Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neurologists often equate movement disorders with disease or dysfunction of the basal ganglia, so no review of movement disorders would be complete without a discussion of these subcortical structures and their connections. In some movement disorders such as parkinsonism, chorea, and ballism, the link to the basal ganglia is supported by clinicopathological, biochemical, functional neuroimaging, and electrophysiological data, whereas in other movement disorders such as tremor, dystonia, and tics, dysfunction of the basal ganglia is implied but not proven. Clinicopathological studies relate the signs of Parkinson disease (PD) to deficient dopaminergic neurotransmission in the striatum consequent to the death of dopaminergic neurons in the substantia nigra pars compacta (SNc). Choreic movements in Huntington disease (HD) are linked to the death of medium spiny neurons in the caudate and putamen. Hemiballism (HB) is typically associated with structural lesions in the contralateral subthalamic nucleus (STN) or its afferent or efferent connections. Changes in basal ganglia neurotransmission are well described in many movement disorders, and deepening understanding of basal ganglia neurotransmission has yielded promising symptomatic therapies in many such conditions. Functional neuroimaging studies with specific radiopharmaceutical agents demonstrate abnormal function of basal ganglia structures, and intraoperative electrophysiology studies demonstrate abnormalities in neuronal firing rates and patterns, particularly in the STN and globus pallidus (GP) of patients with PD, dystonia, chorea, and other movement disorders. Animal models including the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD, excitotoxic and transgenic models of HD, and the STN lesion model of HB confirm the central role of disordered basal ganglia function in these conditions. In other movement disorders such as dystonia, the link with basal ganglia function is more complex. For example, although secondary dystonias may result from structural lesions in the contralateral putamen, other sites of pathology include the thalamus, rostral brainstem, and cerebellum. Functional neuroimaging studies in patients with dystonia show abnormal activation of the lenticular nucleus, but neuroimaging and physiological studies provide support for additional involvement of the cortex, brainstem, and cerebellum. In other movement disorders such as essential tremor (ET), restless legs syndrome (RLS), stiff person syndrome (SPS), hemifacial spasm (HFS), spinal myoclonus, and painful legs–moving toes syndrome (PLMTS), the dysfunction appears to lie outside the basal ganglia, such as in the brainstem, cerebellum, spinal cord, or even in the peripheral nervous system.
There is no clear consensus on which structures should be included in the basal ganglia. For the purposes of this discussion, we consider those structures in the striatopallidal circuits involved in modulation of the thalamocortical projection: the caudate nucleus, the putamen, the external segment of the GP (GPe), and the internal segment of the GP (GPi). In addition, the SNc, the substantia nigra pars reticulata (SNr), and the STN are included in the basal ganglia ( ). The substantia nigra (SN), a melanin-containing (pigmented) nucleus, normally contains about 500,000 dopaminergic neurons. Several transcriptional regulators, including Nurr1, Lmx1a, Lmx1b, Msx1, and Pitx3, are responsible for the development and maintenance of midbrain dopaminergic neurons ( ).
The caudate nucleus is a curved structure that traverses the deep hemisphere at the lateral edge of each lateral ventricle. Its diameter is largest at its head, tapering to a small tail. It is continuous with the putamen at the head and tail. The caudate and putamen together are called the striatum , and they form the major target for projections from the cerebral cortex and the SN. The putamen and the GP together form a wedge-shaped structure called the lenticular nucleus . The GP is divided into two parts, the GPe and the GPi. The GPi is structurally and functionally homologous with the SNr. The SNr and SNc extend the length of the midbrain ventral to the red nucleus and dorsal to the cerebral peduncles. The STN is a small lens-shaped structure at the border between the cerebrum and the brainstem. The basal ganglia and its relation to the thalamus and overlying cortex are illustrated in Fig. 96.1 .
