Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Meconium aspiration syndrome (MAS) follows fetal hypoxic/ischemic stress that leads to intestinal peristalsis, meconium release, contamination of the amniotic fluid, and gasping respirations, which causes aspiration of noxious meconium-stained fluid deep into the fetal lung. Aspiration of meconium manifests as airway obstruction with trapping of gas in the lung during exhalation, chemical pneumonitis, and surfactant inactivation with decreased lung compliance. The newborn typically presents with respiratory distress shortly after birth with or without evidence of pulmonary hypertension.
Observation of meconium-stained amniotic fluid (MSAF) is essential for the diagnosis of MAS. With changes in obstetric practice, specifically reducing delivery after 41 weeks gestation, the incidence of MSAF in the United States has decreased from 10% to 15% of all live births to less than 1%, with MAS occurring in approximately 2.5 infants per 1000 live births. In addition, as a result of advances in neonatal care, MAS mortality has improved from approximately 4% to 7% to less than 1%. Several risk factors for MAS have been identified. MSAF and MAS occur with a higher frequency in post-term infants; approximately 30% of infants greater than 41 weeks are born through MSAF. Some 20% to 33% of MAS cases are associated with nonreassuring fetal heart tones and perinatal depression. Fetal distress is associated with acidosis and decreased muscle tone. Vagal stimulation and parasympathetic tone increase with repeated cord compression, leading to evacuation of meconium. In addition, hypoxia causes fetal gasping, which results in aspiration of MSAF into the lungs. ,
A recent report assessed the relationship between umbilical cord lactate levels and outcomes in infants with MAS. The presence of lactate in both serum and urine can be a sign of asphyxia in neonates; however, the prognostic significance has not been evaluated. In this report the authors found that a serum lactate of 4.1 mmol/L could discriminate between the presence of thin and thick MSAF, with thick MSAF more frequently associated with pulmonary hemorrhage, persistent pulmonary hypertension of the neonate (PPHN), intraventricular hemorrhage, and respiratory failure requiring ventilation support. In addition, infants with thick MSAF had higher gestational age, lower Apgar scores, greater base deficit, higher P co 2 , lower P o 2 , and a lower pH. These findings suggest an association between MSAF and asphyxia, with umbilical cord lactate predictive of severity and adverse neonatal outcomes. Finally, published studies have also confirmed an association between MSAF and oligohydramnios.
