Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver transplantation is used worldwide to treat a broad spectrum of end-stage liver diseases. Nonalcoholic fatty liver disease–induced cirrhosis, alcohol-induced liver disease, and hepatitis C virus (HCV) infection are the leading indications in North America, Europe, and South America. In Asia, hepatitis B virus (HBV)–induced cirrhosis is responsible for most liver transplantation, followed by the previously listed causes. Recurrence of the original disease is common after transplantation, and liver allografts are susceptible to a variety of technical complications that cause dysfunction and produce morphological manifestations.
This chapter focuses on aspects of common diseases in the transplantation setting and on conditions unique to allografts, such as infection, rejection, small-for-size syndrome, preservation/reperfusion injury, and minimization of immunosuppression. We will also introduce the reader to machine perfusion for extended-criteria donors and suboptimal organs, and current advances in immune-tolerance and weaning protocols. Most disorders are discussed in the order in which specimens are received, from donor and then recipient, after transplantation. The terminology and acronyms used in liver transplantation are listed in Table 53.1 . The material in this chapter is based on multiple previous publications and book chapters from our group. Therefore redundancy is difficult to avoid.
| Acronym | Definition |
|---|---|
| ACR | Acute cellular rejection |
| AHR | Acute humoral rejection |
| AMR | Antibody-mediated rejection |
| ASTS | American Society of Transplant Surgeons |
| CIT | Cold ischemia time |
| DAA | Direct-acting antiviral |
| DCD | Donation after cardiac death |
| DDLT | Deceased donor liver transplantation |
| DSA | Donor-specific antibodies |
| ECD | Extended-criteria donors |
| FCH | Fibrosing cholestatic hepatitis |
| FDA | Food and Drug Administration |
| GVHD | Graft-versus-host disease |
| HAT | Hepatic artery thrombosis |
| HSC(T) | Hematopoietic stem cell (transplantation) |
| IPTH | Idiopathic posttransplantation hepatitis |
| IS | Immunosuppression |
| LDLT | Living donor liver transplantation |
| NRH | Nodular regenerative hyperplasia |
| PHP | Portal hyperperfusion |
| PTLD | Posttransplantation lymphoproliferative disorder |
| SFSS | Small-for-size (graft) syndrome |
| SOS | Sinusoidal obstruction syndrome |
| TCMR | T cell–mediated rejection |
| VOD | Venoocclusive disease |
Gross and frozen-section examination can assist in evaluation of nonideal or extended criteria donors (ECDs) as defined by various characteristics. Included are advanced donor age (>60 years), large-droplet macrovesicular steatosis (>40%), cold ischemia time (>12 hours), partial liver allografts, donation after cardiac death (DCD), hemodynamic instability, use of vasopressors, hypernatremia, HBV or HCV infection or hepatitis B core antibody (anti-HBc) positivity, history of cancer, or finding of a liver mass, fibrosis, or other focal lesions.
Feng and colleagues introduced the concept of a donor risk score based on a study of more than 20,000 transplants; the scores inversely correlate with 1- and 3-year recipient survival times. The overall score is the sum of component scores for the following parameters: donor age > 60 years, anoxic and cerebrovascular causes of death, black race, short height, DCD, split or partial grafts, regional or national sharing, and cold ischemic time.
Evaluation of donor livers by a pathologist is most often requested because of the gross appearance, texture, or color of the donor liver, known pre-existing donor disease (e.g., HCV infection), and the clinical history or circumstances of the donor’s death or harvesting procedure. The opioid crisis has greatly expanded the donor pool. Goldberg et al. analyzed the Organ Procurement and Transplantation Network (OPTN) data and showed that the largest relative growth in deceased organs comes from donors suffering from drug overdose. This subset of donors brings in unique challenges and perceptions of increased disease transmission risk (e.g., HCV, HIV). In our experience, the circumstances surrounding their death and hypotension results in organ hypoperfusion and variable ischemic changes involving abdominal organs, especially watershed areas such as the terminal ileum and cecum. Patients and doctors alike may be hesitant to use these organs because of perceptions of increased risk of infection transmission (e.g., HCV, HIV), which are usually covered by donor screening serology and ischemic damage. In reality, these donors tend to be younger and more likely to be Caucasian when compared with donors who die from stroke and cardiovascular disease, and livers donated by those who die from drug overdose are associated with longer graft survival when compared with donors who die from cardiovascular disease. Patients and physicians must weigh the benefits of utilizing these organs against the risk of dying or severe morbidity while on a waitlist. Intraoperative evaluation of organs for evidence of ischemia and necrosis helps in the decision-making process.
Considering the importance of gross examination, the tissue for frozen-section evaluation should be fresh, preferably obtained in the presence of a pathologist, who should also inspect the organ to ensure that the tissue sample represents the liver as a whole. Three tissue samples should be collected if the gross appearance is uniform: two 2.0-cm, 16-gauge needle cores, one each from the right and left lobes, and one 2.0-cm subcapsular right lobe wedge biopsy. The wounds are closed by sutures. Core biopsies are useful for staging the degree of fibrosis, and wedge biopsies are used to evaluate arterial or arteriolar disease, steatosis, and extent of hepatocellular necrosis if present.
A few suggestions can help pathologists avoid introduction of artifacts during sample preparation. Fresh liver tissue should be immediately transported to the frozen-section room on a paper towel moistened with preservation solution or in a plastic specimen container. Storage in physiological saline, air drying, and placement of the tissue sample on an absorbent substrate should be avoided. Air-drying and storage in physiological saline can cause hepatocytes to appear shrunken or necrotic, which can lead to overestimation of ischemic injury. Absorbent substrates also blot fat out of the tissue, resulting in underestimation of the extent of fatty infiltration.
Difficulty cutting the frozen section should alert the pathologist to the possibility of a steatotic donor liver, and fragmentation of sections should also alert the pathologist to the possibility of advanced fibrosis. Recognition of hepatocytes in various stages of injury or necroapoptosis caused by ischemic damage can be enhanced by staining several sections with eosin for increasing lengths of time. This enhances the contrast between viable and damaged or nonviable hepatocytes; the latter are hypereosinophilic and often show early nuclear karyorrhexis.
Histopathological findings should be correlated with the donor’s history and laboratory values. Because partial or fragmented clinical histories can be misleading, the pathologist should aggressively request additional information if the biopsy findings do not correlate with the known history or histopathological findings. However, donor evaluation by biopsy is only one laboratory test. Pathologists are unable to predict the adequacy of organ function after transplantation based solely on the absence of significant histopathological findings on frozen-section evaluation of the donor organ.
Generally, organs are disqualified for transplantation if the donor is positive for certain serologically confirmed infections (e.g., human immunodeficiency virus [HIV], rabies) or has had a history of malignancy with a high transmission risk. , Biopsy findings that usually disqualify organs include diffuse necrosis involving more than 10% of hepatocytes, severe large-droplet macrovesicular steatosis involving 50% or more hepatocytes, moderate or severe atherosclerosis of intrahepatic artery branches, and definite evidence of bridging fibrosis. Large-droplet macrovesicular steatosis is defined as a single fat droplet that distends the involved hepatocyte to a size that is obviously larger than adjacent nonsteatotic hepatocytes. In our experience, polarized light microscopy at the time of frozen-section examination offers a rapid, easy, and accurate estimate of liver fibrosis without the need for a trichrome stain.
Some ECD factors that may have histopathological manifestations are advanced donor age (>60 years), large-droplet macrovesicular steatosis (>40%), HCV infection, cardiovascular instability or ischemic injury, and occasionally DCD. Other ECD factors are not reliably associated with specific histopathological findings and do not justify biopsy evaluation: black race, short stature, cerebrovascular cause of death, hypernatremia (>155 mEq/L), cold ischemia time exceeding 12 hours, and partial-liver allografts.
DCDs represent approximately 4% to 5% of the total donor pool, but their use is fraught with potential pitfalls. Reich and colleagues reviewed the American Society of Transplant Surgeons (ASTS) best practice guidelines. DCD livers are exposed to significant warm ischemia, which optimally should be limited to less than 20 minutes. Even under ideal circumstances, DCDs are still susceptible to ischemic cholangiopathy that can develop several weeks to months after transplantation. It is thought that suboptimal flushing of the peribiliary capillary plexus leads to microvascular thrombosis and subsequently to poor reperfusion and ischemic injury to the biliary tree. Various extracorporeal machine perfusion approaches have the potential to change this outlook. ,
A grossly fatty appearance of the organ is the most common reason for requesting frozen-section evaluation of a cadaveric donor liver ( Fig. 53.1 ). Experienced donor surgeons are usually able to accurately estimate the severity of steatosis before biopsy evaluation, except for donors with small-droplet macrovesicular steatosis. Digital imaging photosharing to estimate steatosis can be hampered by lighting conditions in operating rooms that significantly influence the gross appearance of donor livers and can lead to inaccurate estimation of the degree of steatosis.
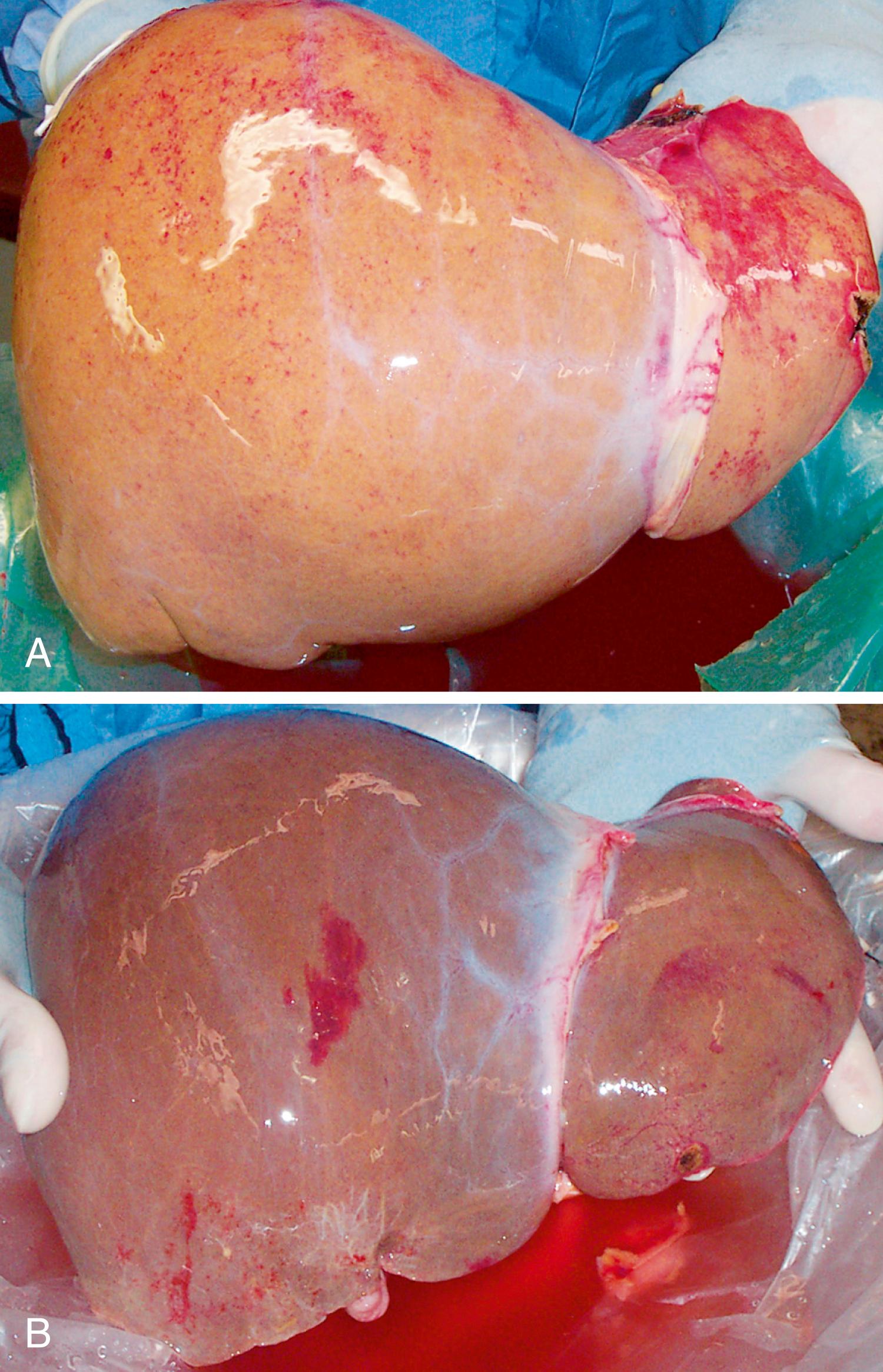
Large vacuolar macrovesicular steatosis is now defined as fat globules that distend the involved hepatocytes to a size that is larger than typical nonsteatotic nearby hepatocytes with peripheral nuclear displacement. Small-droplet steatosis (previously known as microvesicular steatosis ) is defined as all non–large-droplet steatosis. Moderate large-droplet steatosis (>30%) increases susceptibility to preservation/reperfusion injury, impairs regeneration, and is associated with decreased graft survival. , , Small-droplet steatosis is often found after a short period of warm ischemia or other insults and usually does not adversely affect outcome. One study, however, associated high-grade, small vacuolar steatosis with delayed graft function. In our opinion, the severity of large-droplet steatosis can be roughly estimated on hematoxylin and eosin (H&E)–stained slides alone. Fat stains are unnecessary.
