Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute pulmonary hypertension (aPH), classically referred to as persistent pulmonary hypertension of the newborn (PPHN) when occurring during the perinatal transition period, is characterized by failure of the normal postnatal decline in pulmonary vascular resistance (PVR). Elevated pulmonary arterial pressure (PAP) may also occur via alternative mechanisms, including left ventricular diastolic heart failure and excessive pulmonary blood flow.
Hypoxemia, with or without accompanying systemic hypoperfusion, is the clinical manifestation of several different underlying pathophysiological phenotypes of PH. Right ventricular (RV) myocardial hypoxia and ischemia play an important role in the pathophysiology of circulatory impairment.
Treatment strategies for PH should consider the underlying hemodynamic disturbance(s) and be targeted toward individual pathophysiology. Targeted neonatal echocardiography (TnECHO) is an important assessment tool that enables adjudication of the underlying physiology, targeting of treatment, documentation of response to treatment, and identification of when to wean therapy.
The approach to management should include optimization of respiratory support, care of the underlying condition, and specific hemodynamic therapy with cardiotropic agents and/or pulmonary vasodilators in accordance with the underlying phenotypic presentation of PH.
Neonatal pulmonary hypertension is a heterogenous condition affecting both term and preterm neonates with a range of underlying disease processes, the hallmark of which is elevated pulmonary arterial pressure (PAP). Mean PAP (mPAP) is dependent upon pulmonary vascular resistance (PVR), cardiac output (CO) (and shunts), and left atrial pressure. Calculation of mPAP is possible, using pulmonary capillary wedge pressure (PCWP) as a proxy for left atrial pressure:
The primary pathophysiologic event in aPH occurring during the perinatal transition period, classically referred to as persistent pulmonary hypertension of the newborn (PPHN), is interruption to or failure of the normal postnatal decline in pulmonary vascular resistance (PVR). This may be due to maladaptation (e.g., perinatal asphyxia, meconium aspiration syndrome), maldevelopment of the pulmonary vasculature (e.g., in utero closure of the ductus arteriosus), or pulmonary underdevelopment or hypoplasia in the setting of e.g., congenital diaphragmatic hernia or oligohydramnios. Whatever the underlying etiology, there is associated dysregulation of the pulmonary vascular bed and changes in cardiac loading conditions, with significant hemodynamic consequences. Although PPHN is a commonly used descriptor, aPH may be a more appropriate term for the constellation of features that include hypoxemia and right ventricular dysfunction, due to the protracted time taken for physiological decline in PVR after birth in healthy newborn infants. Persistent elevations in PAP after birth are essentially ubiquitous, and for healthy infants, not pathological. In the setting of aPH, the resultant low oxygen saturation is poorly tolerated after birth and affected neonates may develop multi-organ dysfunction/failure because of hypoxemia and inadequate tissue perfusion. The hemodynamic consequences of aPH ( Chapter 25 ) may include right ventricular dysfunction, dilation and hypertrophy due to increased afterload, and impairments in pulmonary blood flow. Adverse systemic effects include hypoxemia, abnormal left ventricular (LV) mechanics, and low cardiac output. Although generally considered to be a primary disorder of elevated PVR, it is evident from the “mPAP equation” that elevations in cardiac output and/or left atrial pressure may also result in raised pulmonary pressure, with implications for management. Accurate delineation of the pathology requires comprehensive hemodynamic assessment and a detailed understanding of the interplay of the neonatal cardiovascular system with the various underlying disease processes ( Table 27.1 ).
