Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
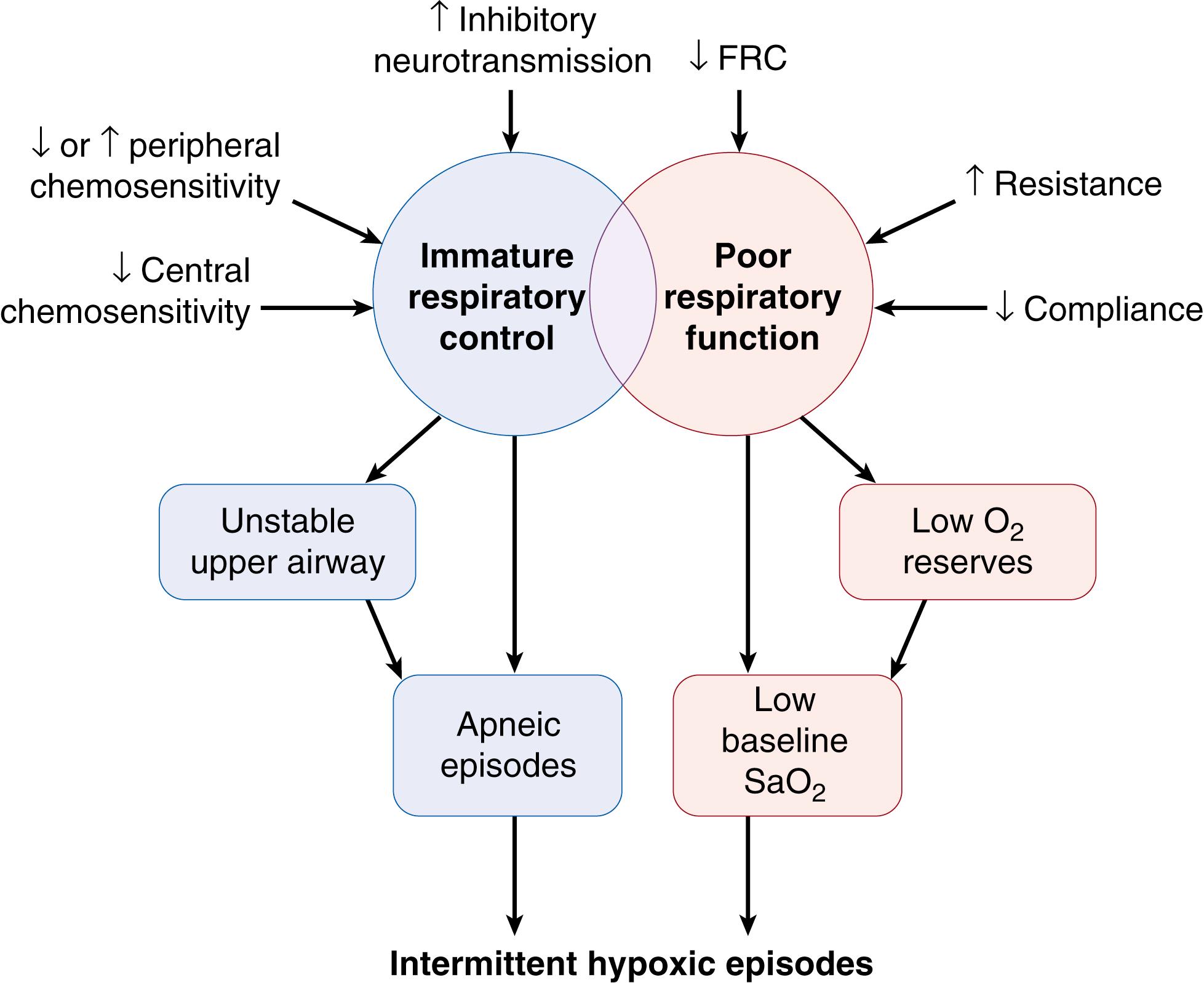
Apnea is an almost universal manifestation of immature respiratory control in premature infants. Such infants may experience respiratory pauses of varying duration, with decreasing gestational age increasing vulnerability to such events. Short respiratory pauses may be self-limiting, while longer episodes may necessitate intervention, especially in the most immature infants. Apnea has traditionally been defined as a respiratory pause lasting at least 20 seconds, or a pause accompanied by bradycardia, cyanosis, or pallor. However, shorter apnea events (e.g., 15 seconds in duration), if recurrent, may also require clinical intervention, particularly if they result in hypoxemia.
