Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
More than half a century after its first description by Northway and colleagues, bronchopulmonary dysplasia (BPD) continues to be the most common respiratory morbidity in extremely preterm infants surviving after different modes of respiratory support. Over the years, the epidemiologic, clinical, and pathologic picture of this condition has changed remarkably. In its current form, BPD is the end result of various antenatal and postnatal factors that interfere with the normal progression of development of lung parenchyma, vasculature, and airways, leading to varying degrees of respiratory failure. This complex, multifactorial pathogenesis has provided unique challenges to not only fully understand the pathophysiology but also to devise effective prevention and treatment strategies for BPD.
BPD, as originally described by Northway and colleagues, resulted from severe injury to the developing lung in the presaccular phase of development, secondary to prolonged mechanical ventilation with high pressures and inspired oxygen concentrations. This resulted in a grossly distorted pathologic picture characterized by emphysema, fibrosis, and marked vascular and airway epithelial changes. Since then, changes in clinical practices such as widespread use of antenatal steroids, exogenous surfactant, and less invasive respiratory support have led to a reduction in injury to the developing lung. In addition, advances in neonatal care have improved the survival of extremely premature infants whose lungs are at the late canalicular or early saccular phase of development exposing them to various antenatal and postnatal factors that can disrupt normal alveolar and vascular development. Hence the pathologic picture of this new form of chronic lung disease, “new BPD,” is markedly different and is characterized by alveolar simplification with fewer and dysmorphic capillaries, but less evidence of emphysema, airway damage, vascular remodeling, and pulmonary hypertension.
Human lung development is a complex process comprising of anatomic structure development, histologic differentiation, and biochemical maturation in conjunction with vascular development. These mechanisms are discussed in detail in other chapters. There are multiple factors that can contribute to an aberration in the normal lung development, including prenatal factors such as genetic predisposition and exposure to maternal smoking, antenatal conditions associated with or resulting in preterm birth, untoward effects of life-sustaining procedures like mechanical ventilation or oxygen supplementation, as well as associated complication such as infections or nutritional deficiencies ( Fig. 159.1 ).
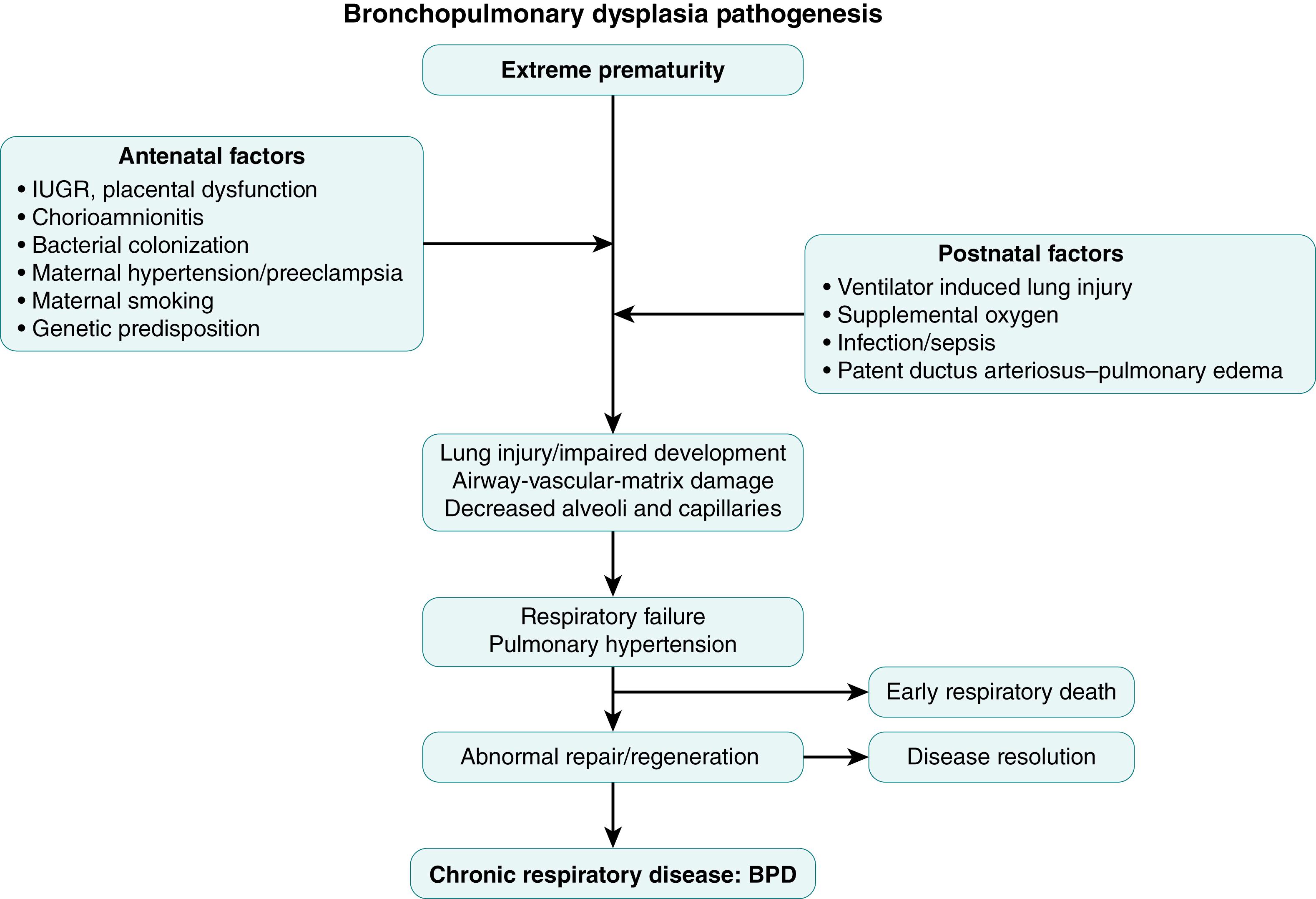
While gestational age at birth continues to be the most significant determinant for the risk of most of the sequelae of prematurity, including BPD, other conditions can have a significant effect on lung development and risk for BPD. One of the more studied in-utero exposures is cigarette smoking. In addition to epidemiologic studies suggesting an association between maternal smoking and increased risk of BPD, several animal models have shown exposure to nicotine in-utero results in abnormal airway branching with increased smooth muscle thickness and collagen deposition.