| PH Phenotype | Pathophysiology | Echocardiography Features | Suggested Treatment Approach |
|---|---|---|---|
| Acute PH | |||
| Arterial (classic) | Hypoxic pulmonary vasoconstriction, V/Q mismatch | Dilated and hypertrophied RV, septal flattening in systole, predominantly R → L PDA and atrial shunts, ↑ PAAT:RVET, PA Doppler notching, ↓ LVO | Optimize lung recruitment and supportive care, offload RV with pulmonary vasodilators, support systemic arterial pressure with vasoconstrictors which do not ↑ PVR |
| Flow-mediated | High volume of blood in a circuit with limited capacitance, endothelial dysfunction, oxidative stress | Dilated RV and/or LV, discordant ventricular outputs with either ↑ RVO, ↑ LVO, or both, septal flattening in diastole if ASD/VSD | Shunt management; maintain ↑ PVR, avoid selective pulmonary vasodilators |
| Left heart dysfunction | Poor LV compliance and high LVEDP, resulting in impaired flow through pulmonary circuit | Dilated LV, LV systolic and diastolic dysfunction [↓ MVE, ↑ IVRT ↓ PV velocities], MR and/or AI, ↓ LVO | LV afterload reduction, inotropy, maintain R → L ductal shunt if ↓ SBF, maintain ↑ PVR, diuretics for pulmonary edema |
| Chronic PH | |||
| Arterial (classic), e.g., associated with BPD | Pulmonary vascular remodeling, impaired angiogenesis, alveolar hypoxia/hyperoxia | Dilated, hypertrophied RV, septal flattening in systole, predominantly R → L PDA and atrial shunts, ↑ PAAT:RVET, PA Doppler notching, ↓ LVO | Optimize lung recruitment and supportive care, offload RV with pulmonary vasodilators, consider diuretics if RV dilation |
| Flow-mediated | High volume of blood in a circuit with limited capacitance, interstitial edema, pulmonary vascular remodeling | Dilated RV or LV, discordant ventricular outputs with either ↑ RVO, ↑ LVO, or both, septal flattening in diastole if ASD/VSD | Shunt management; maintain ↑ PVR, avoid selective pulmonary vasodilators |
| Systemic hypertension | High LV afterload causing ↑ LVEDP, LA hypertension, pulmonary venous congestion | Dilated LV, LV diastolic dysfunction (↓ MVE, ↑ IVRT, ↓ PV velocities), MR and/or AI | Antihypertensive therapy; role for ACE inhibitors/ARBs; avoid pulmonary vasodilators |
The clinical manifestations of aPH depend on the degree of elevation of PAP; the presence, direction, and magnitude of shunting through the patent ductus arteriosus (PDA) and/or patent foramen ovale (PFO); right and left ventricular performance and ability to adapt to changes in cardiac loading conditions; and the presence of associated morbidities. The severity of aPH can run the full clinical spectrum, from mild hypoxemia with varying degrees of respiratory distress to severe hypoxemia and cardiopulmonary instability necessitating advanced intensive care support. Hypoxemia in the newborn may result from lung parenchymal and/or cardiovascular pathology, and differentiating the contributors to poor oxygenation clinically can be challenging. Multiple lung and vascular pathologies may also coexist in the same patient. For example, in the setting of aPH secondary to meconium aspiration, atelectasis, intrapulmonary shunting, alveolar edema, and hyperinflation may all be present together with pulmonary vascular remodeling, hypoxic vasoconstriction, and myocardial dysfunction due to perinatal hypoxia-ischemia. Hypoxemia out of proportion to the degree of underlying lung disease and lability in oxygenation are both characteristic of aPH, and presence of either of these clinical features should raise suspicion of a cardiopulmonary vascular component to the infant’s presentation. Approaches to treatment vary according to the severity of the pathophysiology, the underlying cause(s), and the hemodynamic phenotype. Consequently, an in-depth understanding of the pathophysiology ( Figure 27.1 ) is essential to allow individualization of therapy and appropriate targeting of treatment modalities.