Most studies confirm the reproducibility of identifying donor macrovesicular steatosis of 50% or more of affected hepatocytes by frozen-section evaluation before transplantation, , but reproducibility is decreased at the lower cutoff value of 30%. , One study recommended that pathological evaluation be abandoned because of poor intrapathologist and interpathologist reproducibility for microvesicular and macrovesicular steatosis, inflammation, and hepatocyte ballooning and because of poor correlation between pathologists and computerized morphometric fat assessment. Use of guideline images as a tool to improve assessment of hepatic steatosis can be very useful, particularly in centers with low volumes. Other modalities used to assess steatosis include clinical and biochemical parameters and hepatic computed tomography (CT) in conjunction with other noninvasive clinical data, and magnetic resonance imaging (MRI).
The use of donor livers with >30% large-droplet steatosis is controversial and varies among transplantation centers. The objective measure for assessing risk (Donor Risk Index) incorporates multiple variables such as donor age, DCD, split grafts, and ischemia time, but not steatosis. The extent of large-droplet macrovesicular steatosis might even be more important than DRI in evaluating outcomes. Most disqualify donor livers when large-droplet steatosis exceeds an estimate of 50% ( Fig. 53.2 ) because it has been reliably associated with an increased risk of early graft dysfunction and failure. , , , , This practice, however, has been questioned, , , particularly if other risk factors (e.g., cold ischemia time) or complications are absent or have been mitigated and with careful selection of recipients (donor recipient algorithm).
Utilizing data from United Network for Organ Sharing (UNOS) and the European Liver Transplant Registry databases, Dutkowski and colleagues analyzed outcome of liver transplants with biopsy proven steatosis utilizing the Balance of Risk (BAR) stratification. The BAR score ranges from 0 to 27 and factors in donor and recipient data to define three risk groups; low morbidity/mortality group, increased morbidity but low mortality group, and high mortality/morbidity group. They showed that use of allografts with >30% macrovesicular steatosis is only acceptable in the low morbidity/mortality group. This approach probably explains the acceptable outcomes of utilizing severely steatotic livers at some centers. Importantly, methods of estimating steatosis in various studies are not necessarily similar.
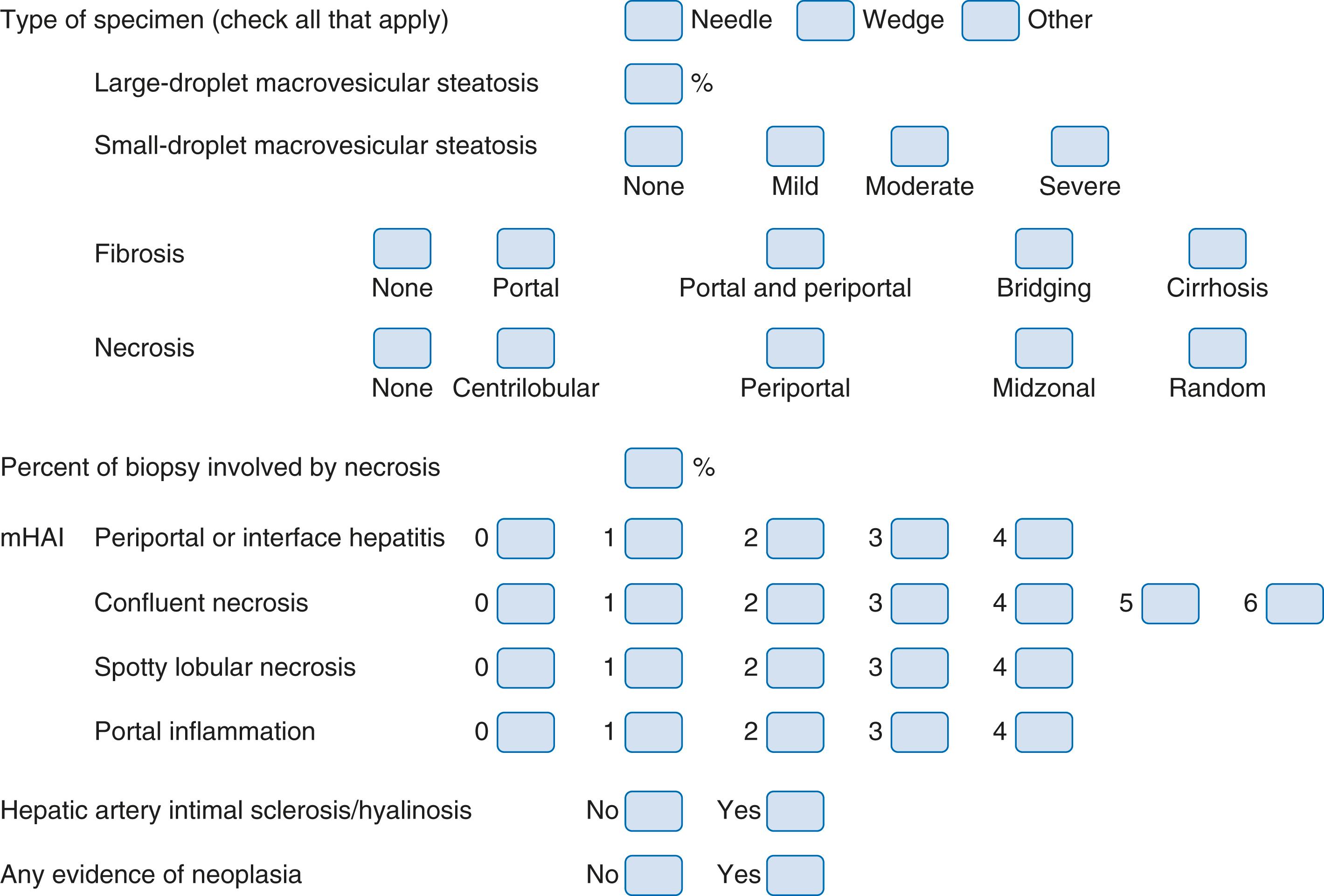
Some studies have applied an evenly distributed range for scoring large-droplet macrovesicular steatosis (mild <30%, moderate = 30% to 60%, and severe >60%), but we and others , use a scale that more closely reflects the triage algorithm for ECD at our center. Mild donor large-droplet macrovesicular steatosis (<10%) does not influence the decision-making process; moderate large-droplet macrovesicular steatosis (10% to 30%) is typically used for transplantation, but other criteria (e.g., ECD characteristics) are also taken into consideration. Livers with more than 30% (severe) large-droplet steatosis are used only in special circumstances, such as when the cold ischemic time is kept to a minimum (<9 hours) and there are few or no other ECD risk factors. Outcomes in these situations are comparable to those for nonsteatotic donor livers. , A sample donor liver evaluation form from our center is shown in Fig. 53.3 .
Necrosis in donor biopsies has negatively affected recipient outcomes in some , but not all studies. , An algorithmic and reproducible method of quantifying the necrosis has not been defined, which may account for differences in observations. In our experience, the liver is usually disqualified if more than 10% of nonsubcapsular hepatocytes are necrotic, and the necrosis diffusely involves the wedge and needle cores. However, assessment should not be based on necrosis limited only to subcapsular areas because this finding is quite common, especially when the harvesting operation is associated with vigorous manipulation. Correlation with serial preharvest donor serum liver injury test profiles can be used as an additional gauge of the extent of necrosis. A rising transaminase trend at the time of harvesting should trigger additional caution.
Many centers use mildly diseased HCV-positive donors to prolong the life of an HCV-positive recipient with end-stage HCV-induced liver failure and in HCV-negative patients with fulminant hepatic failure. , Graft and patient outcomes have been minimally affected by donor HCV status, , but more rapid progression of fibrosis can occur. All HCV-positive donors at our institution are subjected to frozen-section biopsy analysis. Those with nonbridging fibrosis (<3 of 6 on the Ishak scale) are offered to potential recipients after informed consent is obtained. Other groups report a fibrosis cutoff value of one lower stage (<2 of 6). Efforts to expand the donor pool by transplanting HCV-infected donor organs to uninfected recipients (HCV-mismatched transplantation) is being investigated and is gaining acceptance by some programs. This is in part caused by long organ waiting lists, the presence of effective direct-acting antiviral (DAA) therapy, and possibly cost-effectiveness, particularly for kidney recipients. , Although early results are encouraging, few data are available on liver transplantation from infected donors to uninfected recipients. Further studies and trials are underway. Further discussion of use of HCV-infected organs is available here.
Anti–HBc-positive donors can transmit HBV to naïve and unvaccinated recipients. The risk is lower in vaccinated and anti–HBc-positive recipients and can be further reduced by anti-HBV medications and passive antibodies. Donor biopsy evaluation usually is not helpful in this circumstance because most biopsies are normal in the absence of other diseases.
Neoplastic, infectious, and metabolic diseases have been inadvertently transferred from donors to recipients. Examples that may be detectable by pathologists include various cancers, amyloidosis (often subtle histopathological manifestations), hemochromatosis, and fungal, viral, and parasitic diseases. Metabolic diseases such as familial amyloid polyneuropathy, oxalosis, and possibly α 1 -antitrypsin deficiency can be transferred with the donor organ in so-called domino transplants. The rationalization for this approach is the expectation that the latency period between transplantation and onset of disease in the recipient constitutes a gain in life span (years to decades). Post hoc analysis of donor data for recipients with unexpected complications may provide valuable insight.
Efforts to expand the donor pool by using ECD, DCD, marginal, and severely steatotic livers led to advances in liver preservation techniques. Dynamic machine perfusion is the latest in organ protective/preservation strategies, promising to decrease preservation/reperfusion injury, extend organ viability, and promote regeneration. Techniques at various temperatures (hypothermic oxygenated machine perfusion, subnormothermic machine perfusion, and normothermic machine perfusion) for preservation of abdominal organs have been developed and have been well reviewed by Weissenbacher et al. and Linares et al. , Normothermic machine perfusion, however, is the technique that has attracted the most attention. A recent randomized trial of normothermic preservation in liver transplantation showed a 50% lower rate of organ discard, lower graft injury as assessed by hepatocellular enzymes, and no significant difference in bile duct complications.
Living donor liver operations account for approximately 5% of all livers transplanted in North America and Europe but represent most transplantations performed in Asia. According to OPTN data, the number of these operations has been steadily growing in the United States with 401 living donor liver transplantations out of 8250 total liver transplants in 2018. Living donor liver transplantation can address donor organ shortage and decrease wait times. In experienced centers, this has been reported to be associated with better long-term outcome and decreased resource utilization. , Because major liver resection is risky (mortality rate of approximately 2 deaths per 700 patients), most centers routinely subject potential living donors to a rigorous screening protocol. Liver biopsy evaluation is included at some centers to further minimize donor risk, but the popularity of this practice is decreasing.
A thorough stepwise medical and surgical donor evaluation screen is performed for any major medical diseases, obesity, previous major abdominal surgery, anatomical compatibility between the donor and recipient, infectious diseases that could be transmitted to the recipient, psychosocial issues, and any liver function abnormality or disease that may put the donor at risk. , Abnormalities detected during the workup can disqualify a potential donor, signal the need for a liver biopsy, or require further observation and follow-up evaluation (e.g., weight loss in the potential donor).
Several groups have reported histopathological findings for likely living donors. Most are normal or show mild steatosis, but 20% to 50% show mostly minimal or mild abnormalities. Large-droplet macrovesicular steatosis of varying severity is identified in 14% to 53% of potential donors and is the most common reason for donor disqualification. Disqualification rates based on biopsy findings alone vary from 3% to 21%. Most programs try to limit the severity of macrovesicular steatosis in living donors to less than 30% because this level does not adversely affect the postoperative course of the donor or recipient. Candidates with biopsies showing more than 30% of macrovesicular steatosis undergo diet modification and other treatments to reduce hepatic steatosis. Some programs limit macrovesicular steatosis to less than 10% or 20%. ,
Other biopsy findings include low-grade chronic hepatitis of undetermined origin, nonnecrotizing granulomas, and a variety of unexpected findings (e.g., early-stage primary biliary cholangitis [PBC]). Mild (1+ to 2+ on a scale of 0 to 4+) periportal hepatocellular iron deposits occur in approximately 17% of mostly male potential donors. Hepatocyte iron deposits probably represent a normal finding in men that does not preclude transplantation. Unexplained portal tract eosinophilia may be seen, and in two cases, it did not adversely affect the postoperative clinical course of the donor or recipient. A frozen-section examination initiated by the surgeon because of a black-appearing liver usually correlates with the presence of hepatocellular lipofuscin pigmentation; this finding alone should not deter the surgeon from moving forward with the transplant.