Lung angiogenesis is one of the critical components of alveolar development, with increasing evidence that conditions affecting primarily angiogenesis may also contribute to abnormal alveolar development. Disorders affecting placental function, such as gestational hypertension, preeclampsia, and intrauterine growth restriction, have also been associated with impaired vascular growth, with evidence that suggests these effects are mediated by an imbalance in pro- and anti-angiogenic factors. Impaired vascular growth and function may disrupt distal airspace development (the “vascular hypothesis” of BPD).
Exposure of the fetal lung to infection has been shown to result in inflammation and lung injury, , as well as enhanced lung maturation. It is likely that chorioamnionitis has variable effects, depending on the fetal inflammatory response, the organism causing the infection, exposure to antenatal steroids, and the severity and duration of infection. , This is supported by clinical evidence from several studies suggesting an increased risk for BPD in infants born to mothers with evidence of chorioamnionitis , ; others, however, have failed to show this association.
Preterm birth interrupts normal fetal lung development, leading to a premature infant who is exposed to multiple life-saving therapies that are likely to cause lung injury. The relative contribution of different factors has evolved over time, with newer strategies such as less invasive respiratory support and judicious use of supplemental oxygen most likely being responsible for the reduced severity of the chronic lung injury. The two main mechanisms of lung injury from mechanical ventilation are volutrauma, , due to excessive stretching of tissues from over inflation, and atelectotrauma, caused by repetitive opening of closed lung units resulting in shear injury. These can result in damage to the alveolar-capillary barrier, exudation of intravascular fluid resulting in pulmonary edema, release of cytokines, chemokines, and proteases, as well as recruitment and activation of inflammatory molecules. , Oxidative stress continues to be a significant risk factor for lung injury in preterm infants due to a combination of factors, including the transition from a fetal low oxygen environment to a postnatal high oxygen environment, need for supplemental oxygen, as well as inadequate antioxidant mechanisms. The resultant generation of reactive oxygen species may cause lipid peroxidation with cell membrane damage, apoptosis and cell death, surfactant inactivation, protein and DNA damage, as well as activation of inflammatory cascade. The effect of patent ductus arteriosus (PDA) on lung injury has been debated for a long time. As a consequence of the left-to-right shunting through the PDA, pulmonary blood flow and lung fluid increase, negatively affecting lung mechanics and gas exchange and thereby increasing the need for more aggressive mechanical ventilation and the risk for BPD. Furthermore, simultaneous occurrence of both infection and PDA leads to a synergistic interaction that may further increase the risk for developing BPD.
Inflammation continues to be considered as a major mediator in the pathogenesis of lung injury. , The pulmonary inflammatory response can be triggered prior to birth, as in the setting of antenatal infection, or postnatally by a number of factors, including ventilation with excessive tidal volumes, , oxygen free radicals, , and postnatal infections.
A significant increase in inflammatory cells (macrophages, neutrophils), eicosanoids, and various cytokines (IL-1β, IL-6, IL-8, tumor necrosis factor-α) has been demonstrated in the airways of infants in whom BPD develops subsequently. The increase in cytokine concentrations has been documented early after birth, supporting the contention that in many infants the insult may start during fetal life or in the early postnatal period. There is evidence of pulmonary alveolar macrophage (PAM) activation in infants who later develop BPD, and these activated PAMs have been suggested as a source of neutrophil chemo attractants, especially when exposed to high oxygen concentrations.
Alterations in alveolar and vascular development is one of the hallmarks of bronchopulmonary dysplasia. The interaction between epithelium and mesenchyme is critical for lung development, with multiple signaling pathways playing important roles during different stages of lung development. These include members of transforming growth factor (TGF)-β, , bone morphogenic protein (BMP), vascular endothelial growth factor (VEGF), , fibroblast growth factor (FGF), sonic hedgehog (SHH), platelet-derived growth factor, , Wnt, and insulin growth factor (IGF).