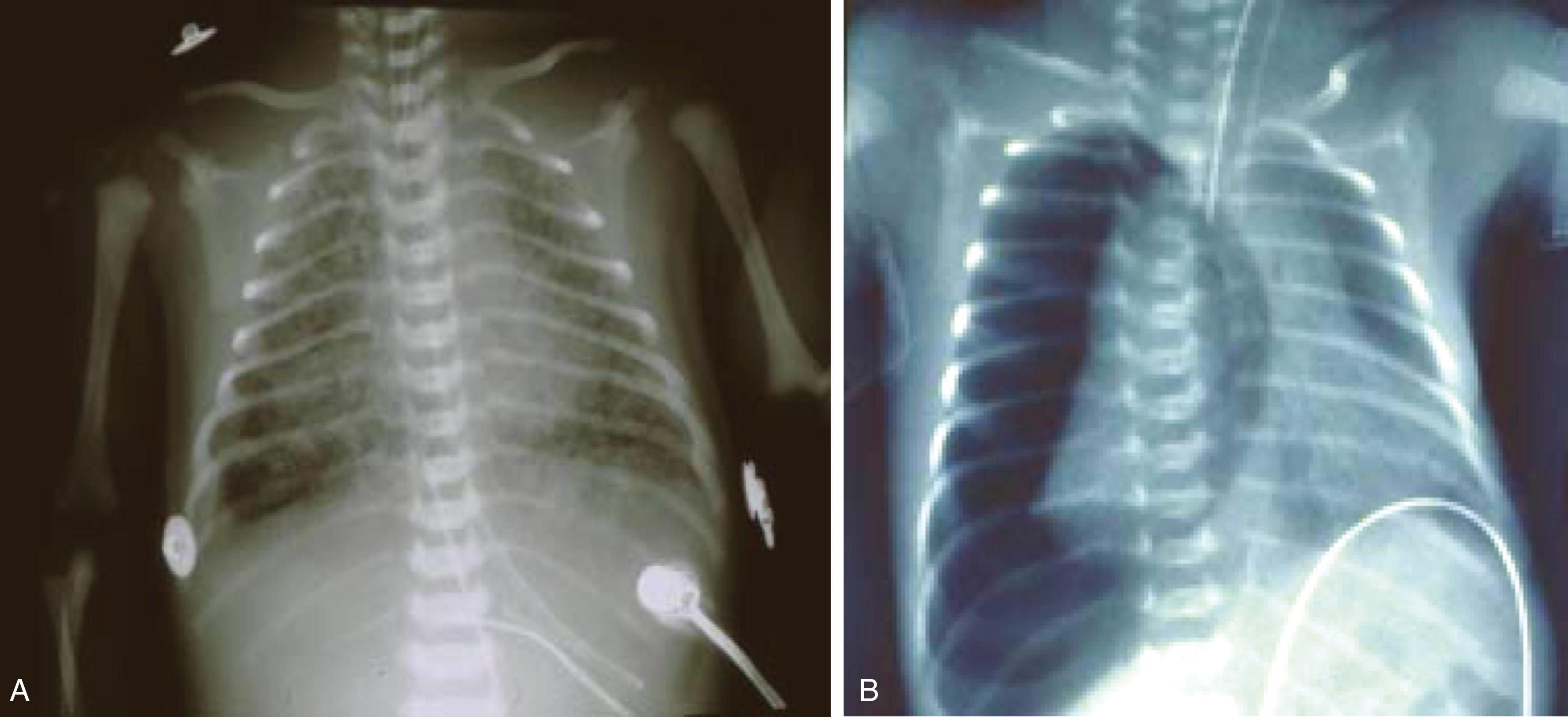
Meconium is a thick, black-green, odorless material first demonstrable in the fetal intestine during the third month of gestation. The characteristics of meconium are key to understanding the pathophysiology of MAS. Meconium results from the accumulation of debris, including desquamated cells from the intestine and skin, gastrointestinal mucin, lanugo hair, fatty material from the vernix caseosa, amniotic fluid, and intestinal secretions, leading to the formation of a viscous, adhesive substance. Meconium also contains blood group-specific glycoproteins and a small amount of lipid and protein that decreases during gestation. , The black-green color results from bile pigments. Meconium may or may not be sterile, but free fatty acids, bile salts, and pancreatic phospholipases in meconium are responsible for the adverse effect on surfactant function. When aspirated into the lung, meconium results in decreased pulmonary function and compliance with secondary surfactant inactivation and small airway obstruction with ball-valve or check-valve phenomena leading to atelectasis or air trapping within the alveoli and bronchioles. , , Progressive hyperinflation ensues, which increases the risk for pneumothoraces. Patients with severe disease are at risk for respiratory failure necessitating intubation and mechanical ventilation ( Fig. 158.1 ).
Inflammation is central to the pathogenesis of MAS, as meconium stimulates production of cytokines and other vasoactive substances that lead to cardiovascular and inflammatory responses in the fetus and newborn. , These secondary effects develop almost immediately after birth and worsen over the first 24 hours of life. Severe pulmonary hypertension develops in 20% to 40% of patients with MAS. Mechanisms responsible for the development of pulmonary hypertension are the release of inflammatory cytokines, hypoxia, hypercarbia, acidosis, and poor lung recruitment. Remodeling of the pulmonary vasculature in utero in severe cases of MAS also contributes to the development of pulmonary hypertension and suggests that chronic intrauterine stress may further account for the severity of cardiopulmonary disease at birth in the setting of MAS.
In addition to the mechanical ball-valve/check-valve effects on the airway and lung parenchyma and inflammation, there are several other mechanisms by which meconium results in respiratory failure after birth. Meconium directly alters pulmonary function, produces a chemical pneumonitis, inactivates surfactant, predisposes to infection, and contributes to the development of PPHN.