Accurate biopsy interpretation requires familiarity with the type of donor and operation because many causes of dysfunction are attributable to agonal events in the donor and to complications during harvesting and implantation of the donor organ. In Western countries, orthotopic liver transplantation with a whole cadaveric donor liver is the most common procedure. The native liver is replaced in an anatomically correct fashion after resection of the donor gallbladder. End-to-end anastomoses connect the recipient and donor portal vein, hepatic artery, bile duct (except for those with primary sclerosing cholangitis [PSC]), and vena cava. Donor and recipient are usually matched for size and ABO blood group, unless the recipient is critically ill, or the donor pool is limited by blood type.
Operative variations include the use of live donors, split livers (single, usually adult liver divided into two portions for two recipients), Roux-en-Y hepaticojejunostomy biliary reconstruction, and alternate vena caval anastomoses. , For example, living donor operations are common in Asia. Because the operative approach can lead to complications that cause graft dysfunction, it is important for the pathologist to understand the histopathological effects of transplantation and the methods used for all vascular and biliary anastomoses.
Regardless of the type of donor or operative approach, technically demanding operations that deviate from reconstruction of normal anatomy increase the risk of complications (e.g., biliary tract issues, suboptimal venous drainage). Reduced-size liver (splits and living donor) transplantations increase the risk of both vascular and biliary tract complications. , Accurate biopsy interpretation requires an understanding of the characteristic periods during which certain causes of allograft dysfunction occur ( Table 53.2 ). The pathologist should be aware of the original disease, time from transplantation, type of graft (whole liver or reduced-size graft), and liver injury test profile, which often provides enough information to generate a reasonably accurate diagnosis, even without biopsy evaluation. Because liver injury often has more than one cause, all clinical parameters should be explored before the final histopathological diagnosis is given.
| Syndrome | Clinical Associations and Observations | Peak Period |
|---|---|---|
| Preservation/reperfusion injury | Long cold (>12 hours) or warm (>120 minutes) ischemic time; older donor age (>60 years), hemodynamically unstable, DCD, repeat anastomosis; poor bile production; prolonged cholestatic phase predisposes to biliary sludge syndrome | Recognized primarily in postreperfusion biopsies and biopsies obtained during the first few weeks after OLT; changes may persist for several months, depending on severity of the initial injury |
| Early acute antibody-mediated rejection | ABO-incompatible donor; high-titer (>1:32) lymphocytotoxic crossmatch DSAs; persistently low platelet counts and complement levels during first several weeks after transplantation | First several weeks to months after transplantation; later onset with de novo DSA can occur, usually with less pronounced features |
| T cell–mediated rejection | Younger, healthier female and inadequately immunosuppressed recipients, long cold ischemic times, and disorders of dysregulated immunity (e.g., PSC, AIH, PBC) | Peak depends on IS regimen; usually 3-40 days; later onset usually associated with inadequate IS |
| Chronic rejection | Usually occurs in inadequately immunosuppressed patients (e.g., infections, tumors, PTLD); patients have a history of moderate or severe or persistent TCMR episodes or are noncompliant | Bimodal distribution; early peak during first year and later increase in noncompliant and inadequately immunosuppressed patients |
| Hepatic artery thrombosis | Suboptimal anastomosis; pediatric or small-caliber vessels; donor and/or recipient atherosclerosis; suboptimal or difficult arterial anastomosis; large difference in vessel caliber across anastomosis; hypercoagulopathy; suboptimal arterial flow (vasospasm caused by small-for-size syndrome) | Bimodal distribution; early peak at 0-4 weeks and later peak at 18-36 months |
| Biliary tract obstruction or stricturing | Arterial insufficiency or thrombosis; long cold ischemia, DCD, difficult biliary anastomosis; AMR; original disease of PSC | Varies, but timing can be used to determine cause: <6 months, usually mechanical, preservation/reperfusion injury (ischemic cholangiopathy), or AMR; >6 months, recurrent disease or mechanical |
| Venous outflow obstruction | Difficult piggyback hepatic vein reconstruction; cardiac failure | Usually during the first several months |
| Opportunistic viral (e.g., CMV, EBV, adenovirus) and fungal infections | Seropositive donors to seronegative recipients (often pediatric); excessive IS | 0-8 weeks; much less common thereafter, except for EBV-related PTLDs and other EBV-related tumors |
| Recurrent or new-onset viral hepatitis (e.g., HBV, HCV, HEV) | Original disease HBV, HCV, or acquired HEV-induced hepatitis in patients with contact with animals or culinary exposures | Usually first becomes apparent 4-6 weeks after transplantation and persists thereafter; earlier onset (<2 weeks) in aggressive cases |
| Recurrent AIH, PBC, and PSC | Original disease of AIH, PBC, or PSC | Usually >6 months after transplantation; incidence of recurrence increases with time after transplantation |
| Alcohol abuse | Recipient psychiatric comorbidity or social instability; noncompliance with treatment protocols; GGT/ALP ratio >1.4 | Usually >6 months |
| NASH | Original disease of NASH or cryptogenic cirrhosis; persistent or worsening risk factors for NASH in the general population | Usually 3-4 weeks and increases with time if risk factors persist |
Allograft biopsies are used to: (1) determine the cause of dysfunction, (2) assess the effect of therapy or progression of disease, and (3) document the immunological and architectural tissue status to help guide immunosuppressive therapy. Tissue triage depends on the reason for the biopsy, the clinical differential diagnosis, and the time after transplantation. Allograft needle biopsy should follow the American Association for the Study of Liver Diseases recommendations for liver biopsies, in general: two passes with a 16-gauge needle for assessment of fibrosis. Fibrosis staging is subject to sampling error for small biopsies (<20 mm long), and those containing fewer than 11 portal tracts may not be representative.
Most diagnostically important histopathological studies can be completed on routinely processed, formalin-fixed, paraffin-embedded (FFPE) sections. A clinical differential that incorporates antibody-mediated rejection (AMR) optimally includes fresh-frozen tissue for C4d staining. , FFPE samples can also be used for C4d immunohistochemistry staining, but is less sensitive than frozen tissue. Typically two H&E-stained slides, each containing two- to four-step sections, a C4d immunohistochemical stain performed on FFPE, one trichrome, and a cytokeratin 7 (CK7)-stained slide are reviewed. The most frequently used special stains, which are ordered only after review of the H&E slides, include trichrome, iron, and copper to detect chronic cholestasis. CK7 is especially useful for programs with a high rate of reduced-size and ECD grafts because it nicely detects ductular metaplasia of periportal hepatocytes in cases with suboptimal biliary drainage, ductopenia, and chronic rejection. Periportal hepatocyte copper deposition helps distinguish chronically suboptimal biliary drainage because of a biliary stricture (usually positive) from chronic ductopenic rejection (usually negative). Perivenular hepatocytes show similar changes with suboptimal venous drainage and with steatohepatitis. Sign-out stations should have access to the electronic medical records, serial laboratory results, immunosuppression drug levels, and donor-specific antibody (DSA) data.
Optimal information needed for interpretation of posttransplantation allograft biopsies includes the original disease, ABO compatibility, DSA status, time after transplantation, and type of donor and transplant operation (e.g., standard whole-organ cadaveric, DCD, reduced-size cadaveric, living related). These variables influence susceptibility to specific complications and consequently affect the morphological differential diagnosis.
A clinical differential diagnosis is valuable, but complete clinical information can bias biopsy interpretation; therefore the slide review should be completed before the pathologist correlates the findings with the clinical history and laboratory results to generate the differential diagnosis. We routinely also evaluate any previous biopsy slides, which greatly assists with interpretation of the current biopsy, and specifically comment in our reports on the effects of therapeutic intervention and disease progression or response. Post hoc multidisciplinary conference review of all liver allograft biopsies is an essential quality assessment tool to track outcomes of clinical interventions based on liver biopsy interpretations, providing feedback to the clinicians and pathologists.
Gross examination of failed allografts should use an approach similar to that for native hepatectomy specimens. Special attention should be paid to dissection and inspection of the biliary, hepatic artery, portal vein, and hepatic vein patency and integrity of anastomotic sites. A thorough examination may require assistance of the operative surgeon. Routine tissue sampling for microscopy should include anastomoses included with the resected specimen, superficial and deep sections of the right and left lobe, at least one deep hilar section with cross-sections of medium-sized bile ducts and arteries, and any grossly obvious defects.
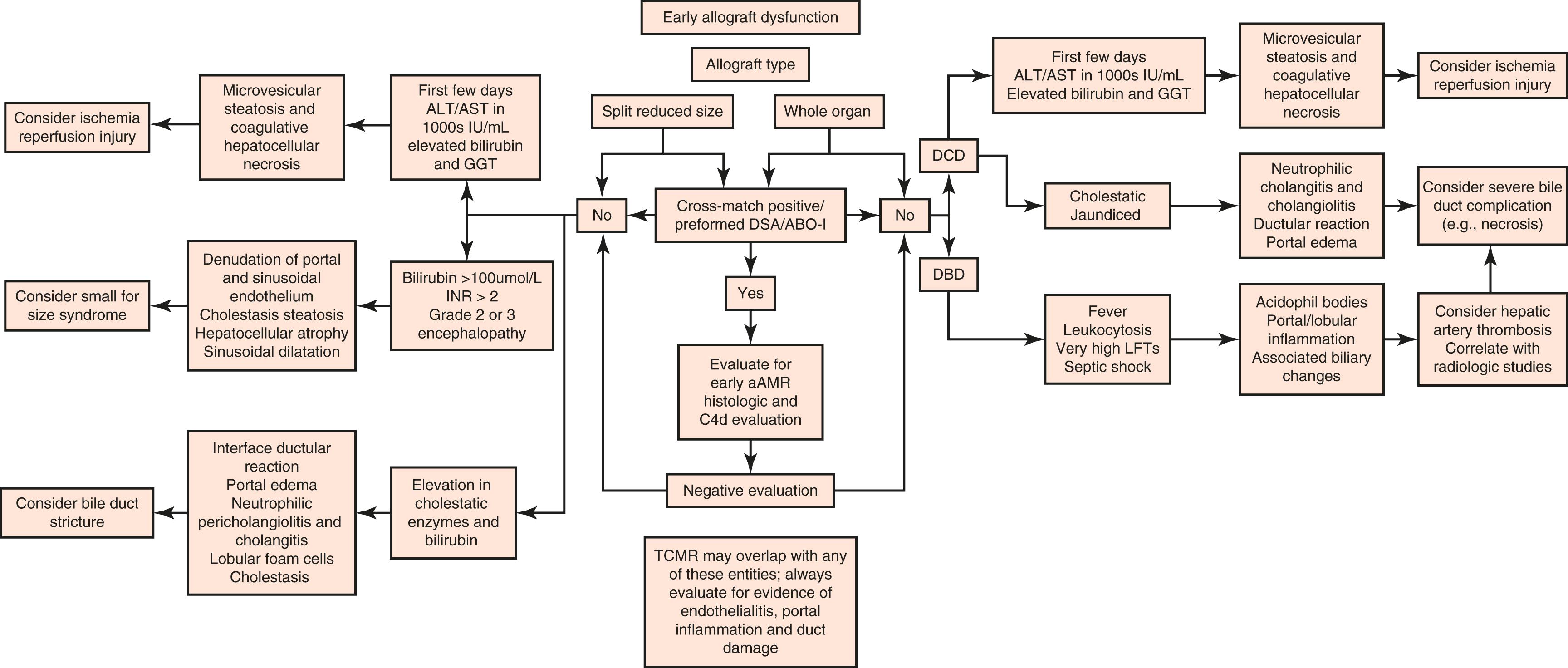
The most common causes of allograft failure vary according to the time after transplantation. Preservation/reperfusion injury or primary dysfunction (currently becoming less common), vascular thrombosis, and death of the patient are the leading causes of allograft failure within the first several weeks after transplantation. , Allograft failure resulting from T cell–mediated rejection (TCMR) or acute AMR has become rare. , Recurrent disease (PSC and autoimmune hepatitis [AIH]), delayed manifestations of technical complications (e.g., vascular thrombosis, biliary sludge syndrome), and patient death are most commonly responsible for late graft failures (>1 year after transplantation). , Typical chronic rejection (see the “Chronic Rejection” section later in this chapter) has become an uncommon cause of graft failure, and its incidence continues to decrease. , Recurrent HCV-induced cirrhosis has historically been the leading cause of allograft failure and retransplantation, and it has been responsible for about 30% of retransplantation procedures in the United States. However, since the introduction of DAA agents, the rates of retransplantation for recurrent HCV have steadily declined from 20.4% in 2005 to 1.25% in 2014, particularly at 1 year posttransplantation. There has also been significant improvement in 1-year graft and patient survival. Figure 53.4 provides a flowchart to simplify diagnosis of early allograft dysfunction.
Normal liver structure and function depends on optimal portal venous and hepatic artery inflow and adequate venous outflow and biliary drainage. Transplantation of a portion of the liver (e.g., living donors, cadaveric splits) necessarily compromises at least one of these vascular or biliary conduits, especially near the cut edge of the residual liver fragment. The pathologist should be aware of operative technical details and the exact origin of the posttransplantation biopsy because sampling errors in reduced-size allografts can be quite misleading. The clinical and laboratory context is important to determine whether there is a sampling artifact.