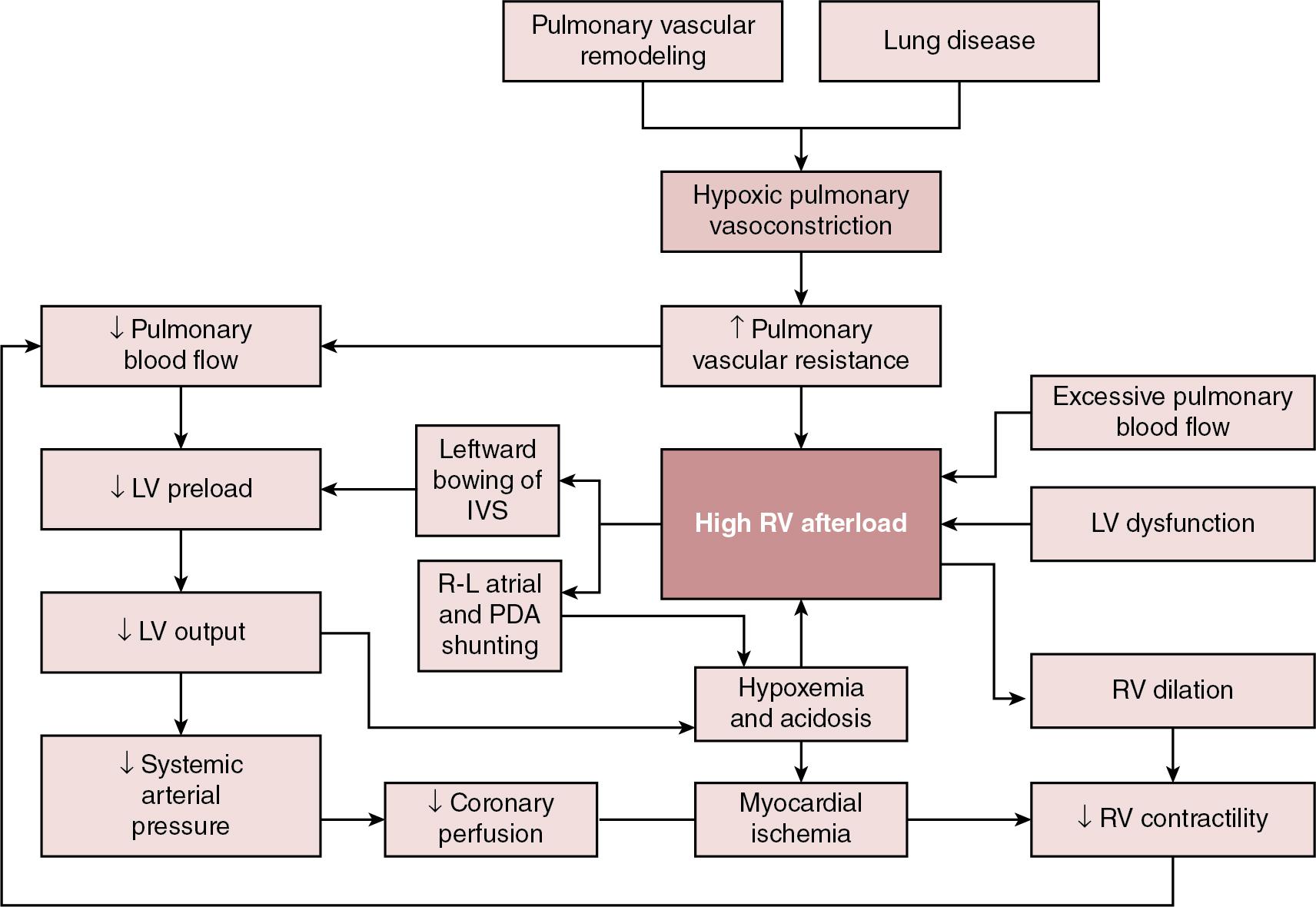
Due to the complexity of interactions between PVR, lung aeration, and myocardial adaptation, perinatal transition is a time of heightened vulnerability for the newborn cardiovascular system. Perturbations in this physiological framework due to any combination of perinatal hypoxia-ischemia, infection, and/or pulmonary parenchymal disease may interfere with the normal physiological decline in PVR and result in development of aPH. As a result of the elevated PVR in arterial (classic) aPH, hypoxic vasoconstriction ensues, which is an adaptive response to optimize ventilation-perfusion (V/Q) matching. Poor lung recruitment and overdistension from positive pressure ventilation can worsen hypoxic vasoconstriction, due to both increases in PVR and impaired carbon dioxide clearance, resulting in acidosis. It is important to realize that PVR is lowest at optimal lung recruitment, that is, at or near FRC. With excessive lung volume, some pulmonary capillaries undergo narrowing and stretching, whereas reduced lung volume can lead to capillary tortuosity or kinking, both of which increase PVR. Careful titration of positive pressure ventilation based on clinical, blood gas, and radiological assessment is therefore necessary. Particular care should be taken to avoid lung overinflation in the setting of hypoxic-ischemic encephalopathy, where lung compliance and FRC may be normal and hypoxemia instead a manifestation of altered pulmonary vascular reactivity. Overly aggressive attempts at lung recruitment in this context may be complicated by further impairments in PBF, as well as systemic compromise from reductions in LV stroke volume and LV end-diastolic volume (LVEDV). Over-distension also has the potential to compromise pulmonary venous flow, which may be difficult to distinguish clinically from progression of aPH driven by high PVR. Additional non-pulmonary factors related to either the underlying disease process, such as sepsis, perinatal asphyxia, and hypovolemia, or iatrogenesis, such as treatment with vasopressors that can cause pulmonary vasoconstriction (e.g., high-dose dopamine), may further drive increases in PVR. Elevated PVR leads to increased RV afterload and bidirectional or right-to-left shunting through the PFO and PDA. Right-to-left atrial shunting contributes to a reduction in RV preload. Right-to-left ductal shunting, though contributing to hypoxemia and reduced PBF, may provide a pop-off mechanism for the RV exposed to high afterload and an additional source of post-ductal perfusion where there is significant LV impairment.