Lung function in MAS is characterized by both hyperinflation and volume loss, with both adversely effecting pulmonary function and gas exchange. Airway obstruction is a major contributor to both hyper- and hypoinflation and is central to the pathogenesis of MAS. Airway obstruction can be complete or partial and occurs by several mechanisms. Meconium induces apoptosis of the airway epithelium, resulting in sloughing of cellular debris from apoptotic cells into the airway and causing airway obstruction and inflammation. In addition, meconium is proinflammatory, further exacerbating airway inflammation. Airway inflammation results in increased mucus production as well as narrowing of the caliber of the airways. Inflammation of the airways also increases resistance and contributes to the development of atelectasis. Another mechanism by which airway obstruction occurs is through particulate meconium, which can completely or partially occlude the airway. Complete obstruction produces a ball-valve effect preventing the passage of air into the lung, leading to distal atelectasis. Partial airway obstruction occurs when the airway diameter is larger in inspiration and gas can enter around the partial obstruction. However, as the airway narrows during exhalation, the meconium plug occludes the airway completely, trapping the gas distally. This process is consistent with a check-valve effect and can lead to overdistension of the lung and alveolar rupture, with a resulting pneumothorax or other air leak complications. This check-valve effect is responsible for air leak in 10% to 30% of infants with MAS. , , Overdistension of the lung with progressive hyperinflation also results in difficulty with carbon dioxide (CO 2 ) elimination.