For example, infarcted parenchyma or morphological features of high-grade biliary or venous outflow obstruction can be localized to biopsies when obtained near the cut surface in otherwise well recipients with normal or near-normal liver injury test results. The histopathological changes are attributable to localized defects of blood or bile flow and are not representative of the entire organ. If the patient has more than one biliary anastomosis, biopsies from one lobe may show obstructive cholangiopathic changes, whereas the other lobe may be normal.
Early after transplantation, reduced-size/living donor allografts usually undergo rapid growth and may be more susceptible to damage from needle biopsies and AMR. Biliary leaks and biloma are also recognized early posttransplantation complications in living donor liver transplants. Late after transplantation, portal venopathy, low-grade ductular reactions, bile duct stricture, and nodular regenerative hyperplasia (NRH) are fairly common.
The term preservation/reperfusion injury refers to organ damage that occurs during agonal events in the donor, graft preservation, sanguineous reperfusion in the recipient, and perioperative events. , Cold ischemia refers to damage that occurs when the donor organ is stored in preservation fluid and immersed in an ice bath. It preferentially damages sinusoidal endothelial cells, causing them to lift from the underlying matrix. In general, optimal cold ischemic time should be less than 12 hours. Warm ischemia refers to the time the organ is exposed to blood but is suboptimally perfused because of hypotension or death of the patient. Pathophysiological mechanisms of warm and cold ischemia and preservation/reperfusion injury have been reviewed elsewhere. , Preexisting steatosis increases susceptibility to warm and cold ischemic injury. ,
DCD donors merit special mention because these organs suffer from a different type of warm ischemia insult. Instead of a relatively smooth transition from adequate blood perfusion to cold preservation solution, blood flow stops completely in the DCD donor. Ideally, the time from pronouncement of cardiac death to infusion with preservation solution should be less than 20 minutes. But even in the best of circumstances, considerable blood component sludging and subsequent damage can occur in the peribiliary plexus, which often results in ischemic cholangiopathy, the bane of DCD donor livers.
Dysfunction attributable to preservation/reperfusion injury usually occurs shortly after transplantation. Technical or vascular insults, such as arterial or venous thrombosis, alloimmunological or adverse drug reactions, toxin exposure, and infections, must be excluded. Donor and recipient hypotension, warm ischemia, metabolic abnormalities, cold ischemia during organ preservation, and reperfusion injury contribute to the syndrome of preservation/reperfusion injury.
Reliable early (during the first week) signs of significant preservation/reperfusion injury after complete revascularization include poor bile production and markedly (>2500 IU/mL) elevated serum aminotransferases that persist for several days and elevated serum lactate levels followed by a rapid normalization of the ALT/AST ratio during the first week. This is typically followed by a prolonged cholestatic phase characterized by persistent elevation of total bilirubin and GTP values. Grafts that recover from early significant injury have gradual resolution of abnormal liver injury test results, but they are also at risk for ischemic cholangiopathy and the biliary sludge syndrome. ,
Postreperfusion needle biopsies obtained within several hours of complete revascularization can reliably gauge the extent of preservation/reperfusion injury. , ,
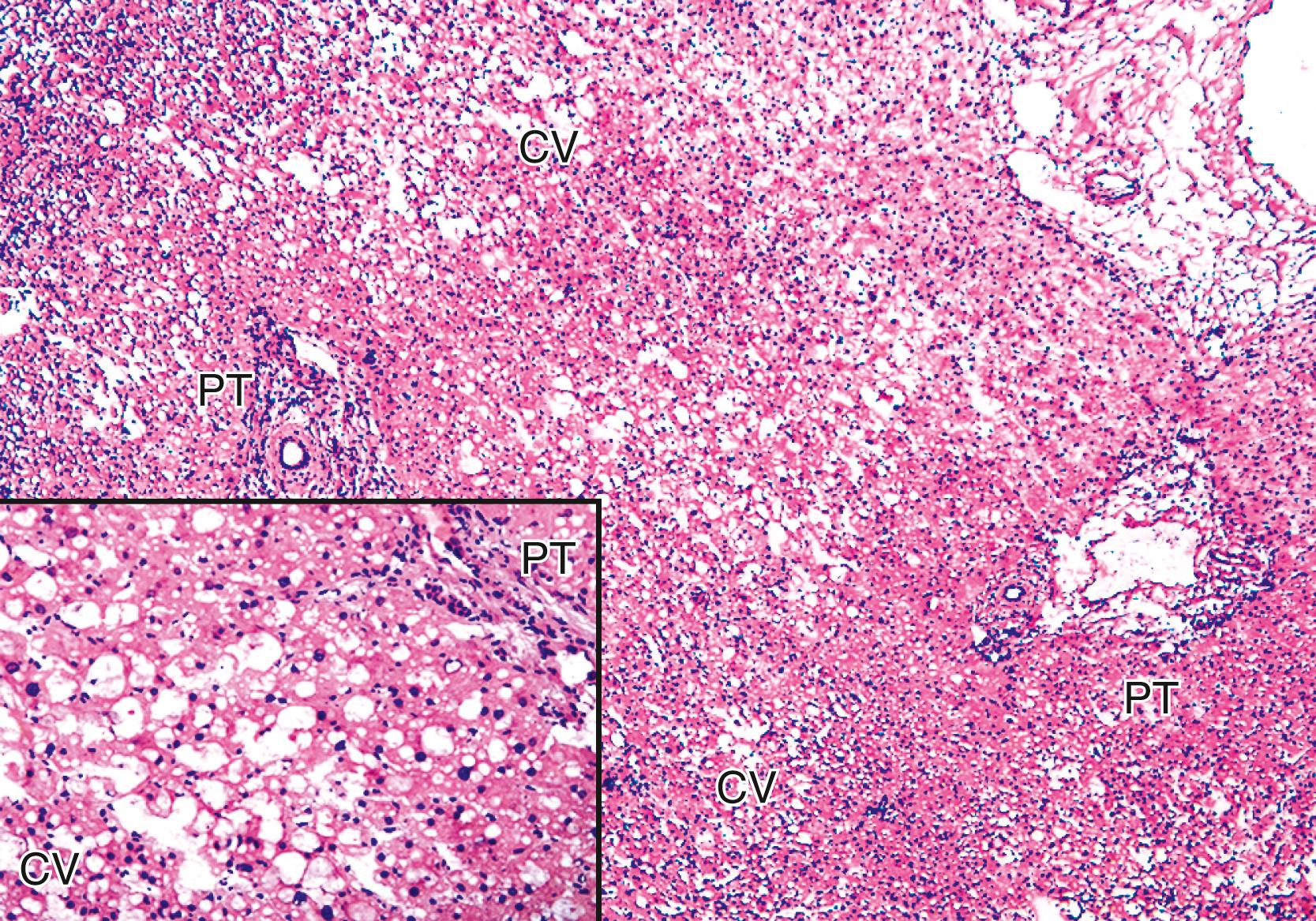
Mild damage that occurs in many liver allografts includes microvesicular steatosis, which is usually attributable to warm ischemia, hepatocellular cytoaggregation (i.e., detachment and “rounding up” of individual hepatocytes from each other), and hepatocellular swelling. , Severe injury is characterized by confluent, often zonal, coagulative necrosis, particularly if it is periportal or bridging, and severe neutrophilic inflammation. , Surgical hepatitis or manipulation injury, characterized by perivenular sinusoidal neutrophilia without nearby hepatocyte necrosis, and necrosis and neutrophilia in the immediate subcapsular parenchyma in wedge biopsies, should not be included in the assessment of preservation/reperfusion injury.
Repair responses usually begin 1 to 2 days after injury and are directly proportional to the severity of the hepatocyte damage and necrosis. Mild injury is usually followed by hepatocellular mitosis and hepatocyte plate thickening. The “cholestatic” phase includes mild centrilobular hepatocellular swelling, and hepatocanalicular cholestasis often coexists and persists for several weeks ( Fig. 53.5 ). Severe preservation/ reperfusion injury is usually accompanied by marked centrilobular hepatocellular swelling and hepatocanalicular and cholangiolar cholestasis. , These features often persist for 1 or 2 months ( Fig. 53.6 ). Cholangiolar proliferation is usually triggered by severe injury (e.g., periportal and confluent bridging necrosis). , Coexistent sepsis (common during this period) can also contribute to the pattern of injury. If the graft recovers, normal architecture can be restored, but patients are at risk for ischemic cholangiopathy.
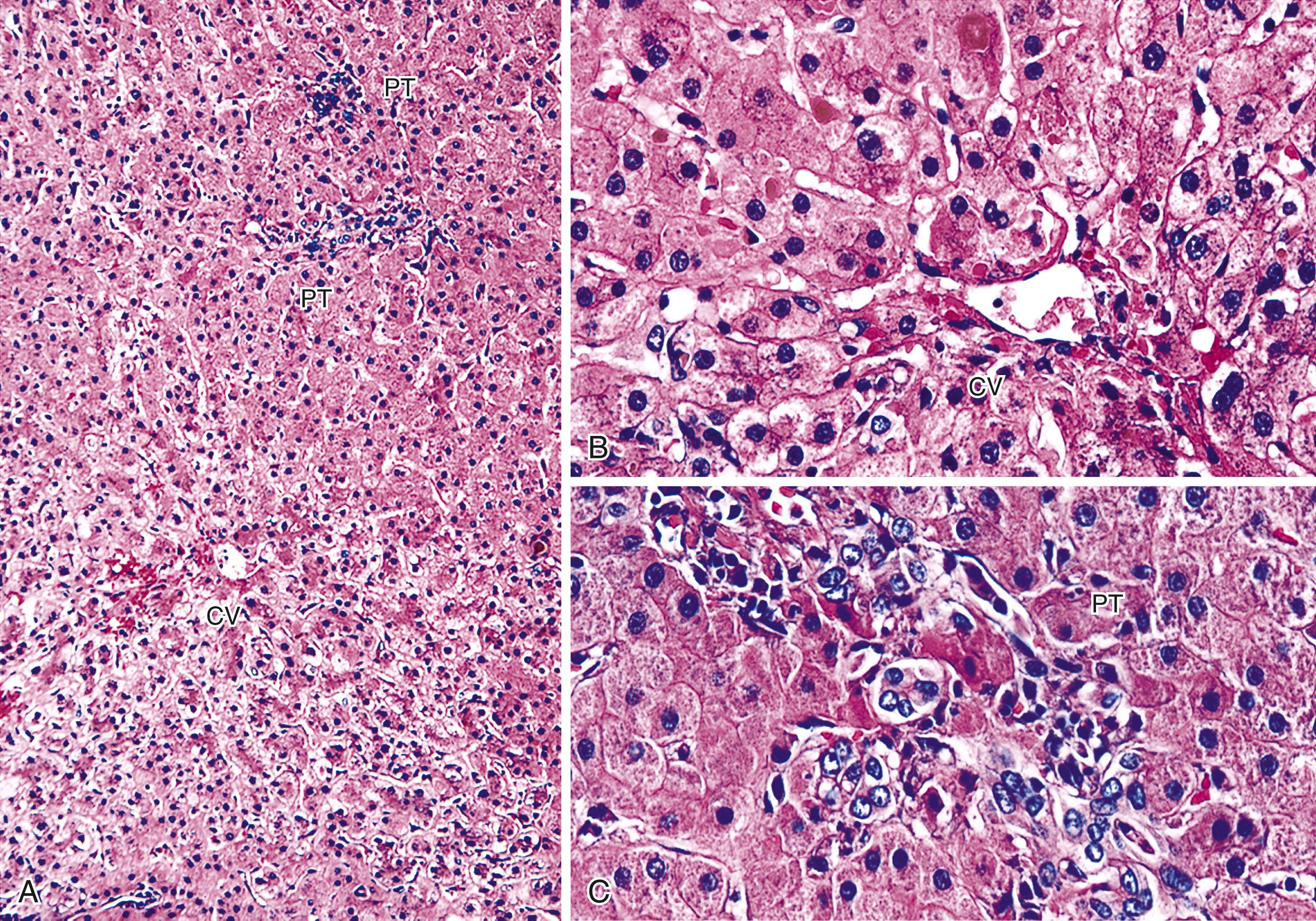
Preservation/reperfusion injury of steatotic (>20% large-droplet macrovesicular steatosis) donor livers causes death of some fat-containing hepatocytes. This causes release of large lipid droplets into the sinusoids that coalesce into even larger globules, which trigger local fibrin deposition, neutrophilia, red blood cell congestion, and local obstruction of sinusoidal blood flow, referred to as lipopeliosis ( Fig. 53.7 ). If the liver recovers, the large fat globules become surrounded by macrophages and eventually resolve over several weeks. Because normal hepatocytes require only 4 to 6 hours to undergo the entire apoptotic cycle, recognition of apoptotic hepatocytes or coagulative necrosis in a biopsy obtained more than several days after transplantation should arouse suspicion of another, usually ischemic, insult.

Suboptimal biliary drainage, pancreatitis, sepsis, acute AMR, and cholestatic hepatitis can produce histopathological changes that resemble preservation/reperfusion injury. Detailed donor information, including age and organ type (e.g., ECD, DCD), cold and warm ischemic times, operative difficulties, recipient’s clinical profile, blood culture, blood crossmatch, and results for DSAs and C4d staining help determine the likely source or sources of injury. However, preservation/reperfusion injury and operative technical difficulties most often are the causes of liver injury early after transplantation.