Apnea should be distinguished from periodic breathing, in which the infant exhibits regular cycles of rapid respiration for approximately 10 seconds interspersed with pauses of similar duration, in a recurring pattern (≥3 cycles). Periodic breathing has been considered to represent a benign respiratory pattern in the premature or young term infant, although there may be accompanying hypoxemia and bradycardia. The respiratory pause of apnea, unlike that of periodic breathing, may not be self-limiting and may produce significant physiologic changes. Such changes are considered in detail in the following discussion.
Apneic events are distinguished not only by their duration but also by the presence or absence of airway obstruction during the episode of apnea. Thach and Stark initially described an increase in the frequency of apnea when the premature infant’s neck was flexed. Subsequently, upper airway obstruction was found to accompany apnea in preterm babies, even though neck flexion was not present. The location within the upper airway at which obstruction occurs is usually within the pharynx but may vary between pharyngeal and laryngeal structures.
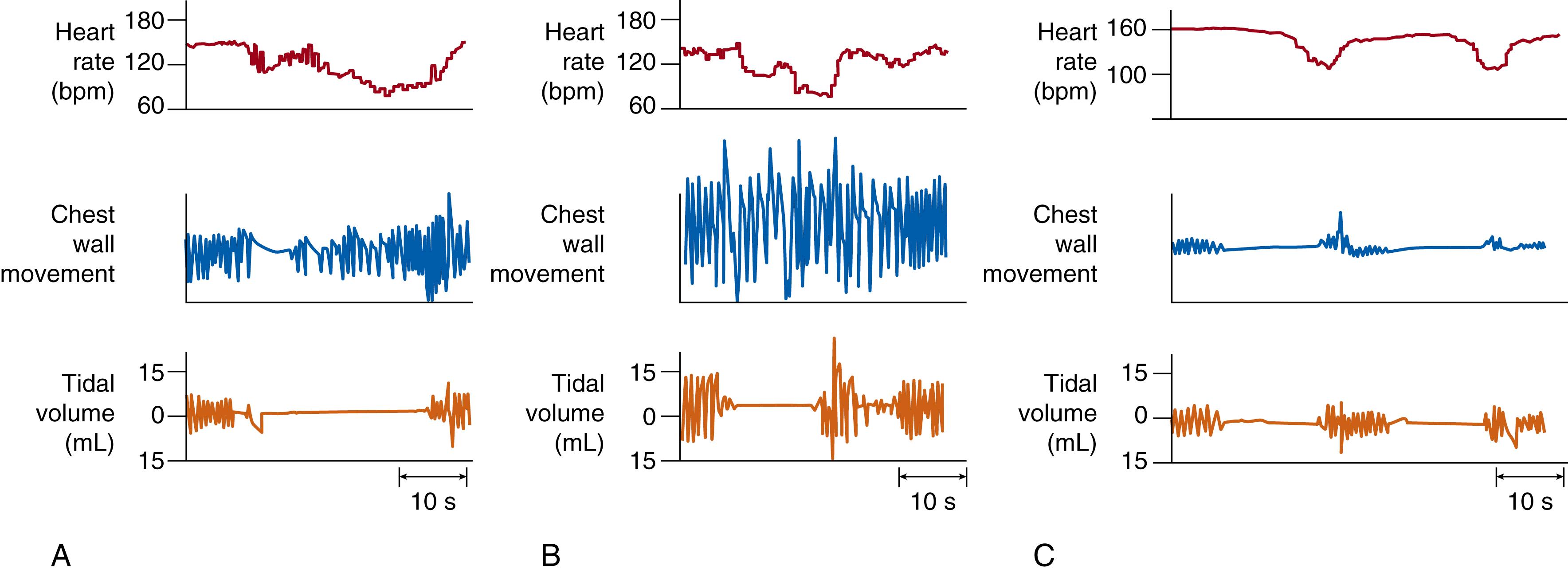
The presence or absence of upper airway obstruction forms the basis of the classification of apnea into three types ( Fig. 156.1 ). Mixed apnea is the most commonly observed clinically significant event in small premature infants and consists of obstructed inspiratory efforts as well as a central pause (see Fig. 156.1A ). In obstructive apnea, obstructed breaths characterized by chest wall motion without nasal airflow continue throughout the entire apnea (see Fig. 156.1B ). In central apnea, inspiratory efforts cease entirely, and obstructed breaths are not observed (see Fig. 156.1C ). Mixed apnea accounts for approximately 50% to 75% of all instances of apnea in premature infants; obstructive apnea, 10% to 20%; and central apnea, 10% to 25%. The longer an apneic event lasts, the more likely that it is a mixed-type apnea rather than a purely centrally mediated event.