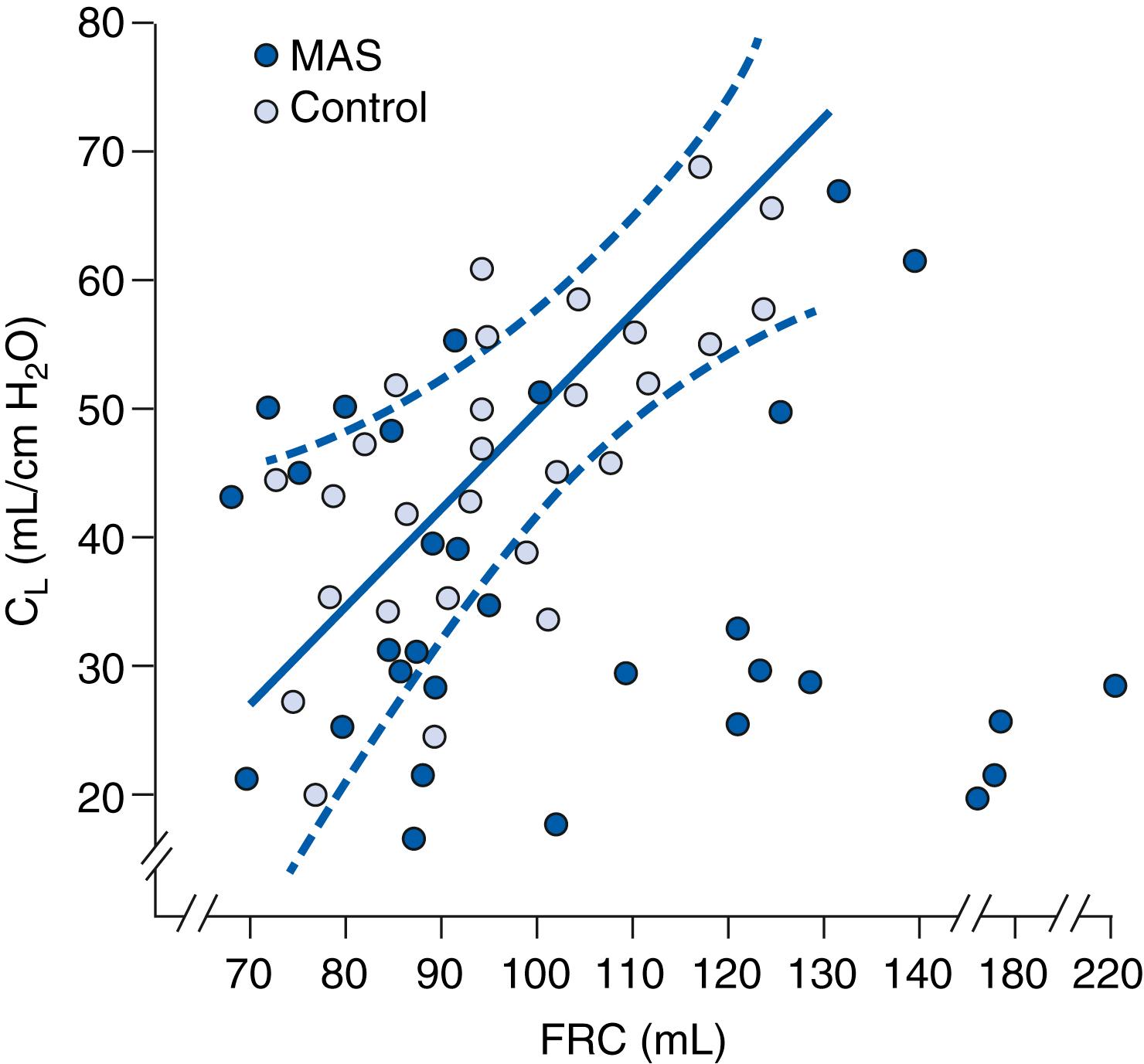
In addition to the lung function abnormalities that result secondary to airway inflammation, decreased pulmonary compliance secondary to surfactant inactivation also contributes to hypoinflation and atelectasis in MAS. Fig. 158.2 demonstrates the relationship between lung compliance and functional residual capacity (FRC) in infants with MAS and control patients. FRC was determined by a closed loop helium method, and compliance was determined using a pneumotachograph, an esophageal pressure probe, and a pressure manometer. When the FRC was low and compliance remained low, the patients were found to be atelectatic on chest x-ray. However, when the FRC was high, they were hyperinflated. These changes in lung function ultimately lead to ventilation/perfusion (V/Q) mismatch and worsening hypoxia and hypercarbia.
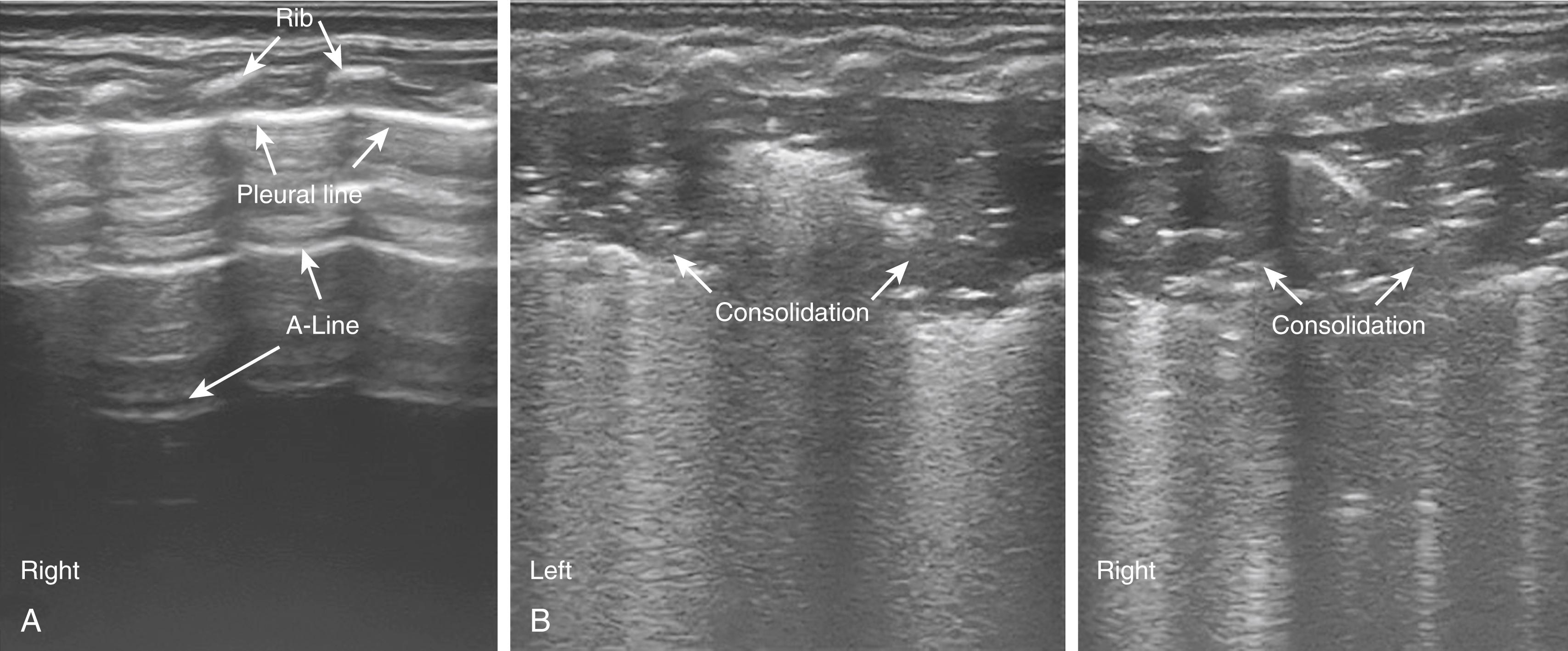
More recent reports have evaluated the sensitivity of lung ultrasound for the detection of MAS. The following ultrasound findings were observed: (1) B-pattern (alveolar-interstitial syndrome [AIS]) either coalescent or sparse; (2) lung consolidation with irregular edges; (3) atelectasis and air bronchograms. The latter ultrasound findings reliably predicted the presence of MAS when compared to a routine chest x-ray. A follow-up study on a larger cohort of 117 patients demonstrated that consolidation with irregular edges, disappearance of the pleural or A line, and/or the presence of AIS or B-line in the nonconsolidation area predicted the presence of MAS with 100% sensitivity and specificity ( Fig. 158.3 ).