Examination of true bile ducts (not cholangioles) contained within the true portal tract stroma and cholangioles at the interface zone provide useful clues for distinguishing preservation/reperfusion injury from suboptimal biliary drainage. The latter usually cause at least some degree of periductal lamellar edema or produce stellate-shaped septal bile duct lumens with neutrophils in the lumen or infiltrating between biliary epithelial cells. Preservation/reperfusion injury does not usually show true bile duct changes unless it is associated with or leads to ischemic cholangiopathy. Instead, neutrophils surround the interface zone cholangioles. Centrilobular hepatocanalicular, cholangiolar cholestasis and intralobular neutrophil clusters are common to both disorders. The clinical context, history, and laboratory results are often needed to distinguish sepsis from preservation/reperfusion injury.
TCMR superimposed on preservation injury is recognized by the typical TCMR-type inflammatory infiltrate in portal and perivenular regions (blastic and smaller lymphocytes and occasional eosinophils), which are an excellent early marker of an emerging rejection reaction, especially when associated with lymphocytic cholangitis and lymphohistiocytic central perivenulitis. Distinguishing preservation injury from AMR is discussed later (see Antibody-Mediated Rejection).
Drug- and/or viral-induced cholestatic hepatitis can be difficult to distinguish from preservation injury without knowledge of the clinical history, but the typical periods of onset differ substantially. Viral-associated cholestatic hepatitis has been reported only for patients infected with HBV or HCV and is currently rare because of effective DAA agents. In this context, cholestatic hepatitis usually worsens with time unless the patient is treated with decreased immunosuppression or antiviral therapy, whereas the trend is gradual improvement for patients with preservation/reperfusion injury. Drug-induced cholestatic hepatitis early after transplantation is rare.
Normal liver structure, function, and growth depend on finely balanced portal and hepatic artery inflow and hepatic venous outflow and biliary tract drainage. It is difficult for surgeons to divide reduced-size donor livers (e.g., living donor, splits) with sufficient precision such that all of these requirements are satisfied in the donor and recipient. This is especially true when a reduced-size or living donor allograft (<30% of the expected recipient’s liver volume or <0.8% of the recipient’s body weight) is placed into the hyperdynamic and hypertensive portal circulation characteristic of a cirrhotic recipient. The donor liver fragment may be unable to accommodate the increased portal inflow, especially if hepatic venous drainage is compromised. , Too much portal venous inflow can injure portal venous and periportal sinusoidal endothelium , and contribute to allograft dysfunction. This is referred to as portal hyperperfusion/small-for-size syndrome (PHP/SFSS).
The arterial buffer response, which refers to reciprocal regulation of portal venous and hepatic artery hepatic blood flow, is an important aspect of PHP/SFSS. , Increased portal venous flow, as occurs in SFSS, causes arterial constriction and diminishes hepatic arterial flow, which results in suboptimal oxygenation. This predisposes the patient to arterial thrombosis and ischemic cholangitis, particularly if the arterial anastomosis is imperfect. Conversely, decreased portal venous flow, as occurs in cases of portal venopathy, causes hepatic arterial dilation and increased arterial flow.
The arterial buffer response is thought to be mediated by portal venous flow and the relative washout rate of adenosine, , an arterial vasodilator produced in the portal tracts. Other compounds, such as adenosine triphosphate (ATP) and hydrogen sulfide (H 2 S), and sensory innervation are likely to be involved. Temporarily reducing portal venous flow by transient portal-caval shunting, banding, splenic artery ligation, and pharmacological manipulation can ameliorate some PHP/SFSS manifestations. , Conversely, low portal inflow can impair liver regeneration and cause graft steatosis because the hepatic parenchyma depends on portal venous blood. , Primary alterations in hepatic artery flow do not cause flow changes in the portal circulation. Reduced-size or living donor allografts are usually required to grow after transplantation and are likely to experience PHP/SFSS to some extent, although it may not be clinically evident. Portal hyperperfusion is an important physiological stimulus of liver regeneration. Portal pressure elevation after partial hepatectomy inversely depends on the size of the graft. The rate of subsequent hepatocyte regeneration is directly proportional to increased portal pressure and flow. , Failure of liver regeneration is usually not the major clinical problem associated with PHP/SFSS, although regeneration can be impeded by suboptimal hepatic venous drainage in severe cases. Instead, PHP/SFSS becomes clinically significant when the structural integrity of the hepatic vasculature is compromised by the arterial buffer response, in which hepatic arterial flow counteracts changes in portal venous flow. Decreased arterial flow causes parenchymal or biliary ischemia and infarction. Splanchnic venous stasis and perfusion of the liver with endotoxin-rich blood may contribute to the evolving cholestasis.
The optimal approach to avoid this complication is to gradually titrate venous inflow and have adequate venous drainage, which can be achieved surgically or pharmacologically. This creates venous inflow high enough to trigger and sustain regeneration but not high enough to directly cause sheer stress injury or indirectly compromise arterial flow because of sustained spasm as part of the arterial buffer response.
Dahm and colleagues defined PHP/SFSS as at least two of the following complications occurring on 3 consecutive days in the first several weeks after transplantation: elevated bilirubin (>100 μmol/L), international normalized ratio (INR) higher than 2, and grade 3 or 4 encephalopathy after exclusion of technical, immunologic, or infectious complications. Surgeons are usually aware of the potential for PHP/SFSS, but the diagnosis can be difficult to establish with certainty. Clinical manifestations are not specific and may be caused by other insults (e.g., portal vein thrombosis).
It is often difficult to determine whether PHP/SFSS contributes to complications that may otherwise be deemed technical, especially because the latter are encountered with increased frequency in reduced-size allografts. For example, a strong arterial buffer response can lead to significant arterial vasospasm and predispose to thrombosis, especially if blood vessels are a smaller caliber than normal. This event may be misinterpreted as a technical complication. Risk factors for PHP/SFSS in living donor liver transplants include recipients of the left lobe (compared with the right lobe), higher portal reperfusion pressure, high preoperative bilirubin, older donor age, and higher donor BMI. Recognition and amelioration of these factors using a variety of proper planning and surgical and pharmacological approaches has decreased the incidence and severity of this complication.
PHP/SFSS has been observed reliably in experimental animal models , , , and occasionally in postreperfusion or early posttransplantation allograft biopsies obtained within the first several days in humans. Sheer stress resulting in portal vein and periportal sinusoidal endothelium denudation occurs as early as 5 minutes after transplantation. , , , , In severe cases, microvascular rupture at the portal vein–sinusoidal junction can result in hemorrhage into the portal and periportal connective tissue and dissect into hepatic parenchyma. ,
If the allograft survives the initial crisis, reparative changes occur, including endothelial cell hypertrophy and subendothelial edema accompanied by growth of myofibroblasts and endothelial cells into the subendothelial space. Eventually, this process leads to fibrointimal hyperplasia or intimal thickening and luminal obliteration or recanalization of thrombi ( Fig. 53.8 ). Venous findings typical of PHP/SFSS are uncommon in peripheral core-needle biopsies. Instead, a constellation of nonspecific findings can be seen including centrilobular hepatocanalicular cholestasis, centrilobular hepatocyte steatosis or hepatocyte atrophy with sinusoidal dilation, centrilobular hepatocyte necrosis, and a low-grade ductular reaction ( Fig. 53.9 ).


Review of operative and radiographic reports and clinical history followed by a discussion with the surgeon will help determine whether PHP/SFSS is contributing to biopsy findings. Hilar sections of allografts that fail because of PHP/SFSS frequently show changes of traumatic injury to large portal vein endothelium, focal fibrointimal hyperplasia of vein branches, evidence of arterial vasospasm, and, in some cases, ischemic cholangitis, particularly if the hepatic artery has thrombosed.
If the graft recovers, portal hypertension and ascites resolve over a period of several weeks. Although a near-normal architecture usually occurs, NRH as a result of portal venopathy from the initial injury can occur.
Suboptimal arterial flow resulting from arterial thrombosis or stricturing, sepsis, hypotension, suboptimal biliary tract drainage from a variety of causes, or ischemic cholangitis can cause pathological changes similar to those of PHP/SFSS. However, arterial vasospasm is usually detectable microscopically only in failed allografts and only when severe primarily in medium-sized perihilar arteries.
Preservation/reperfusion injury is a differential diagnostic consideration, but living donor grafts are usually not affected significantly. Because portal hyperperfusion can lead to diminished arterial flow and thrombosis, it is not surprising that more than one complication may occur. Suboptimal biliary tract drainage alone is usually not accompanied by portal tract connective tissue hemorrhage, centrilobular hepatocyte ischemic changes, or significant NRH. When these changes are found, PHP/SFSS should be considered.
Most vascular complications occur within the first several months after transplantation; they are related to anastomotic imperfections (e.g., narrowing, flaps, dramatic caliber reductions), preexisting donor atherosclerotic disease, vascular tree trauma, creation of kinks or abnormal tortuosity, metabolic or physiological abnormalities that predispose to thrombosis, or a combination of these factors. Potential problems often manifest because of factors that increase the technical difficulty of the vascular anastomoses (e.g., small-caliber vessels in pediatric recipients, reduced-size grafts, abnormal anatomy such as piggyback venal caval anastomosis). Physiological or metabolic abnormalities that decrease hepatic blood flow or promote coagulation, such as cardiac failure, clotting abnormalities, TCMR, and infections, also increase the risk of vascular complications.
Vascular interposition arterial grafts or small venous segments, which are used to link the donor and recipient arteries or veins, respectively, can be a significant source of problems. Vascular grafts increase the need for sutured anastomoses; some vessel segments might have been cryopreserved or stored in preservation fluid for one to several days before implantation and may be marginally viable. These factors increase the risk of thrombosis, stimulate atherogenesis, and serve as a nidus of infection.
Hepatic artery thrombosis is the most common major vascular complication and an important cause of early allograft damage, failure, and retransplantation. It occurs in 2% to 20% of transplants, usually within 30 days of transplantation. , Early hepatic artery thrombosis maybe detected by routine use of Doppler ultrasonography in the early postoperative period, allowing for timely intervention and decreasing complications associated with hepatic artery thrombosis. Allografts are more susceptible to arterial ischemia than native livers because they are devoid of a collateral arterial circulation early after transplantation.
The hepatic artery exclusively supplies the extrahepatic and intrahepatic bile ducts, hilar and portal tract connective tissue, and hilar lymph nodes. These structures are preferentially damaged by inadequate arterial flow or by arterial thrombosis, often resulting in ischemic cholangitis , or ischemic cholangiopathy, terms that denote ischemic damage to the biliary tract. Ischemic cholangiopathy manifests as frank necrosis, poor anastomotic healing, biliary leaks, and cholangitic abscesses, resulting in biliary sludge syndrome and recurrent bacteremia.
A second, smaller wave of technically related arterial thromboses occurs 1 to 3 years after transplantation. , Suboptimal arterial anastomoses can cause turbulent arterial flow downstream from the suture line, which eventually leads to arterial fibrointimal hyperplasia, luminal narrowing, and thrombosis. Fibrointimal hyperplasia often develops more quickly in arterial interposition grafts and predisposes to thrombosis.
Reported risk factors for early hepatic artery thrombosis include cytomegalovirus (CMV) mismatch (i.e., seropositive donor liver in a seronegative recipient), low weight of a pediatric recipient, procoagulant activity, retransplantation, use of arterial conduits, prolonged operation time, variant arterial anatomy, and low-volume transplantation centers. Late hepatic artery thrombosis is not uncommon, with an estimated incidence of 4.8%. Risk factors for late hepatic artery thrombosis include prior episode of thrombosis, small donor size, split/reduced-size grafts, history of TCMR, prolonged operation time, previous upper abdominal surgery, and possibly retransplantation. ,
Most early hepatic artery thromboses manifest clinically with one or more of the following manifestations: fever, leukocytosis, septic shock, ischemic cholangiopathy, and severe elevations of liver injury laboratory test results. , Late hepatic artery thrombosis may be asymptomatic or may present insidiously along with cholangitis, relapsing fever, sepsis (related to hepatic infarcts), abscesses, and ischemic cholangiopathy with subsequent impaired bile flow. ,
Ultrasonography is mostly used as a screening tool to inspect hepatic arterial blood flow, but angiography is the most reliable method of establishing a diagnosis. Early after transplantation, urgent surgical revascularization is often attempted to salvage the graft, but even if successful, the graft can develop ischemic cholangiopathy.
Hepatic artery thrombosis can be uncommonly diagnosed on peripheral core-needle biopsies because needle biopsies sample subcapsular parenchyma, which is only sometimes affected. , Structures more susceptible to ischemic injury include the perihilar tissue and large bile ducts, which are not routinely sampled by biopsy.