Elevated pulmonary pressure results in a reduction in the pressure gradient between the RV cavity and the pulmonary vascular bed. The initial increase in RV afterload results in compensatory RV hypertrophy and increased contractility (referred to as “coupling” or homeometric adaptation). Increased contractility is mediated by changes in the sarcomere length-tension relationship and cardiomyocyte force-frequency relationship and increased calcium sensitivity. The force-frequency relationship describes the adaptive response of the neonatal myocardium to tachycardia, leading to improved force generation with increasing heart rate. To maintain cardiac output in the face of increased afterload, the RV dilates and heart rate increases. These changes preserve stroke volume initially by way of the increase in stroke volume associated with increased preload – via the Frank-Starling mechanism, with a larger ventricle emptying proportionally less blood – and an intact force-frequency response. This process is referred to as heterotopic adaptation. Unable to generate sufficient pressure for complete ejection of blood with each cardiac beat, RV emptying becomes progressively inadequate, leading to an increase in RV end-systolic pressure and, because less blood is ejected during systole, an increase in RV end-diastolic pressure. In addition, the force-frequency response is blunted as cardiac dysfunction develops. Progressive dilatation increases wall stress and myocardial oxygen consumption and eventually leads to a reduction in stroke volume. RV dilatation also changes the configuration of the cardiac chamber into one that is more spherical, which is associated with functional regurgitation across the tricuspid valve and increased right atrial pressure. Where elevation of PVR is excessive and sustained, ischemia, hypoxemia, and myocardial stretching lead to progressive RV dysfunction and uncoupling. , This is associated with leftward bowing of the interventricular septum (IVS), which is caused by prolonged contraction of the RV free wall when compared with the septum or LV free wall, impairing diastolic LV filling. Together with RV dilation, this leads to further impairment of RV function and decreased PBF. Through ventricular interdependence, the leftward shift in septal configuration also reduces LVEDV, LV stroke volume, and RV systolic contractile force, , resulting in systemic hypoperfusion, acidosis, and further reductions in PBF. In some infants a cycle of reduced PBF, worsening acidosis, hypoxemia, ventilation-perfusion mismatch, and cardiac dysfunction may develop, with a clinical phenotype of hypoxemic respiratory failure and cardiocirculatory impairment.
