Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hypertrophic cardiomyopathy (HCM) is characterized by the presence of left ventricular (LV) hypertrophy in the absence of another cardiac or systemic etiology. It is a genetic condition with an autosomal dominant inheritance, affecting 1 in 500 individuals of the general population. The commonly used threshold for HCM diagnosis is unexplained maximal wall thickness of 15 mm or greater in one or more myocardial segments or 13 mm or greater in the presence of positive family history. In the setting of systemic hypertension, a ratio of septal to posterior wall thickness exceeding 1.5:1 is also strongly suggestive of the diagnosis, reflecting that one of the key features in this condition is the asymmetric nature of the hypertrophy pattern. This chapter reviews the different anatomic and physiologic variants of HCM.
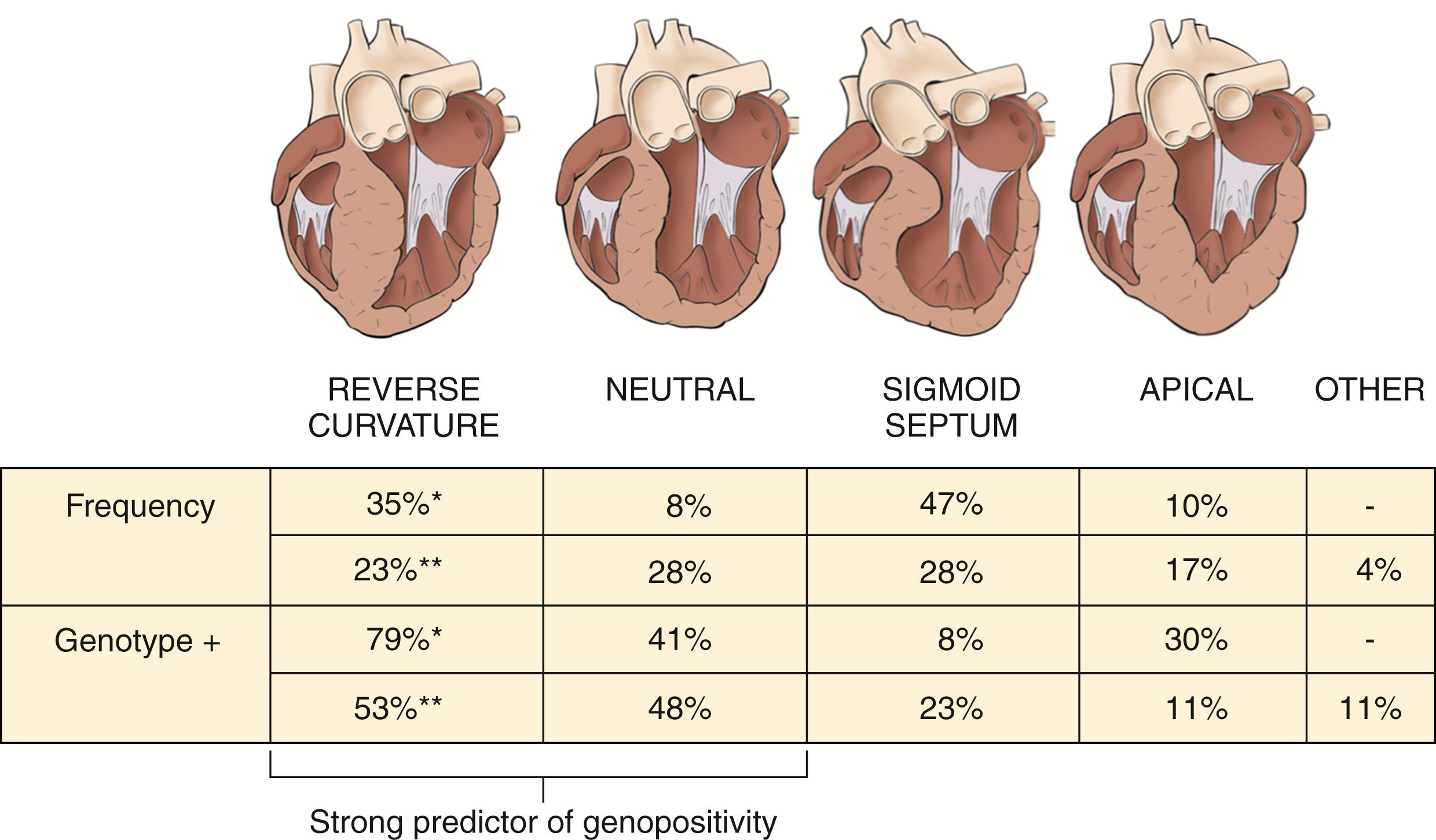
HCM is a heterogeneous condition with multiple anatomic variants, including reverse curvature, a neutral septum, a sigmoid septum, and apical hypertrophy ( Figs. 58.1 and 58.2 ). The reverse curvature morphology involves a predominant midseptal convexity toward the LV cavity, with an overall crescent shape, whereas a sigmoid septum has an overall ovoid shape with a prominent septal bulge, and a neutral septum has a relatively straight shape. Patients with HCM who are diagnosed at a young age are more likely to have the reverse curvature morphology than the sigmoid septum, which is more commonly seen in older adult patients with HCM. Patients in the same family may present with more than one type of anatomic variant; the exact reason for this is unclear. It has also been shown that various anatomic forms of HCM have different genotype-positive rates. Studies from the Mayo Clinic and Toronto showed that 53% to 79% of patients with reverse septal curvature and 41% to 48% of patients with a neutral septal type were found to have an identifiable HCM-causing mutation compared with only 8% to 23% of patients with a sigmoid septum and 11% to 30% of patients with apical HCM ( Fig. 58.3 ). ,