Apnea duration and classification also may correlate with the infant’s neurologic status. Apnea in a subset of infants may possibly be a sign of a diffuse neurologic insult in prenatal or postnatal life that leads to disordered control of breathing. In most infants, however, an underlying neuropathologic process is unlikely, because apnea frequency decreases as the infant matures. Whether apnea with accompanying hypoxemia and bradycardia contributes to later adverse outcome remains speculative. Hypothetically, apnea may resolve when central and peripheral chemoreceptors develop to the point at which appropriate responses to change in blood gas status can occur, possibly with accompanying arousal. A further important developmental contribution to resolution of apnea may be the increasing ability of medullary respiratory control centers to activate upper airway–dilating musculature in synchrony with increasing ventilatory drive.
Hypoxemic events resembling apneic spells also occur in intubated, mechanically ventilated preterm infants. Bolivar and co-workers described episodes of hypoxemia preceded by an increase in total pulmonary resistance, a decrease in compliance, and apnea in intubated infants. Furthermore, Dimaguila and colleagues reported that such episodes may be preceded by subtle spontaneous movement and are characterized by both central respiratory depression and obstruction to airflow (the latter features being analogous to mixed apnea). These hypoxemic episodes in intubated infants are a consequence of hypoventilation and are frequently associated with arousal. They further illustrate the vulnerability of premature infants to imbalance of central respiratory control and altered pulmonary function.
Cessation of respiration during apnea has significant ventilatory and reflex cardiovascular consequences for the preterm infant. Both hypoxemia and hypercarbia accompany prolonged apnea. The decrease in oxygenation with apnea may be directly related to the duration of the apnea and baseline respiratory status ( Fig. 156.2 ). The reflex effects of apnea include characteristic changes in heart rate. Bradycardia may begin within seconds of the onset of the apneic episode ( Fig. 156.3 ). The bradyarrhythmia most often is sinus in character, with an occasional infant showing a nodal escape. Dorostkar and associates have observed that 1.8% of preterm infants may even have asystolic events