Increased pulmonary blood flow, such as that observed in left-to-right shunt lesions, may result in elevations in pulmonary arterial pressure. The most obvious mechanism for this is an increase in pressure driven by increased flow through a circuit with a finite capacity to expand to accommodate the additional circulating volume. Muscular hypertrophy of the pulmonary vasculature, with secondary elevation in PVR, also occurs in response to a sustained increase in PBF. , An additional contributor is a disturbance of endothelial function resulting in altered pulmonary vasoreactivity; specifically, selective impairment of endothelium-dependent pulmonary vasodilation. Based on animal models, this may be attributable to both high basal activity of nitric oxide (NO) that is not readily increased and dysregulation of endothelial NO synthase, induced by increased pulmonary blood flow and pulmonary hypertension. This attenuates the fetal endothelial release of NO in response to sheer stress such as that generated by excessive pulmonary blood flow and may contribute to elevated mPAP in this setting.
Newborns with large preductal systemic to venous connections resulting in high-volume shunts are particularly at risk of complications from right heart pressure and volume loading. For example, the vein of Galen aneurysmal malformation (VGAM) is a rare cerebral arteriovenous malformation (AVM) with major hemodynamic implications. In utero, the low resistance of a cerebral AVM is balanced by the low-resistance placental circuit. With removal of the placenta and transition to the high afterload systemic neonatal circulation, up to 70% of the cardiac output is directed toward the cerebral vasculature. Superior vena caval flow may be up to 10 times normal, reflecting high flow through the AVM. This may result in a profound reduction in lower body perfusion, with lactic acidosis and post-ductal arterial hypotension. The ventricles receive increased preload, which in the case of the RV must be ejected against increased afterload, predisposing to RV dysfunction and failure. Supra-systemic pulmonary hypertension occurs, which is associated with right to left shunting at atrial and ductal levels. , This perpetuates hypoxemia, which further compromises ventricular performance. Chronic volume and pressure loading leads to RV dilation, which increases susceptibility to afterload-mediated dysfunction as adaptive mechanisms fail. RV coronary perfusion normally occurs throughout systole and diastole and is characterized by a lower flow and oxygen extraction when compared with the LV. The maladaptive, dilated, and hypertrophied RV has a higher oxygen requirement and inefficient oxygen utilization, with coronary perfusion limited to diastole by the increased RV pressure. Elevations in RV end-diastolic pressure, leading to increased right atrial and central venous pressure, and therefore reduced diastolic coronary flow to the RV constitute a potential additional source of impaired perfusion in the setting of PH. Intracranial vascular “steal” through the AVM, which is associated with retrograde diastolic descending aortic flow, may further compromise tenuous coronary perfusion via low coronary root pressure, creating a “pseudocoarctation” physiology. This effect is additive with the increased ventricular pressure in reducing subendocardial perfusion, contributing to myocardial ischemia and RV dysfunction. Non-judicious use of pulmonary vasodilators may exacerbate these effects, particularly in the setting of an open ductus. Despite the increase in PVR, the shunt in VGAM is obligatory, such that pulmonary vasoconstriction does not offset the high-output cardiac failure, as might occur in congenital cardiac disease. This is postulated as a contributor to the severity of cardiac failure in infants with VGAM.