Peripheral core-needle biopsies can show a variety of changes; included are frank centrilobular coagulative necrosis, marked centrilobular hepatocyte swelling, acidophil bodies, “ischemic hepatitis” (similar to that seen in viral hepatitis), lobular and portal inflammation, biliary tract complications such as cholestasis, cholangiolar proliferation, acute cholangiolitis, and obstruction or stricturing. The peripheral biopsy may be unremarkable because arterial collaterals render the thrombosis inconsequential or the necrosis of bile ducts and biliary sludging have not developed in the peripheral liver parenchyma. Chronic suboptimal arterial flow can cause centrilobular hepatocellular atrophy and sinusoidal widening.
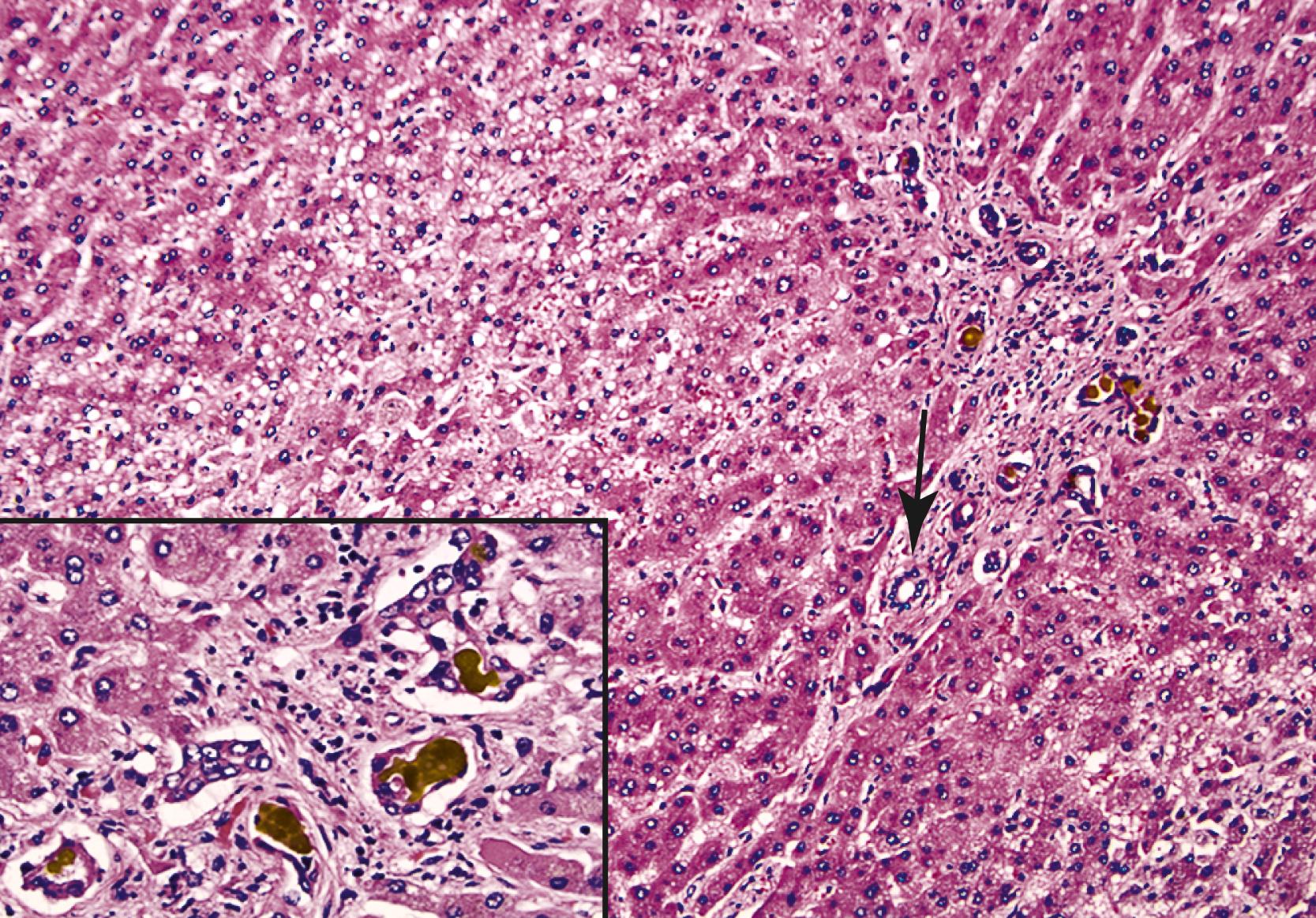
Examination of failed allografts with hepatic artery thrombosis ( Fig. 53.10 ) often reveals necrosis of the hilar or perihilar bile ducts ( Fig. 53.11 ) with leakage of bile into the surrounding connective tissue and veins. Biliary sludge, biliary abscesses seeded with fungi and bacteria, infarction of hepatic hilar lymph nodes, and patchy parenchymal infarction may also be seen.


Because hepatic artery thrombosis or arterial narrowing can mimic almost every type of liver allograft syndrome, uncommon manifestations can easily be overlooked or misdiagnosed. Centrilobular ischemic necrosis most reliably indicates hepatic artery thrombosis (HAT), but HAT can also result in centrilobular hepatocyte swelling and biliary tract obstruction or cholangitis. Chronic suboptimal arterial flow can cause biliary epithelial cell senescence that resembles chronic rejection and centrilobular hepatocyte atrophy and sinusoidal widening. Ischemic hepatitis can appear similar to the lobular phase of acute or recurrent viral hepatitis (e.g., HCV infection, which is now rare). However, Liu and colleagues suggest that a combination of apoptosis and mitosis (i.e., low ratio of apoptosis to mitosis) without inflammation is more common in ischemic hepatitis than in recurrent hepatitis C, in which apoptosis predominates. The relationship between arterial thrombosis and biliary tract complications is common; therefore examination of hepatic arterial patency should be performed when biliary tract complications are encountered.
Portal vein complications, which affect 1% to 2% of liver allograft recipients, are less common than hepatic artery complications. , Early complications include thrombosis, strictures, and poor flow because of persistent collateral circulation or hypotension. The incidence is increased in reduced-size grafts and when cryopreserved venous interposition grafts are used. Risk factors include problems with the portal venous anastomosis, small portal vein diameter, pediatric recipients, previous portal vein thrombosis, surgical shunting before transplantation, and splenectomy. Similar to native livers, long-surviving liver allografts with cirrhosis are also susceptible to portal vein thrombosis.
Portal vein thrombosis in a noncirrhotic allograft can cause widespread necrosis with massive elevation of liver injury tests and lactic acidosis ( Fig. 53.12A ). Clinical manifestations can include bleeding varices, fulminant hepatic failure, and portal hypertension with massive ascites and edema. If the thrombus is partial, small infarcts can develop, or the liver can become seeded by intestinal bacteria, which may cause relapsing fever. The clinical presentation of portal vein thrombosis in a cirrhotic allograft is the same as that in a native liver with cirrhosis: variceal hemorrhage, splenomegaly, and ascites.

Findings depend on the severity of portal vein flow compromise, time after transplantation, and the structural integrity of the allograft. Complete portal vein obstruction early after transplantation in a noncirrhotic allograft often causes massive coagulative necrosis. Suboptimal portal vein flow because of strictures, kinks, or persistent collateral circulation can result in periportal or midzonal linear-shaped zones of hepatocyte atrophy and/or necrosis ( Fig. 53.12B ), focal confluent coagulative necrosis, unexplained zonal or panlobular macrovesicular steatosis, and NRH. In fact, when macrovesicular steatosis rapidly appears early (within several weeks) in a nonsteatotic donor liver after transplantation, persistent venous collaterals that have failed to close or have reopened after transplantation should be suspected as an underlying cause. Bacterial or fungal infection of a partial portal vein thrombus can result in miliary hepatic abscesses.
Suboptimal portal vein blood flow can be difficult to distinguish from suboptimal hepatic venous drainage. Linear-shaped zones of ischemic necrosis or hepatocellular atrophy favor the former, whereas red blood cell congestion in and around central veins and centrilobular sinusoids and obliterative central venopathy suggest suboptimal hepatic venous drainage. Ultrasonography and angiography often are needed to specify the cause of the vascular abnormality. Cases of suboptimal portal vein flow that manifest with intrahepatic steatosis must be distinguished from recurrent or de novo steatosis and steatohepatitis.
Suboptimal hepatic venous outflow, including the hepatic veins and vena cava, are relatively uncommon. Risk factors include reduced-size or living donor allografts without inclusion of the middle hepatic vein, difficulties with reconstruction of the venous outflow tract, and alternative anastomoses such as the piggyback approach. Late hepatic vein stenosis at the anastomotic site can be seen as a result of extrinsic compression by an enlarging liver, fibrosis, and fibrointimal hyperplasia. Significant stenosis or thrombosis of the outflow tract is associated with significant clinical and pathological manifestations.
The clinical presentation depends on the severity of outflow tract compromise. Severe stenosis or thrombosis can lead to Budd-Chiari syndrome, with hepatic enlargement, tenderness, ascites, and edema. Less severe stenosis may cause only histopathological manifestations or an increase in the portal vein–vena cava pressure gradient. In less severe cases, it is often difficult to determine whether surgical intervention is required.
Congestion and hemorrhage involving the perivenular sinusoids are the most reliable histopathological findings of suboptimal hepatic venous drainage ( Fig. 53.13 ). Bland centrilobular hepatocyte necrosis and dropout are also usually present. Chronically suboptimal hepatic venous drainage can include perivenular fibrosis and central vein occlusion with venocentric cirrhosis, particularly if outflow obstruction is severe or of long duration. Chronic changes can also be accompanied by NRH changes, and a ductular reaction at the interface and/or centrilobular areas; this can make it difficult to recognize architectural landmarks and to exclude suboptimal biliary drainage.

If any of the previously described perivenular changes are not accompanied by noticeable inflammation, suboptimal hepatic venous drainage should be suspected. A major differential consideration is chronic AMR, which can also contribute to noninflammatory perivenular subsinusoidal fibrosis. Recognition of an immune-mediated cause may be aided by the finding of interface activity, portal collagenization and portal vein and capillary uptake of C4d staining, and detection of serum DSAs of relatively high MFI (>10,000 mean fluorescence intensity [MFI]) , (see the Chronic Rejection section). If acute or chronic centrilobular vascular changes are accompanied by significant lymphocytic, histiocytic, or lymphoplasmacytic inflammation, an immunologically mediated cause of injury such as acute or chronic TCMR, plasma cell–rich rejection or AIH, or an adverse drug reaction should be suspected.
Perivenular inflammation can be transient in some cases of immune-mediated centrilobular injury and later manifest as bland perivenular fibrosis, when it might be indistinguishable from mechanical venous outflow obstruction. Finally, pathologists should not cavalierly recommend invasive radiographic studies of vena caval patency in their reports.
Despite improvements in surgical techniques, the biliary tree continues to be the “Achilles heel” of liver transplantation. Biliary complication rates have been declining in recent years but continue to represent a major source of postoperative mortality and morbidity. Complications vary by center and occur in approximately 20% of recipients in whole-organ cadaveric donors, and an even higher incidence is observed for living and reduced-size allografts, but center-specific effects are seen. , , Biliary tract reconstruction approaches vary considerably among surgeons and depend on primary liver disease, graft type, and previous transplants/biliary surgery.
The most common biliary anastomosis used for cadaveric whole organs are end-to-end, duct-to-duct, and donor biliary–recipient enteric anastomoses. Duct-to-duct anastomoses are fairly common because of ease of surgical anastomosis and presentation of the sphincter of Oddi, and it allows for postoperative diagnostic and therapeutic instrumentation (e.g., endoscopic retrograde cholangiopancreatography [ERCP], stent placement). A more time-consuming choledochojejunostomy anastomosis (donor biliary–to–recipient enteric anastomosis) is usually performed on recipients with a history of PSC or other biliary tract abnormalities. Most biliary tract complications are attributable to ischemic or traumatic injury, immunological injury, surgically introduced abnormal anatomy, or a combination of these factors. All predispose to poor biliary tract wound healing, suboptimal drainage, and/or reflux, occurring singly or often in combination. ,
The extrahepatic donor bile duct immediately adjacent to the anastomosis is particularly vulnerable to ischemic injury. To ascertain adequate arterial flow and duct viability after completion of the hepatic arterial anastomosis, the terminal end of the donor extrahepatic bile duct is progressively trimmed back toward the liver until bleeding occurs from the cut surface to assure adequate arterial flow.
The peribiliary arterial plexus is particularly vulnerable to preservation/reperfusion injury during operative manipulation. In DCD donors, blood component sludging or use of the more viscous University of Wisconsin solution , can promote clogging of the peribiliary capillary plexus and prevent adequate reperfusion after transplantation. Machine perfusion (still in its infancy) might offer improved outcomes, particularly when DCD and ECD organs are utilized for transplantation. ,
Other causes of biliary tract ischemia resulting from arterial or peribiliary plexus injury include small-for-size syndrome and severe arterial vasospasm, prolonged cold ischemia, hepatic artery thrombosis/stenosis, older donors with atherosclerotic disease, and preformed or de novo donor anti-antibodies (e.g., ABO isoagglutinin, anti–human leukocyte antigen [HLA] antibodies). , , Once the myriad of other causes of biliary tract complications have been excluded, recurrent primary disorders such as recurrent PSC or primary biliary cholangitis (PBC) should be considered, but recurrent disease usually occurs more than 6 months after transplantation.