during apnea, defined as absence of QRS complex for more than 3 seconds during apnea. This subgroup of infants appears to have a benign clinical outcome. Henderson-Smart and co-workers noted a significant correlation between the decrease in oxygen saturation and heart rate and postulated that bradycardia during apnea could result from hypoxic stimulation of the carotid body chemoreceptors.
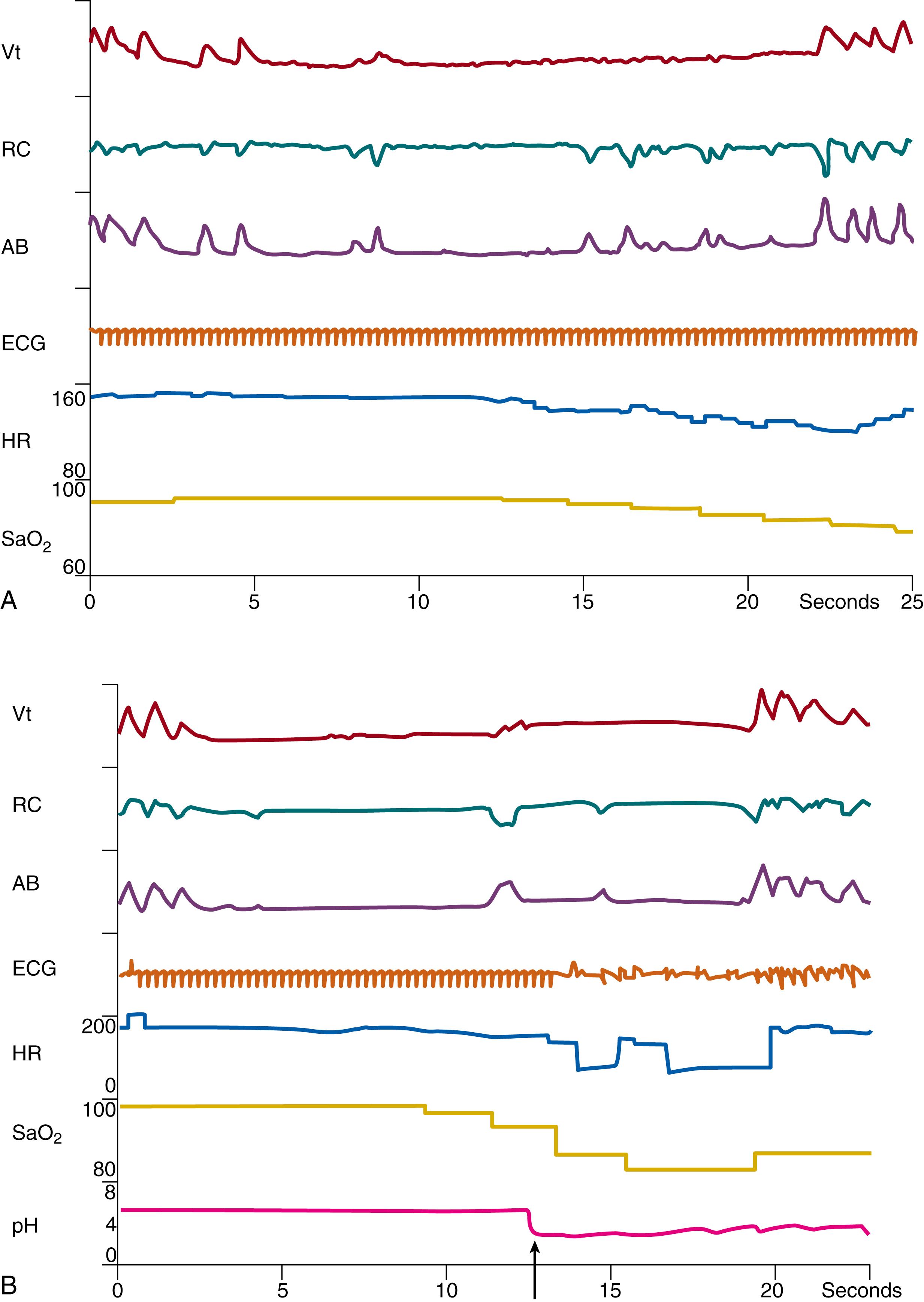
The relationship between reflex control of heart rate and breathing is complex. When ventilation is allowed to increase in response to hypoxia, tachycardia occurs. When this reflex increase in ventilation is prevented, bradycardia results. At the onset of apnea, at which time cessation of ventilation and onset of hypoxemia occur almost simultaneously, hypoxemia would be expected to produce bradycardia.
Other reflex input may accentuate the bradycardia during hypoxemia. For example, the reflex effects of apnea in infants also have been likened to the physiologic responses in diving mammals. During reflex apnea in these animals, upper airway afferent input from superior laryngeal and trigeminal nerve stimulation may produce greatly enhanced bradycardia. The contribution of upper airway reflexes to the bradycardia that occurs during apnea is difficult to study in human infants. In summary, the rapid onset of bradycardia during apnea may be a complex reflex deriving from multiple sources, including trigeminal receptors and carotid chemoreceptors.
A change in blood pressure also accompanies apnea in newborn infants. The decrease in heart rate during apnea may be accompanied by a concomitant rise in pulse pressure, usually owing to an increase in systolic pressure, occasionally accompanied by a fall in diastolic pressure. During bradycardia, filling volume of the heart may increase, leading to a rise in stroke volume and pulse pressure in accordance with the Starling law. With prolonged apnea and more severe bradycardia (less than 80 beats/min), a decrease in systemic blood pressure may occur, accompanied by a fall in cerebral diastolic and systolic blood flow velocity. In premature infants with poor cerebrovascular autoregulation, cerebral blood flow may mirror systemic blood flow, and cerebral perfusion may decrease during prolonged apnea, although potential consequences remain speculative. Resolution of apnea may then be accompanied by cerebral hyperperfusion.