Lung injury and repair mechanisms play a key role in the development of BPD. While effective repair helps preserve structure and cellular function, defective tissue repair can contribute to the impaired lung function seen in BPD. Connective tissue growth factor (CTGF) is one of the downstream mediators of lung injury, with increased expression seen in infants with BPD as well as different animal models of lung injury. Overexpression of CTGF in neonatal mice results in thickened alveolar septae, decreased alveolarization, reduced capillary density, and pulmonary hypertension. An increase in elastase and an imbalance between elastase and α 1 proteinase inhibitor in the lung has also been postulated as a possible mechanism for neonatal lung injury. ,
The major obstacle in preventing BPD is that as lung damage progresses, the deterioration in lung mechanics and gas exchange requires an increase in respiratory support and use of higher inspired oxygen concentrations. A vicious cycle is thereby created in which the required interventions to improve respiratory failure, mechanical ventilation, and increased inspired oxygen induce more lung damage and exacerbate the respiratory impairment.
Since BPD is a multifactorial disease process, there are several potential prevention strategies. So far, the majority of the prevention and treatment strategies, like less invasive respiratory support, prevention of infection, or judicious use of oxygen supplementation, aim to reduce iatrogenic injury to the developing lung. Additional therapies like corticosteroids have multiple potential effects, including antiinflammatory activity, reduction of lung mesenchyme resulting in improved lung function, and maturation of surfactant system. ,
Caffeine, a nonselective inhibitor of adenosine receptor commonly used for treatment or prevention of apnea of prematurity, has also been shown to have an antiinflammatory effect in newborn animals and possibly reduce BPD in infants. Nitric oxide (NO) is another drug that has been tried for the prevention of BPD, with inconclusive results. NO has multiple potential mechanisms of actions, including having antiinflammatory effects; serving as a mediator for several angiogenic factors, including VEGF; and reducing pulmonary vascular tone.
Specific interventions to modulate growth factors or target inflammatory pathways have been used in animal models of BPD with variable success. These include administration of molecules to antagonize the effect of IL-1, such as receptor antagonist (IL-1Ra), competitive inhibitor anakinra, or upstream pathways inhibiting the production of IL-1β. , Other pathways that have been targeted include CTGF pathway with inhibition of β catenin signaling, Fgf10 pathway with micro RNA-421 inhibition, recombinant Club cell-10Kilodalton protein (CC-10) as an immunomodulatory agent, and recombinant IGF-1 , with some success. There continues to be concern regarding the effects of these drugs on other organ systems, as well as the differentiation between physiologic need and the pathologic role in the disease process.
There is increasing evidence of the repairability of the lung following injurious stimuli, and endogenous stem cells play an important role in this process. , In contrast to full-term infants, stem cells in preterm infants at risk for BPD have impaired replication and differentiation potential, possibly contributing to the pathogenesis of BPD. This has resulted in concerted efforts to explore stem cell treatment for the prevention and treatment of BPD in animal models of hyperoxia-induced lung injury, showing reduced lung inflammation, attenuation of fibrosis, improved lung alveolar and vascular structure, and improved lung function with the use of mesenchymal stem cells (MSCs). , It is increasingly clear that the mechanism of action of stem cells is not mainly by engrafting and replacement of damaged cells but by paracrine effect with secretion of antiinflammatory and trophic factors. Extracellular vesicles (EVs), small nano-sized particles secreted by the MSC, are responsible for these paracrine effects. MSC-derived EVs have been shown to have similar beneficial effects on the prevention as well as treatment of hyperoxia-induced lung injury in neonatal animal models.
The alterations in pulmonary function in infants with BPD are nonspecific and result from the severe disruption of lung development and architecture that occurs in these infants. The degree of abnormality in lung function may range from mild to severe, paralleling the clinical and radiographic presentation. Few studies have described the early changes in lung function in infants who subsequently develop BPD or have longitudinally followed the course of lung function in these infants.
Infants who develop BPD have more severe respiratory failure and worse lung function early in their evolution than infants who recover without lung sequelae. Studies from the presurfactant era have shown higher airway resistance during the first weeks of life in infants in whom BPD developed subsequently compared to infants who recovered without lung damage, , suggesting that early airway obstruction may be a marker or a predisposing factor for the development of more severe pulmonary damage. Studies evaluating longitudinal lung function in preterm infants in the post-surfactant era have shown decreased lung compliance and functional residual capacity (FRC), but no difference in resistance between infants who go on to develop BPD versus those who did not. These results are consistent with the pathologic picture of new BPD, which is characterized by alveolar simplification but less airway injury. Lung mechanics measurements during the first week of life have been used to improve the accuracy of BPD prediction models with inconsistent results, likely reflecting differing severities of disease and different methods used to assess lung function. The worse initial respiratory function observed in infants who subsequently develop BPD most likely reflects the more severe initial respiratory illness in these infants.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here