Biliary tract complications, the majority of which start within 3 months after transplantation, include anastomotic dehiscence, transmural necrosis, bile leakage, cholangitic abscesses, ascending cholangitis, bile casts ( Fig. 53.14 ), strictures, ampullary dysfunction, and biliary-vascular fistulas. , These complications occur in approximately 15% of whole cadaveric allografts and as many as 30% of reduced-size or living donor allografts, but the incidence is decreasing as surgical approaches improve. , , ,

Bile leaks mostly occur at anastomotic sites. The former are more common and are likely caused by technical issues or ischemia at the anastomosis. Nonanastomotic leaks may occur at the site of cystic duct, T-tube, or as a complication of acute hepatic artery thrombosis. , Another source of bile leaks is from small transected bile ducts at the hepatic resection surface in recipients of living or reduced-size grafts.
Strictures are the most common biliary complication. , They are categorized according to location and the time after transplantation as intrahepatic or extrahepatic, nonanastomotic or anastomotic , and early or late (>30 days postoperative). Intrahepatic strictures are further categorized as perihilar or peripheral . Peripheral intrahepatic strictures are less common and tend to occur later after transplantation.
Anastomotic strictures usually appear within the first several months after transplantation, but they appear at a reduced rate for many years. , Risk factors for anastomotic strictures include postoperative bile leaks, female donor–male recipient combinations, and a more recent year of transplantation. , , Compared with nonanastomotic strictures, anastomotic strictures are more amenable to correction either by surgical or radiological intervention; anastomotic strictures also have less of a negative impact on long-term graft and patient survival. , ,
Nonanastomotic strictures usually (1) occur later after transplantation (>1 year), (2) are less amenable to treatment, (3) are generally progressive, and (4) negatively affect graft and patient survival. These usually occur in the more peripheral biliary tree and are associated with vascular/microvascular and immunological risk factors, alone or in combination. The major vascular risk factor is hepatic artery thrombosis/stenosis. Immunological damage at the microvascular level induces ischemia by disruption of the microvascular circulation at the peribiliary capillary plexus level by circulating donor-specific antibodies (DSA) and isoagglutinin and direct cytotoxic lymphocytic damage. , Immunological risk factors also include PSC and AIH as the original disease. Other risk factors for nonanastomotic strictures include use of high-viscosity preservation solution, Roux-en-Y biliary anastomoses, and CMV infection. ,
Nonanastomotic strictures occurring earlier (<1 year) are often associated with preservation-related injury , and are usually located in perihilar bile ducts. Similar to the nonanastomotic strictures that occur later, risk factors for early stricture include long cold and warm ischemic times, high-viscosity preservation solution, older recipient age, a duct-to-duct biliary anastomosis, and bile leaks.
Liver injury tests that show relatively selective elevation of γ-glutamyl transferase (GGT)/alkaline phosphatase (ALP) versus ALT/AST levels is the usual method of detecting early or minor biliary tract complications such as strictures and stones. Clinical signs and symptoms are uncommon unless the patient develops jaundice or acute cholangitis. Biliary tract problems are also often first suspected on routine or protocol biopsy evaluation, especially with the use of routine CK7 staining (see later). However, cholangiography (e.g., magnetic resonance cholangiopancreatography [MRCP], duct-to-duct ERCP, percutaneous transhepatic cholangiography [PTC]) is often used to confirm the diagnosis and localize the defects. , ,
More serious biliary tract complications, such as obstruction, cholangitic abscesses, and ascending cholangitis, usually manifest with fever, jaundice, right upper quadrant pain, and intermittent bacteremia. During the first several months after transplantation, T-tube stents provide access for cholangiograms, which are routinely performed before clamping at 1 week postoperatively and again at the time of T-tube removal.
Allograft biliary tract complications are histopathologically identical to those seen in native livers. Portal, periportal, periductal, and pericholangiolar edema; predominantly neutrophilic portal inflammation; intraepithelial and intraluminal neutrophils in true bile ducts; stellate-shaped lumens in small septal ducts, an interface ductular reaction; centrilobular hepatocanalicular cholestasis; and small clusters of neutrophils and foam cells throughout the lobules are typical findings of stricturing or obstructive cholangiopathy (see Fig. 53.14 ). With time, chronic biliary tract strictures are often associated with mixed or predominantly chronic portal inflammation, biliary epithelial cell senescence changes, and low-grade ductopenia involving small bile ducts. More than 1 year after transplantation, in addition to the classic features described previously, biliary strictures are a relatively common cause of portal eosinophilia.
Ancillary stains are extremely helpful and a very sensitive method of pointing toward suboptimal biliary drainage. We routinely perform CK7 staining on all allograft biopsies because of our frequent use of living donor allografts at our centers; if positive, we follow up with a copper stain. CK7 immunostaining is used to detect ductular metaplasia of periportal hepatocytes when there is suboptimal biliary drainage, either at the lobular level or at larger branches of the biliary tree (supralobular). Rhodamine copper stain highlights periportal hepatocellular copper deposition that mostly occurs after chronically (more than several months) suboptimal biliary drainage. We usually perform CK7 and copper stains in tandem with the following interpretation: (1) CK7+/copper–: recent biliary tract stricture and/or intrahepatic ductopenia (usually chronic rejection); (2) CK7+/copper+: supralobular biliary stricture/suboptimal biliary drainage; and (3) CK7–/copper+: recently decompressed biliary stricture.
Biliary-vascular fistulas are recognized by red blood cells in the lumen of bile ducts or by bile concretions in blood vessels. Occasionally, an inordinate elevation of the serum bilirubin level is associated with a fistula. In contrast, periductal hemorrhage surrounding small interlobular bile ducts is an inconsequential finding in asymptomatic patients when a biopsy is obtained within 1 or 2 days after transhepatic cholangiography.
The differential diagnosis is influenced by the time after transplantation, the patient’s original disease, previous operative and complication history, rejection history, and DSA analyses. Within the first several weeks after transplantation, suboptimal biliary drainage with or without cholangitis can be difficult to distinguish from preservation/reperfusion injury, TCMR, and acute AMR, particularly if the patient was treated with increased immunosuppression before the biopsy was obtained.
Features that favor suboptimal biliary drainage over TCMR include neutrophilic-predominant portal inflammation, periductal edema, retention of the normal nucleus-to-cytoplasm ratio in biliary epithelial cells, and an absence of perivenular mononuclear inflammation and endothelialitis. TCMR is favored when the mixed portal inflammation is composed of blastic and small lymphocytes, plasma cells, and eosinophils. Lymphocytic cholangitis, an increased nucleus-to-cytoplasm ratio in biliary epithelial cells, and perivenular inflammation also are usually seen. Portal eosinophilia in early TCMR and AMR can be quite striking, especially in patients treated with steroid-sparing immunosuppressive regimens. In acute AMR, portal capillary dilation, capillaritis, and importantly, strong and diffuse portal capillary C4d staining can be used to distinguish acute AMR from suboptimal biliary drainage/cholangitis (see Antibody-Mediated Rejection section). Late-onset chronic biliary tract complications occasionally manifest with predominantly mononuclear portal inflammation, biliary epithelial cell senescence changes, and low-grade ductopenia. Late-onset chronic biliary strictures can also cause portal eosinophilia and mimic acute TCMR and chronic rejection, viral hepatitis, and recurrent autoimmune disorders.
It can be difficult to differentiate chronic rejection from biliary strictures/suboptimal drainage from other causes, especially recurrent PSC, in peripheral core-needle biopsies. The at-risk populations are similar. Both conditions can cause intrahepatic cholestasis, biliary epithelial cell senescence changes, and small bile duct loss. Careful examination of the clinical history, evaluation of serial biopsies, and analysis of the histopathology are needed to distinguish between biliary strictures and chronic rejection.
In our experience, features that favor biliary strictures and/or recurrent PSC over chronic rejection include a history of biliary tract complications, PSC as the original disease, periductal lamellar edema involving true bile ducts, stellate portal expansion, portal neutrophilia, a ductular reaction affecting at least some portal tracts, and deposition of copper or copper-associated protein in periportal hepatocytes. Features that favor mixed TCMR with chronic rejection over suboptimal biliary drainage include: a previous history of rejection; inadequate immunosuppression; lymphoplasmacytic portal inflammation; small portal tracts; absence of a ductular reaction; ductular metaplasia of periportal hepatocytes on CK7 staining, but negative copper staining; and active central perivenulitis with or without perivenular fibrosis.
Findings in failed allografts that can be used to favor biliary strictures or recurrent PSC over chronic rejection include significant enlargement/increased weight; bile-pigmented sinus histiocytosis in hilar lymph nodes, if present; mild focal eccentric fibrointimal hyperplasia in perihilar hepatic artery branches versus foam cell arteriopathy; and significant concentric fibrointimal hyperplasia. In addition, suboptimally draining extrahepatic or large intrahepatic bile ducts in recurrent PSC/biliary strictures often show focal ulceration, periductal lymphoplasmacytic inflammation, and fibrosis. In chronic rejection, large-duct inflammation and ulceration are unusual. When the infiltrate shows plasma cell–rich infiltrates, the possibility of recurrent or de novo immunoglobulin G4 (IgG4)-associated sclerosing disease should be considered.
Angiography and cholangiography (e.g., ERCP) are also useful in making the distinction between obstructive cholangiopathy and chronic rejection. Pruning of peripheral arterial and biliary trees and poor peripheral filling are seen in chronic rejection. Suboptimal biliary drainage, however, usually shows some intrahepatic duct dilation on cholangiography, but arterial changes are not seen or are insignificant.
Clinicopathological correlation can also help distinguish late-onset biliary tract complications from late-onset acute rejection. Adequate immunosuppressive drug levels with preferential elevation of GGT and alkaline phosphatase levels favor suboptimal biliary drainage because late-onset acute rejection is unusual in this circumstance. Finally, in our experience, it is not possible to reliably distinguish recurrent PSC from other causes of biliary tract obstruction or stricturing on the basis of peripheral core needle biopsy evaluation alone.
Liver allograft rejection has been traditionally categorized as follows: hyperacute (occurring immediately after revascularization), acute (onset of graft dysfunction over a period of days), and chronic (evolves over weeks to months). Rejection is also broadly categorized on the predominant pathophysiological mechanism of injury: AMR, TCMR, mixed AMR and TCMR, and chronic rejection, which also often shows a mixed pattern of antibody and T cell–mediated injury. AMR can be further subcategorized into hyperacute, acute (aAMR), and chronic active AMR (cAMR). TCMR can occur early or late in the allograft age, and corresponding histological features are also different. These different types of rejection occur as a continuum and often show overlapping features.
This section will reflect recent advances in pathophysiology and diagnostic criteria of acute and chronic AMR as well as changes in rejection terminology ( Table 53.3 ). Discouraged terminology includes humoral rejection, acute cellular rejection, de novo AIH, and plasma cell hepatitis in favor of AMR, TCMR, and plasma cell rich–rejection. It is also clear now that many cases that were previously labeled as idiopathic posttransplantation hepatitis can now be categorized as late-onset TCMR and/or chronic AMR. , , ,
| Older Discouraged Terminology | Newer Encouraged Terminology |
|---|---|
| Acute cellular rejection | T cell–mediated rejection |
| Humoral rejection | Antibody-mediated rejection |
| De novo autoimmune or plasma cell-rich hepatitis | Plasma cell–rich rejection |
| Idiopathic posttransplant hepatitis | More specific terminology when possible
|
Crossing ABO blood group barriers predictably causes severe liver allograft injury, unless plasmapheresis is used to lower isoagglutinin titers to below 1:16. Without these efforts, crossing ABO barriers leads to an approximately 60% incidence of significant AMR and graft failure. It should be noted that ABO-incompatible liver allografts are routinely used in Asian programs in which the donor pool is limited.
Advances in antibody and complement depletion and desensitization protocols utilizing rituximab, intravenous immunoglobulins, plasmapheresis techniques, local graft infusion, splenectomy, and vigorous antirejection and microcirculatory protective therapy have shown acceptable results, in some cases comparable to ABO-compatible transplants. Short- and long-term outcomes in pediatric cases (particularly children younger than 2 years of age) undergoing ABO-incompatible liver transplantation are identical to those with ABO-compatible allografts. , Despite improvement, ABO-incompatible recipients are still at risk for biliary tract complications.
