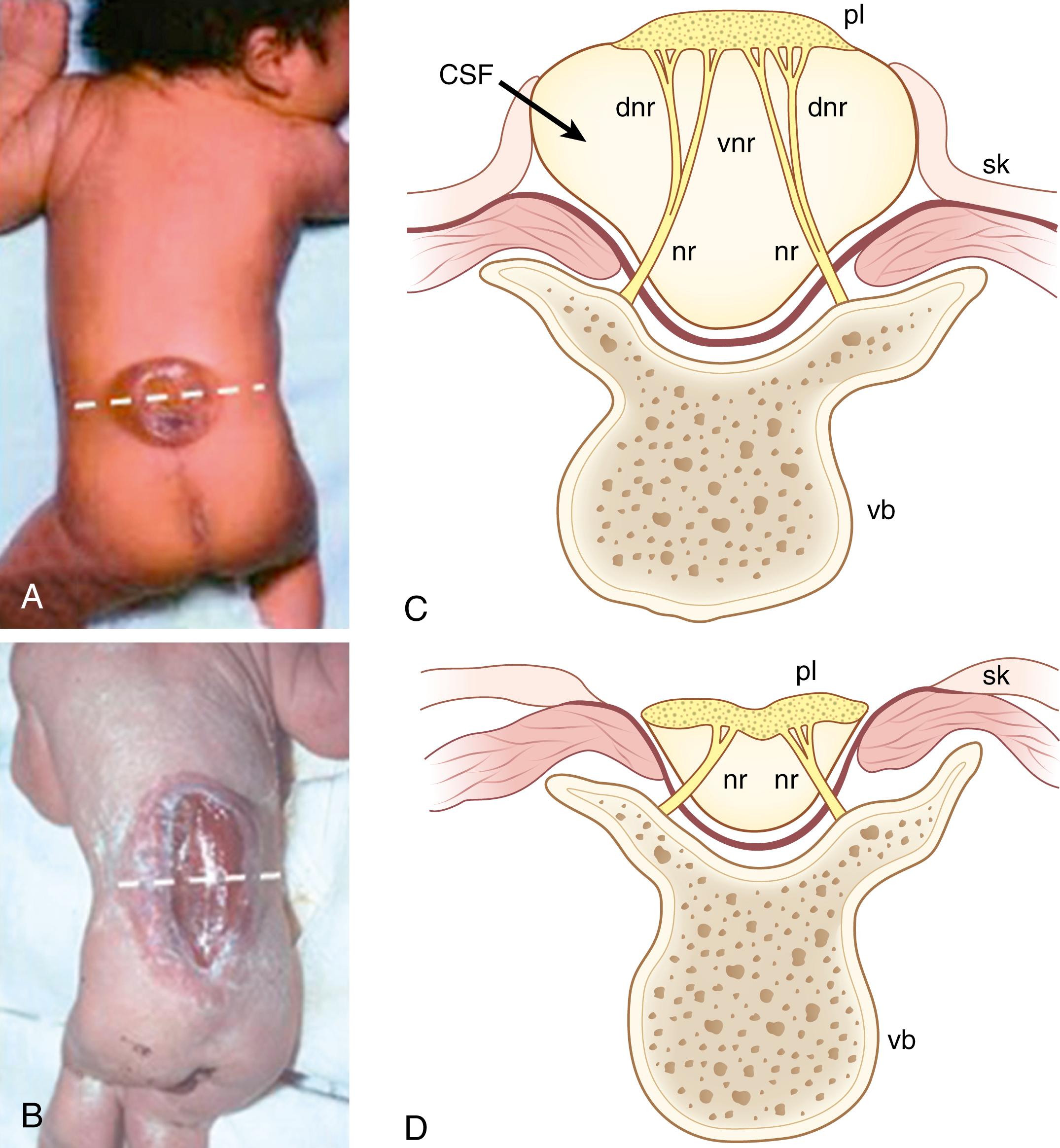
The spinal neuroepithelium in open spina bifida is initially healthy and undergoes normal differentiation and spinal nerve development, with onset of lower sensory and motor function in both humans and mice. However, neurodegeneration then intervenes, due to prolonged exposure of the neuroepithelium to amniotic fluid, in the same way as for open cranial defects; by birth, neurologic function has been lost from the rostral level of the lesion downward. One rationale for the fetal surgical approach to spina bifida management, therefore, is to cover the lesion as early in pregnancy as possible, in order to minimize exposure to CSF and the spinal neurodegeneration that leads to disability. Patients with “high” lesions (e.g., open from thoracic to low spine) typically exhibit lower limb paralysis and incontinence, whereas those with “low” lesions (e.g., confined to low lumbar or sacral regions) often have preserved limb function, although continence can still be affected. Following postnatal surgical closure, the 40-year survival is significantly lower for individuals with high spina bifida than for those with low lesions.
While not an NTD itself, the Chiari II malformation is present in around 90% of open spina bifida cases and can be a life-threatening complication: brainstem compression may lead to respiratory distress and death. An abnormally small posterior skull fossa is associated with herniation of the cerebellar vermis and brain stem (including the fourth ventricle) into the spinal canal, through an enlarged foramen magnum. Associated brain anomalies include agenesis of the corpus callosum, enlargement of the massa intermedia, cortical heterotopia, and polymicrogyria. While the developmental origin of Chiari II has not been experimentally determined, the predominant hypothesis considers chronic leakage of CSF from the open spina bifida lesion as the primary causal factor. Hydrostatic pressure is reduced in the developing ventricular system, producing a relatively “deflated” hindbrain that induces formation of a small posterior skull fossa. This cannot accommodate the enlarging hindbrain, leading to herniation. Fetal surgery to close the open spina bifida lesion in utero is associated with a high frequency of resolution of Chiari II hindbrain herniation. Since the surgery includes sealing the site of CSF leakage, this provides support for the “hydrostatic pressure” hypothesis.
For infants born with open spina bifida, whether the lesion is closed surgically before or after birth, a number of lifelong disabilities are typically encountered. Paraplegia and sensory loss in the lower body, together with neurogenic bladder and sexual dysfunction, are direct sequelae of the neurologic defect in the lower spinal cord. Urinary incontinence is common, with the need for children to learn “clean intermittent self-catheterization” early in life. Nevertheless, many individuals progress to chronic kidney disease with renal failure as a common cause of death. The development of latex sensitization, which affects around half of individuals with open spina bifida, may be related to this frequent instrumentation, although more fundamental immunologic causation has not been ruled out. Other morbidities include hydrocephalus, for which CSF shunt insertion is often required, pulmonary and bowel dysfunction, and skeletal deformations. A significant proportion of individuals with open spina bifida exhibit cognitive impairment. Hence, establishment of an independent lifestyle for these individuals can be extremely challenging. With optimum health and social care support systems in place, life expectancy for a person with open spina bifida can be 30 to 40 years or more, although this varies widely depending on the degree of disability.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here