The airway inflammation in MAS may persist for weeks, after the parenchymal disease has recovered, with resultant airways reactivity and wheezing. Steroids and bronchodilator therapy are often effective in this setting of prolonged airway hyperresponsiveness. ,
Aspiration of meconium into the lung also results in a chemical pneumonitis, which occurs secondary to macrophage and neutrophil activation. , Influx of macrophages and neutrophils influx results in increased free radical formation and the production of cytokines, interleukin (IL-1), IL-6, IL-8, and tumor necrosis factor-α. , , Increased production of proinflammatory cytokines causes an exudative and inflammatory pneumonitis with epithelial disruption, proteinaceous exudation with alveolar collapse, and cellular necrosis. Cytokine production is central to the pathogenesis of MAS, such that improvement in pulmonary function directly correlates with a fall in proinflammatory cytokines over the first 96 hours of life. MAS also results in activation of the arachidonic acid pathway and through cycloxygenase-2 results in the release of thromboxanes and leukotrienes. Meconium can also activate the complement system, which exacerbates inflammation and results in further tissue damage. In addition to its proinflammatory effect, meconium induces apoptosis on airway epithelium as well as type 2 pneumocytes in the lung. , In the first 24 hours after aspiration of meconium, caspase 3 activity—a direct marker for apoptotic activity—increases with peak expression seen at 8 hours. , , Cellular debris from apoptotic cells sloughs off and is released into the airway, exacerbating airway obstruction and inflammation.