Poor function of the left ventricle results in inefficient ejection of end-diastolic volume, which results in a progressive increase in left ventricular end-diastolic pressure (LVEDP). This leads to pulmonary venous congestion, increased pulmonary capillary pressure, and a hydrostatic gradient across the interstitium, resulting in fluid transudation to alveolar spaces, i.e., pulmonary edema. Similar to left-to-right shunt lesions, endothelial dysfunction is also implicated as contributory to elevated pulmonary pressure in obstructive pulmonary venous hypertension. Left and right ventricular dysfunction may coexist in transitional aPH, although there are subgroups in whom LV disease may occur independently. Fetal and neonatal echocardiographic studies of infants with congenital diaphragmatic hernia (CDH) support disturbances of fetal LV development and postnatal LV dysfunction as part of the pathogenesis of hypoxemia in affected infants. Early LV systolic function correlates with markers of clinical disease severity in CDH and may be a primary determinant of illness severity rather than a secondary consequence of cardiovascular instability. These findings have implications for treatment, and use of pulmonary vasodilators, since increasing pulmonary blood flow and LV preload may risk exacerbating existing LV diastolic dysfunction and pulmonary venous hypertension. , Flash pulmonary edema has been observed with pulmonary vasodilator therapy in pulmonary venous disease. Conversely, improvements in RV performance by afterload reduction may indirectly augment LV performance through ventricular interdependence. A multicenter randomized trial of sildenafil and inhaled NO in CDH (CoDiNOS trial) utilizing cardiac function assessment may provide additional guidance on use of pulmonary vasodilators in this setting. In general, pulmonary vasodilators should be used with caution in infants with LV dysfunction and are likely to be harmful where LV impairment is severe.
The pathophysiology of cPH ( Chapter 26 ) is influenced by the development of the pulmonary vasculature and the status of the lung parenchyma. Maldeveloped pulmonary vasculature with abnormal lung parenchyma and elevated PVR is typical of BPD and pulmonary hypoplasia. Maldeveloped pulmonary vasculature is less commonly encountered and is observed in, for example, alveolar capillary dysplasia (ACD) and primary surfactant deficiencies. ACD with misaligned pulmonary veins may, however, account for a greater proportion of unexplained PH in infants than previously thought. Chronic PH secondary to pulmonary venous congestion may be due to either increased PBF (e.g., left-to-right shunts), or increased pulmonary capillary wedge pressure). Elevated PCWP, or left atrial pressure, in the newborn most commonly occurs due to LV dysfunction or acquired pulmonary vein stenosis, although left-sided cardiac lesions may also produce this cPH phenotype.
Chronic PH associated with BPD (BPD-cPH) is characterized by abnormal remodeling and growth arrest of the pulmonary vasculature, , and impaired angiogenesis and alveolarization, , resulting in abnormal pulmonary vascular function with increased PVR, high RV afterload, and RV dysfunction. Abnormal pulmonary vascular remodeling is triggered by postnatal hypoxia/hyperoxia. Additive insults, such as endothelial dysfunction, and inflammatory stimuli, such as infection and ventilator-induced lung injury, are thought to perpetuate alveolar hypoxia , , in at-risk infants. Antenatal factors, including inflammation, intrauterine growth restriction, and the lung microbiome, are also implicated in the pathogenesis. There is evidence that echocardiographic evidence of PH within the first 7–14 days after birth may predict subsequent moderate-severe BPD or death at 36 weeks postmenstrual age, , underscoring the importance of abnormal pulmonary vascular development in the development of BPD. Conversely, a very low risk of late PH has been observed in the absence of PH at 7 days of age. The approach to management includes lung optimization alongside treatment with pulmonary vasodilators (e.g., sildenafil). Both clinical improvement and reductions in pulmonary artery pressure have been documented with sildenafil therapy. In the presence of RV dilatation, diuretics may also be considered for optimizing RV configuration. Although long-term benefits of diuretics have not been demonstrated in infants with BPD, improvements in both oxygenation and pulmonary mechanics have been observed. In addition, a retrospective study demonstrated high rates of symptomatic improvement in BPD-cPH, with diuretic treatment where RV dilatation was used as the treatment threshold.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here