Liver allografts are much less susceptible than other solid organ allografts to damage from anti-HLA AMR as a result of many heterogenous tolerance mechanisms. , , However, depending on the circumstances, the liver is not completely spared from injury by these and other non-HLA antibodies. Although DSAs do not routinely influence organ triage or recipient selection at most centers, , , there are short- and long-term implications to preformed and de-novo DSAs. They are associated with decreased 5-year patient and graft survival, increased incidence of TCMR and AMR, graft fibrosis and chronic rejection, and late-onset T cell–mediated rejection and plasma cell–rich rejection (probably mixed T cell–mediated and antibody-mediated rejection). ,
Two distinct patterns of AMR are increasingly being recognized in liver allografts: (1) acute AMR, and (2) chronic AMR. , , , , Hyperacute rejection is now exceedingly rare as ABO barriers are not crossed without desensitization protocols and complement depletion. , Acute AMR can present early with pronounced manifestations or late with more subtle morphological and clinical presentation ( Fig. 53.15 ). Early acute AMR is increasingly being recognized as a cause of allograft dysfunction in about 5% of allografts. It generally occurs in highly sensitized recipients within the first several weeks after transplantation and is characterized by DSA that persists after transplantation, refractory thrombocytopenia, hypocomplementemia, and allograft dysfunction. , Initial evidence of acute AMR in humans was shown in ABO-incompatible transplants and in recipients with preformed lymphocytotoxic antibodies. , Infrequently, late-onset acute AMR with more attenuated features can occur months to years after transplantation in patients who do not adhere to prescribed immunosuppression therapy and is associated with de-novo as well as preformed DSA. , , Criteria and categories for diagnosis of acute AMR have been defined by the Banff Working Group based on a combination of clinical, histopathological, and serological findings( Box 53.1 ).

Definitive for acute/active AMR (all four criteria required):
Histopathological pattern of injury consistent with acute AMR, usually including portal microvascular endothelial cell hypertrophy, portal capillary and inlet venule dilation, monocytic eosinophilic and neutrophilic portal microvasculitis, portal edema ductular reaction, and cholestasis (see Pathological Features for h-scoring and other associated pathological features).
Positive serum DSA (MFI usually >5000)
Diffuse microvascular endothelial C4d deposition (C4d score = 3) on formalin-fixed or frozen tissue in ABO-compatible allografts and/or portal stromal C4d in ABO-incompatible allografts (see Pathological Features for C4d scoring)
Reasonable exclusion of other insults that may cause a similar pattern of injury (see Differential Diagnosis)
Suspicious for AMR (both criteria required)
DSA is positive
Nonzero h-score with (C4d-score + h-score = 3 or 4)
Indeterminate for AMR (requires a + b and c or d)
C4d-score + h-score = >2
DSA not available, equivocal, or negative
C4d staining not available, equivocal, or negative
Coexisting insult may be contributing to the injury
Improvements in immunosuppression, surgical techniques, and control of original disease recurrence have resulted in frequent long-term (beyond 10 years) survival and increasing concern regarding allograft structural integrity and function, particularly in pediatric transplant recipients. Greater understanding of chronic AMR emerged from long-term follow-up of pediatric liver transplant recipients, immunosuppression weaning trials, study of DSA and chronic rejection, and outcomes of suboptimal immune suppression, , , , , with incidence of class II DSA in long-term allograft survivors ranging from 38% to over 50% in recent studies. , Multiple studies have linked progressive allograft fibrosis to anti–class II DSA, angiotensin II type 1 receptor (AT1R), and portal inflammation, providing further evidence of chronic long-term consequences of immunological injury. , , ,
The exact incidence of cAMR is not entirely clear yet, but it is believed to be around 8% at 10-year post transplantation. Risk factors for development of de novo DSA include retransplantation, young age, , cyclosporine as opposed to tacrolimus, low calcineurin inhibitor levels, and Model for End-Stage Liver Disease (MELD) scores. , The effect of immunosuppression on de novo DSA is reviewed here. A recent study describing the histopathology of 460 liver allografts identified chronic fibrosing hepatitis as the leading cause of retransplantation >10 years posttransplantation in pediatric recipients and the second leading cause (after original disease recurrence) in adults. cAMR is thought to occur in a small percentage of recipients with persistent de novo DSA, especially those directed at DQ. , The 2016 Banff Working Group proposed criteria for the diagnosis of probable cAMR ( Box 53.2 ). , , , (In the presence of only minimal or absent C4d staining, cases are classified as possible cAMR ).
Histopathological pattern of injury consistent with chronic AMR (both a and b required):
Otherwise unexplained pattern of injury and at least mild mononuclear portal and/or perivenular inflammation with interface and/or perivenular necro-inflammatory activity
At least moderate portal/periportal, sinusoidal, and/or perivenular fibrosis
Recent circulating HLA DSA in serum samples (MFI usually >5000)
At least focal C4d positivity (involving >10% of portal tract microvascular endothelia)
Reasonable exclusion of other insults that may cause a similar pattern of injury
Given our expanding knowledge of AMR and its short- and long-term implications on liver allografts, more granular diagnostic criteria and diagnostic categories similar to those available for renal allograft pathology are likely forthcoming.
The consequences of preexisting and/or de novo antidonor antibodies depend on the class, subclass, specificity, titer, C1q binding characteristics, and timing of the antibody response, and, importantly, on the density and distribution of target antigens in the liver tissue. , , Antibodies can cause various degrees of damage and can even be protective in some circumstances. Preformed, complement-fixing IgG3 DSA is associated with the greatest risk. , , MHC class I antigens and ABO antigens are moderately to strongly expressed on the vasculature in normal livers. The former can be upregulated when the graft is inflamed for other reasons. MHC class II antigens are expressed at low levels in the hepatic portal microvasculature in the normal liver, but upregulated significantly after transplantation, especially when there is a coexistent insult, such as TCMR.
Living donor liver allografts are more susceptible to AMR than whole-liver cadaveric donors. Increased susceptibility is probably related to several factors unique to reduced-size or living donor allografts, including smaller-caliber blood vessels; enhanced microvascular injury and arterial vasospasm because of a combination of portal hyperperfusion and pathophysiological mediators of AMR (see Reduced-Size and Living Related Allografts) and upregulation of major histocompatibility complex (MHC) antigens on the microvasculature and bile ducts.
ABO incompatibility places recipients at risk for early acute AMR, especially when isoagglutinins are present in high titers (>1:32). , Isoagglutinins also cause more severe damage than lymphocytotoxic antibodies; the latter do not reliably cause clinically relevant allograft dysfunction unless present at relatively high MFI (>10,000) and do not routinely influence short-term survival, unless multiple specificities are present. , , ,
Resistance of liver allografts to AMR from anti-HLA antibodies (DSA) has been attributed to: (1) secretion of soluble MHC class I antigens that bind to and neutralize anti–class I HLA ; (2) phagocytic properties of Kupffer and liver sinusoidal endothelial cells (LSECs), which express abundant Fcγ receptors that enable them to clear different immune complexes, complement-fixing donor-specific HLA antibodies, and platelet aggregates; (3) a dual afferent hepatic blood supply and a large microvascular surface area effectively diluting antigen-antibody complexes; (4) unique regenerative ability of the liver; and (5) low level of MHC II expression. , ,
Preformed DSA are encountered in about 8% to 12% of mostly female recipients. , , , Only 5% to 20% of recipients with a positive crossmatch have strong enough sensitization to cause clinically significant liver injury. ,
The patient population at risk for severe AMR because of anti-HLA antibodies is small; therefore acute AMR is rare (occurring in approximately 5% among patients harboring anti-HLA antibodies ) and often overlooked as a cause of early liver dysfunction or failure. Many programs do not routinely conduct pretransplantation crossmatching or DSA determinations. Even if crossmatches or DSA assays are performed, further studies (e.g., dilution runs, C1q binding) and posttransplantation monitoring for DSA persistence are not routinely carried out.
Antibodies cause damage by binding to allograft endothelium and fixing complement. In severe cases, antibody-complement binding triggers the complement cascade with direct endothelial damage, deposition of platelet-fibrin thrombi, and initiation of the clotting and fibrinolytic cascades. In rapidly evolving cases, this leads to microvascular thrombosis, arterial vasospasm, and coagulopathy that act in concert to impair blood flow and cause hemorrhagic necrosis of the liver. , DSA binding can also facilitate antibody-dependent cellular cytotoxicity (ADCC) in which antibodies facilitate microvascular sludging and binding of Fc-receptor–expressing cells such as macrophages and NK cells. ADCC is recognized in tissue sections as microvascular inflammation or capillaritis, which is distinct from endotheliitis. Less precipitous injury can occur during the transition from acute to chronic rejection when destruction of peribiliary capillary plexuses contributes to ductopenic or chronic rejection. , Microvascular injury can also lead to obliterative portal venopathy and NRH changes. ,
Hyperacute AMR, the most severe form of acute AMR, has been largely eliminated by avoiding high-risk ABO-incompatible transplants without pretreatment. , When it does occur, hyperacute rejection begins immediately after reperfusion. Even in severe cases, allograft dysfunction evolves during a period of hours to days, in contrast with hyperacute rejection in kidney allografts, which occurs immediately after reperfusion. However, initial signs of dysfunction can develop in the operating room after complete revascularization, recognized by uneven reperfusion, swelling, a dusky appearance, and cessation of bile flow. These signs are often accompanied by coagulopathy, difficulty in achieving hemostasis, and an inordinate need for platelets and other blood components. However, precipitous liver allograft failure is rare.
A more common manifestation of acute AMR is a persistent elevation of serum bilirubin and ALT/AST levels (depending on whether the antibodies are isoagglutinin or anti-HLA) during the first several days to weeks after transplantation. This is often accompanied by persistence of circulating DSA, the presence of multiple DSA specificities, unexplained and often refractory thrombocytopenia, and low serum complement activity levels. , , , In severe cases, hepatic angiograms, obtained to exclude an arterial thrombosis, usually show segmental narrowing (indicative of immunologically mediated arterial vasospasm) and diffuse luminal narrowing with poor peripheral blood flow leading to bile duct loss and chronic rejection. ,
Histopathological manifestations of AMR depend on the timing of the biopsy; the subclass, titer or DSA MFI, and specificity of the anti-donor antibodies (HLA, ABO); and whether TCMR coexists. Isoagglutinins usually cause more red blood cell congestion and necrosis than anti-HLA or lymphocytotoxic antibodies ( Fig. 53.16 ).

Hyperacute rejection is rare and largely limited to the era of ABO-incompatible transplants without pretreatment , ; therefore the next few paragraphs are largely of historical importance. However, hyperacute rejection can reappear in the current era if isoagglutinin titers rebound after transplantation. Despite its rarity, early morphological evidence of AMR and its complications comes from studies of liver transplantation across ABO barriers. , , High-titer isoagglutinin (>1:64) often causes prominent red blood cell and focal neutrophil sludging in the sinusoids and platelet-fibrin thrombi in periportal sinusoids, portal veins, and central veins. , , Acidophilic hepatocyte necrosis develops within 2 to 6 hours after reperfusion in untreated recipients. This is often quickly followed by portal or periportal edema, necrosis, focal hemorrhage, C4d deposits in portal stroma, and diffuse endothelial C4d deposits in portal veins, capillaries, and sinusoids. ,
In severe untreated cases, confluent coagulative necrosis, prominent sinusoidal and venous congestion, and edema and hemorrhage into the portal tracts appear within 1 to 5 days. , , Portal veins often show fibrin deposition. If sampled, medium-sized arteries show endothelial cell hypertrophy and evidence of arterial vasospasm, such as mural myocyte vacuolization, wrinkling of the elastic lamina, and thickening of the wall with narrowing of the lumen. Neutrophilic or necrotizing arteritis is only occasionally observed. Portal neutrophilia, cholangiolar proliferation, and small areas of confluent hepatic necrosis begin to appear at 2 to 3 days after transplantation. If untreated, progressive hemorrhagic infarction occurs in ABO-incompatible organs during the next 1 to 2 weeks.
ABO-incompatible allografts that fail because of untreated AMR are often grossly enlarged, cyanotic, and mottled, and they show areas of geographic necrosis. Capsular ruptures, hepatic artery, or portal vein thrombosis can develop in extreme cases. Microscopically, changes in the hilum or perihilar region can be particularly helpful in recognizing that acute AMR caused or contributed to allograft failure. Included are congestion and leukocyte margination in the peribiliary vascular plexus (e.g., evidence of ADCC), partially organized thrombi in arterial branches, focal mural necrosis of large septal bile ducts, and inflammatory and necrotizing arteritis. Late sequelae of acute AMR include biliary sludge and stricturing caused by obstructive cholangiopathy, obliterative arteriopathy, loss of small bile ducts, and chronic rejection. , ,
Reperfusion biopsies from patients with high-MFI multispecificity DSA can show platelet aggregates in the portal and terminal hepatic veins more often than crossmatch-negative controls. , In those destined for dysfunction, diffuse portal microvascular C4d positivity is seen in postreperfusion biopsies. Other findings, such as portal microvascular endothelial cell hypertrophy, eosinophilic and histiocytic portal microvasculitis, inlet venulitis, portal edema, ductular reaction, and diffuse portal microvascular C4d staining, combined with spotty acidophilic necrosis of hepatocytes and centrilobular hepatocellular swelling, accompanied by cholangiolar proliferation and hepatocanalicular cholestasis, often occur during the first week after transplantation in patients with high MFI and multispecific DSA. Inflammatory and necrotizing arteritis is rare, but it is almost diagnostic when detected. Coexistent TCMR with portal eosinophilia is common. Clinicopathological correlation with exclusion of other causes of dysfunction and staining for C4d and other immune deposits are needed to confirm the diagnosis. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here