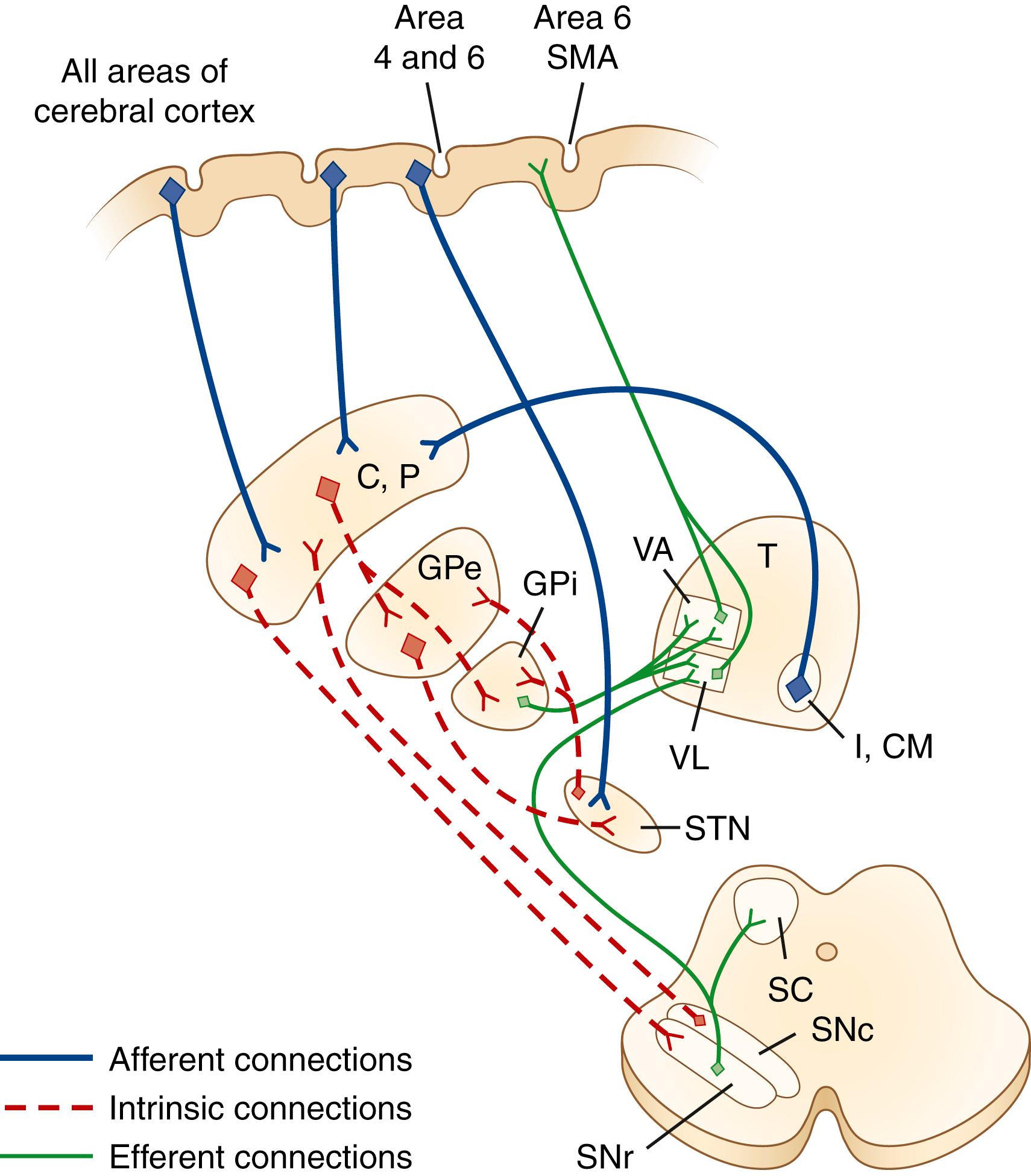
Afferent projections to the striatum arise from nearly all areas of the cerebral cortex, the intralaminar nuclei of the thalamus, mesencephalic SN, and from the brainstem locus coeruleus and raphe nuclei. There is also a projection from the cerebral cortex to the STN ( ). The major efferent projections are from the GPi and SNr to the thalamus and brainstem nuclei such as the pedunculopontine nucleus (PPN). The GPi and SNr project to ventral anterior and ventrolateral thalamic nuclei. The GPi also projects to the centromedian thalamic nuclei, and the SNr projects to the mediodorsal thalamic nuclei and superior colliculi. The ventral anterior and ventrolateral thalamic nuclei then project to the motor and premotor cortex. Throughout, these projections are somatotopically organized ( ).