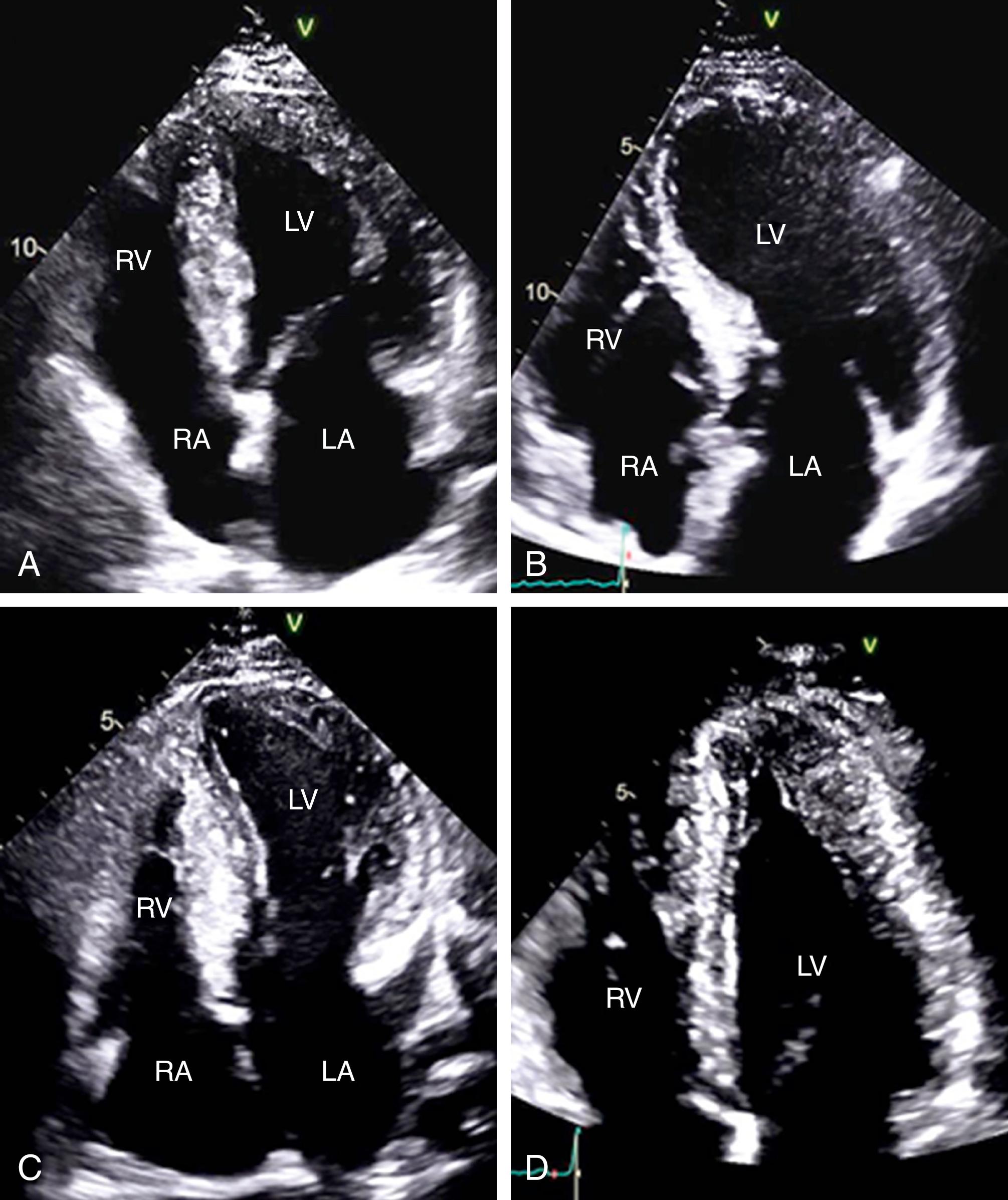
Apart from myocardial hypertrophy, mitral valve and papillary muscles abnormalities have been well described in this condition. Elongation of mitral valve leaflets, asymmetrical enlargement of either leaflet, anterior or apical displacement of the papillary muscles, and direct insertion of the papillary muscle into the anterior mitral valve leaflet are common in this condition. These structural changes can contribute to the degree of left ventricular outflow tract obstruction (LVOTO) and mitral regurgitation (MR) and influence the type and extent of intervention when required.
Diastolic dysfunction, microvascular ischemia, autonomic dysfunction, LVOTO, and MR are the key pathophysiologic mechanisms underlying HCM ( Fig. 58.4 ). Diastolic dysfunction is a main component in all variants of HCM. Myocardial hypertrophy is characterized by increase in muscle mass and decrease in ventricular volume, often combined with different degrees of myocardial fibrosis that cause impaired ventricular relaxation. Patients with HCM have also been shown to have abnormal handling of intracellular calcium, leading to delayed inactivation, which contributes to diastolic dysfunction. Typical echocardiographic findings include reduced E velocity, enhanced A velocity, increased duration and velocity of pulmonary A-wave reversal, and a higher ratio of mitral inflow to annular velocity (E/E′). In addition, patients with HCM have abnormal diastolic myocardial mechanics, with decreased peak early diastolic to peak systolic strain rates, prolonged LV untwisting time, and a lower apical reverse rotation fraction. With time, left atrial remodeling and increased left atrial volume can result in atrial fibrillation and atrial flutter, which can lead to adverse long-term consequences, such as systemic embolization and heart failure symptoms.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here