Continuous recording of oxygen saturations via pulse oximetry has revealed a much higher incidence of intermittent hypoxemic (IH) events associated with apnea than was previously thought, and permitted analysis of high-risk IH patterns that correlate with undesirable clinical outcomes. Previous studies examining the long-term effects of apnea may in fact be proxy for the effects of IH. IH events are associated with multiple negative outcomes including retinopathy of prematurity (ROP), bronchopulmonary dysplasia (BPD), sleep-disordered breathing, wheezing, unfavorable neurodevelopmental outcomes, and death, but it can be difficult to separate the effects of IH itself from the therapies, such as oxygen, used to treat it. Furthermore, the association between IH events and adverse outcomes may not be causal. Although the mechanisms underlying the pathologic correlates of IH are still under investigation, there is evidence from rodent models that IH causes changes in inflammatory signaling and generation of reactive oxygen species. Normally only a small amount of oxygen is incompletely reduced to form reactive oxygen species, which in most cases are neutralized by endogenous antioxidants and free radical scavengers. However, preterm infants do not have well-developed antioxidant defenses, and under metabolically stressful situations the free radicals generated could cause direct tissue damage and trigger proinflammatory and proapoptotic pathways. ATP is exhausted during hypoxia, leading to the generation of purine derivatives (e.g., hypoxanthine); upon reoxygenation, oxidases that metabolize these derivatives are reactivated and generate a burst of superoxide anion.
IH exposure appears to have different effects depending on the specific developmental window in which it occurs. For example, a higher incidence of IH within the first 3 days of life is associated with increased risk of airway hyperreactivity, whereas increased frequency of IH events after 21 days of life (especially in infants with intrauterine growth restriction [IUGR]) is associated with development of BPD. Increased frequency of IH episodes in the first 8 weeks of life (especially after 5 weeks of age) in preterm infants is associated with severe ROP requiring laser surgery. The pattern of IH events appears significant, as well; the relationship between IH and severe ROP was even stronger when there was a time interval of at least one minute between events, possibly reflecting the time interval required for development of oxidative stress affecting neovascularization. In addition, BPD is associated with more frequent, longer, but less severe IH events, combined with exposure to increased oxygen within the first 26 days after birth. Prolonged IH episodes lasting at least one minute in the first 2 to 3 months of life were associated with worse outcomes (late death or disability) at 18 months of age. Isolated bradycardic events were not prognostically significant. Interestingly, not all IH patterns are detrimental. Some rodent studies have shown improved long-term spatial learning and memory with brief, mild hypoxic exposures. , The most damaging IH events appear to be those that last longer than 1 minute and are 1 to 20 minutes apart.