The basal ganglia has dense internuclear connections (see Fig. 96.1 ). Five parallel and separate closed circuits through the basal ganglia have been proposed. These are the motor, oculomotor, dorsolateral prefrontal, lateral orbitofrontal, and limbic loops ( ). It is now generally accepted that these loops form three major divisions—sensorimotor, associative, and limbic—that are related to motor, cognitive, and emotional functions, respectively ( Table 96.1 ). The functions of the sensorimotor striatum are subserved mainly by the putamen, which derives its afferent cortical inputs from both motor cortices. Sensorimotor pathways are somatotopically organized, and the pathway ultimately terminates in the premotor and primary motor cortices and the supplementary motor area (SMA). Cognitive functions are largely mediated by the associative striatum, particularly the dorsal caudate nucleus, which receives afferent input from the homolateral frontal, parietal, temporal, and occipital cortices. Projections from this pathway ultimately terminate in the prefrontal cortex. The limbic striatum subserves emotional and motivational functions. Its input derives from the cingulate, temporal, and orbitofrontal cortices, the hippocampus, and the amygdala. It mainly comprises the ventral striatum, with ultimate projections to the anterior cingulate and medial orbitofrontal cortices ( ). Whether these divisions are interconnected or organized in parallel remains a topic of debate.
| Origin of Striatal | Termination of Basal | |||
|---|---|---|---|---|
| Division | Afferents | Striatal Nucleus | Ganglia Efferents | Function |
| Sensorimotor | Motor cortex | Putamen | Premotor cortex | Movement |
| Primary motor cortex | ||||
| Supplementary motor area | ||||
| Associative | Frontal cortex | Dorsal caudate | Prefrontal cortex | Cognition |
| Parietal cortex | ||||
| Temporal cortex | ||||
| Occipital cortex | ||||
| Limbic | Hippocampus | Ventral striatum | Anterior cingulated cortex | Emotion |
| Amygdala | Medial orbitofrontal cortex | Motivation | ||
| Cingulate cortex | ||||
| Temporal cortex | ||||
| Orbitofrontal cortex | ||||
Within each basal ganglia circuit lies an additional level of complexity. Each circuit contains two pathways by which striatal activity is translated into pallidal output. These two pathways are named the direct and indirect pathways , depending on whether striatal outflow connects directly with the GPi or first traverses the GPe and STN before terminating in the GPi. The direct and indirect pathways have opposite effects on outflow neurons of the GPi and SNr ( Fig. 96.2 , A ).
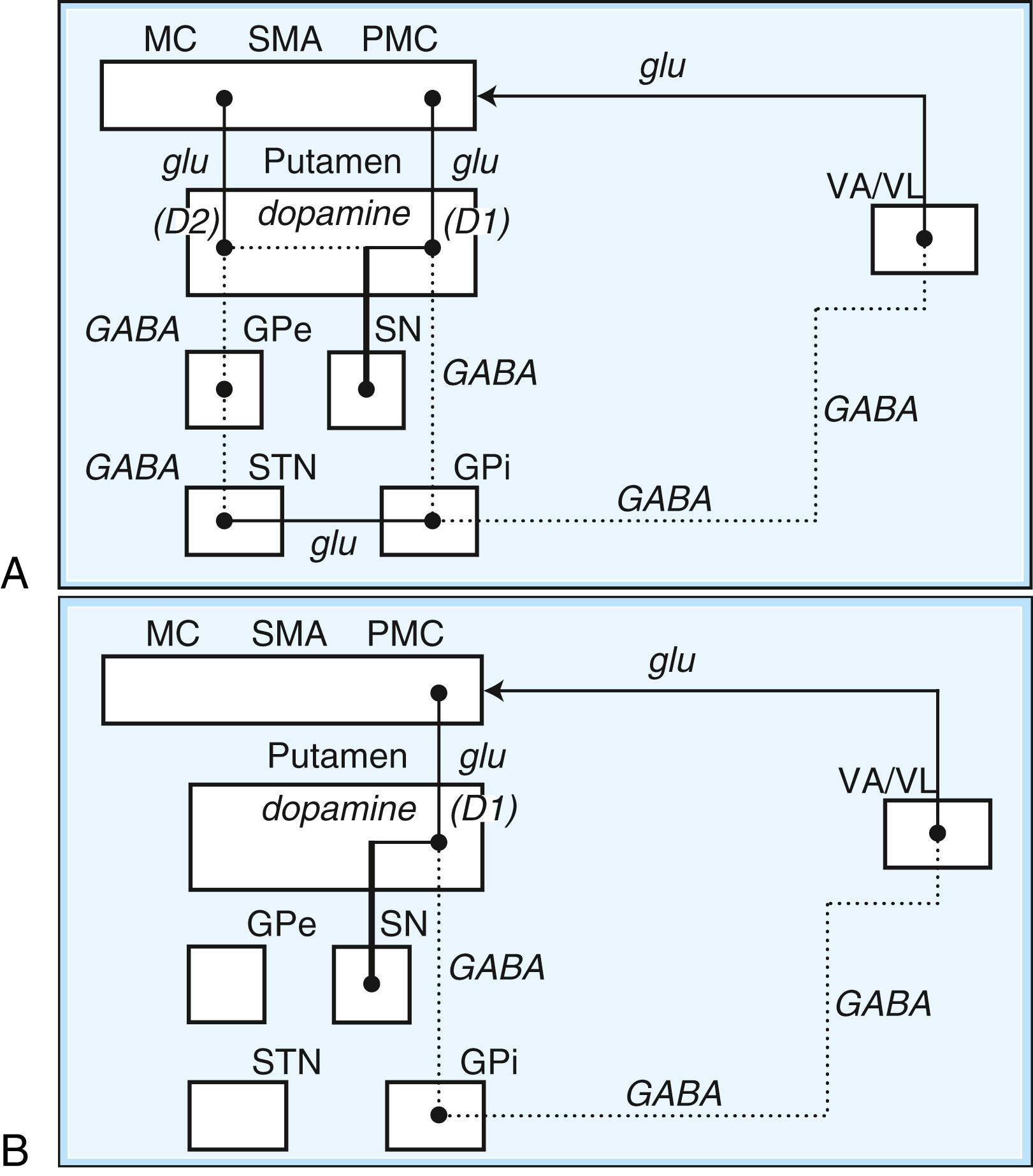