While meconium is usually sterile, the presence of meconium in the lung predisposes to the development of infection. The mucopolysaccharide component provides an excellent growth medium for microorganisms, especially Escherichia coli. However, two recent review articles evaluated the efficacy and safety of antibiotics for the prevention of infection, morbidity, and mortality among infants born through MSAF who were both asymptomatic at birth or had signs and symptoms compatible with MAS. , Prior to these reviews antimicrobial administration was recommended for all symptomatic patients with MAS. While the evidence was weak, in both reviews the antibiotics did not decrease the risk of thromboxane’s early- and late-onset neonatal sepsis in neonates who were either asymptomatic or symptomatic with a diagnosis of MAS. In addition, there were no significant differences in mortality or duration of hospital stay between groups given antibiotics and control groups.
Surfactant is a macroaggregate molecule secreted by type 2 pneumocytes as the infant approaches term gestation. Surfactant is made up of 90% phospholipid and 10% proteins (surfactant protein [SP] A, B, C, and D). The primary function of surfactant is to reduce surface tension in the lung. Dipalmitoyl phosphatidylcholine (DPPC) is the key phospholipid in surfactant for lowering surface tension. SP-B and SP-C are also hydrophobic and necessary for lowering surface tension in the lung. By lowering surface tension in the lung, surfactant prevents alveolar collapse during expiration, thus maintaining FRC and improving compliance of the lung. The estimated surfactant stores of term infants is approximately 100 mg/kg compared with preterm infants who have approximately 4 to 5 mg/kg. Surfactant deficiency results in respiratory distress syndrome (RDS), which can occur due to primary deficiency such as occurs with prematurity or to inactivation, as seen with MAS, pneumonia, asphyxia, etc. In addition to reducing surface tension in the lung, surfactant plays a key role in regulating the immune response in the lungs, with SP-A and SP-D primarily responsible. A deficiency in SP-A or SP-D predisposes to the development of pulmonary infection. There are several mechanisms by which meconium interferes with surfactant function. Meconium directly decreases the synthesis and secretion of surfactant by alveolar type 2 cells, disrupts formation of the lamellar layer, and increases metabolism and degradation of phospholipases. Rodent studies have confirmed decreased SP-A and SP-B in the setting of MAS, and a more recent human study confirmed decreases in DPPC in infants with MAS needing extracorporeal membrane oxygenation therapy (ECMO).
Free fatty acids and bile salts are theorized to be key ingredients of meconium, which contribute to surfactant dysfunction. Palmitic, stearic, and oleic acids are commonly found in meconium. In experimental studies, when these substances were administered by bronchoalveolar lavage, they were found to significantly increase surface tension by stripping surfactant from the alveolar wall. In addition, free fatty acids produce a fluidizing effect that interferes with the ability of spread surfactant films to reach low surface tensions.
Bilirubin present in meconium also inhibits surfactant in a dose-dependent manner, by altering the effects of SP-B and SP-C. Pancreatic phospholipases, especially phospholipase A2, are present in meconium and have been investigated for their role in surfactant degradation. Their unique properties allow them to hydrolyze phospholipids, including DPPC in surfactant, and induce type II pneumocyte apoptosis. , In addition, meconium stimulates inflammatory cells such as macrophages and neutrophils to produce oxidative damage through the formation of peroxynitrite and hypochlorous acid, with resultant type 2 pneumocyte apoptosis and surfactant breakdown. , , In these studies, protein carbonyl concentration, previously shown to correlate with the severity of bronchopulmonary dysplasia in preterm infants was used as a marker of oxidative damage.
Minimal amounts of cholesterol are normally present in surfactant, and it is required for natural pulmonary surfactant membranes to adopt their structure and dynamics. Meconium contains a substantial amount of cholesterol, and exposure to meconium results in incorporation of cholesterol into surfactant membranes and films. Elevated cholesterol may form complexes with surfactant phospholipids, thus increasing the surfactant film fluidity. Higher fluidity results in collapse rather than multilayer formation during lateral compression in breathing cycle. Besides enabling the insertion of cholesterol into surfactant membranes, bile acids in lungs lead to a decrease in DPPC portion and shift in the ratio between phosphatidylglycerol and sphingomyelin. Taurocholic acid, one of the most abundant bile acids in humans, was found to affect the structure of both surfactant monolayers at the interface and surfactant aggregates in solution , thus contributing to loss of surfactant function.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here