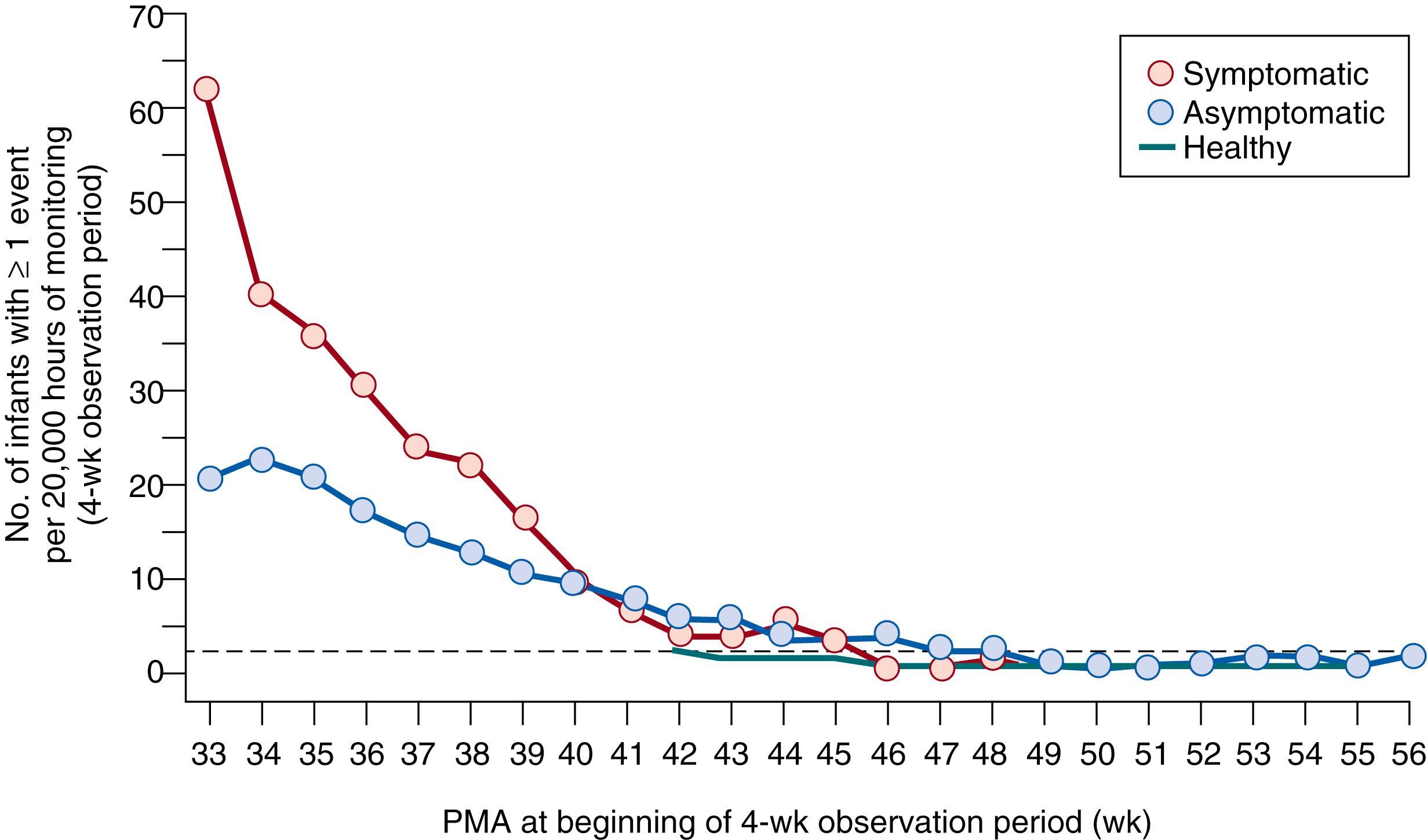
In defining the epidemiology and developmental correlates of apnea, part of the difficulty lies in the numerous definitions of apnea that have been used by various investigators. However, some trends do emerge. Apnea is more frequent in more immature infants, and almost all who weigh less than 1000 g will experience apnea during the neonatal period. Onset of apnea may be as early as day 1 of life in infants without respiratory distress syndrome. By contrast, spontaneously breathing infants with respiratory distress syndrome may show a delay in the peak frequency of apnea. Consistent with this observation, Di Fiore and associates have shown that IH episodes in very preterm infants are infrequent in the first week, followed by a progressive increase over weeks 2 to 4 before decreasing in weeks 6 to 8. Thereafter, both the frequency and duration of apnea and IH events decrease with advancing postnatal age ( Fig. 156.4 ). These observations serve to emphasize the developmental immaturity of respiratory control that underlies infantile apnea, as well as the resolution of this disorder over time. Idiopathic apnea and IH events are thought to be related to prematurity. In rare infants, however, an underlying specific familial neuropathology may be identified. Disorders affecting the brain stem that may manifest with apnea include olivopontocerebellar atrophy, myotonic dystrophy, and syringobulbia, as well as brain stem infarction resulting from asphyxia.
Apnea can recur in premature infants after the neonatal period in response to specific clinical situations in which respiratory drive is altered. Respiratory syncytial viral infection is well known to elicit apnea. These spells may be severe, necessitating endotracheal intubation. The cause of respiratory depression in respiratory syncytial viral infection is unknown; however, inhibitory reflexes from upper airway afferents have been implicated.
Former premature infants also may experience apnea during recovery from anesthesia. This form of apnea most commonly occurs in the first few months of life, particularly when general anesthesia is used during surgery. For this reason, in former preterm infants, cardiorespiratory monitoring during the acute postoperative period is an important part of their care. Routine care of the premature infant before discharge includes several practices that may cause temporary recurrence of apnea. For example, eye examination for retinopathy of prematurity may be associated with cyanosis, apnea, and gastrointestinal side effects. Immunization also has been found to be associated with apnea, bradycardia, and desaturation. With such practices, therefore, infants require continued monitoring until they are stable, but evidence of a subsequent detrimental effect is lacking.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here