In the motor direct pathway, excitatory neurons from the cerebral cortex synapse on putaminal neurons, which in turn send inhibitory projections to the GPi and its homolog, the SNr. The GPi/SNr sends an inhibitory outflow to the thalamus (see Fig. 96.2 , B ). Activity in the direct pathway disinhibits the thalamus, facilitating the excitatory thalamocortical pathway and enhancing activity in its target, the motor cortices. Thus, the direct pathway constitutes part of an excitatory cortical-cortical circuit that likely functions to maintain ongoing motor activity. In the indirect pathway, excitatory axons from the cerebral cortex synapse on putaminal neurons. These neurons send inhibitory projections to the GPe, and the GPe sends an inhibitory projection to the STN. The net effect of these projections is disinhibition of the STN. The STN in turn has an excitatory projection to the GPi (see Fig. 96.2 , C ). Activity in the indirect pathway thus excites the GPi/SNr, which in turn inhibits the thalamocortical pathway. Thus, the net effect of increased activity in the indirect pathway is cortical inhibition. There is growing appreciation of the importance of direct connection from the cortex to the STN, the so-called hyperdirect pathway, and to the thalamus ( ).
The striatum also receives robust afferent input from the SNc. This projection from the SNc, an important modifier of striatal activity, facilitates activity in the direct pathway, mediated via D 1 dopamine receptors, and inhibits activity in the indirect pathway via D 2 dopamine receptors (see Fig. 96.2 , A ).
Disorders of the basal ganglia result in prominent motor dysfunction, though not generally in frank weakness. The absence of direct primary or secondary sensory input and lack of a major descending pathway below the level of the brainstem suggest that the basal ganglia moderates rather than controls movement. The direct pathway is important in initiation and maintenance of movement, and the indirect pathway apparently plays a role in the suppression of extraneous movement. From this model of basal ganglia connectivity, hypotheses about the motor function of the basal ganglia have been proposed. One hypothesis is that the relative activities of the direct and indirect pathways serve to balance the facilitation and inhibition of the same population of thalamocortical neurons, thus controlling the scale of movement. A second hypothesis proposes that direct pathway-mediated facilitation and indirect pathway-mediated inhibition of different populations of thalamocortical neurons serve to focus movement in an organization reminiscent of center-surround inhibition. These hypotheses relate activity in the direct and indirect pathways mainly to rates of firing in the STN and GPi. Thus, death of neurons in the SNc, as in nigrostriatal degeneration associated with PD, decreases activity in the direct pathway and increases activity in the indirect pathway. These changes cause an increased rate of firing of subthalamic and GPi neurons, with excessive inhibition of thalamocortical pathways, and produce the behavioral manifestations of bradykinesia in PD ( Fig. 96.3 , A ).
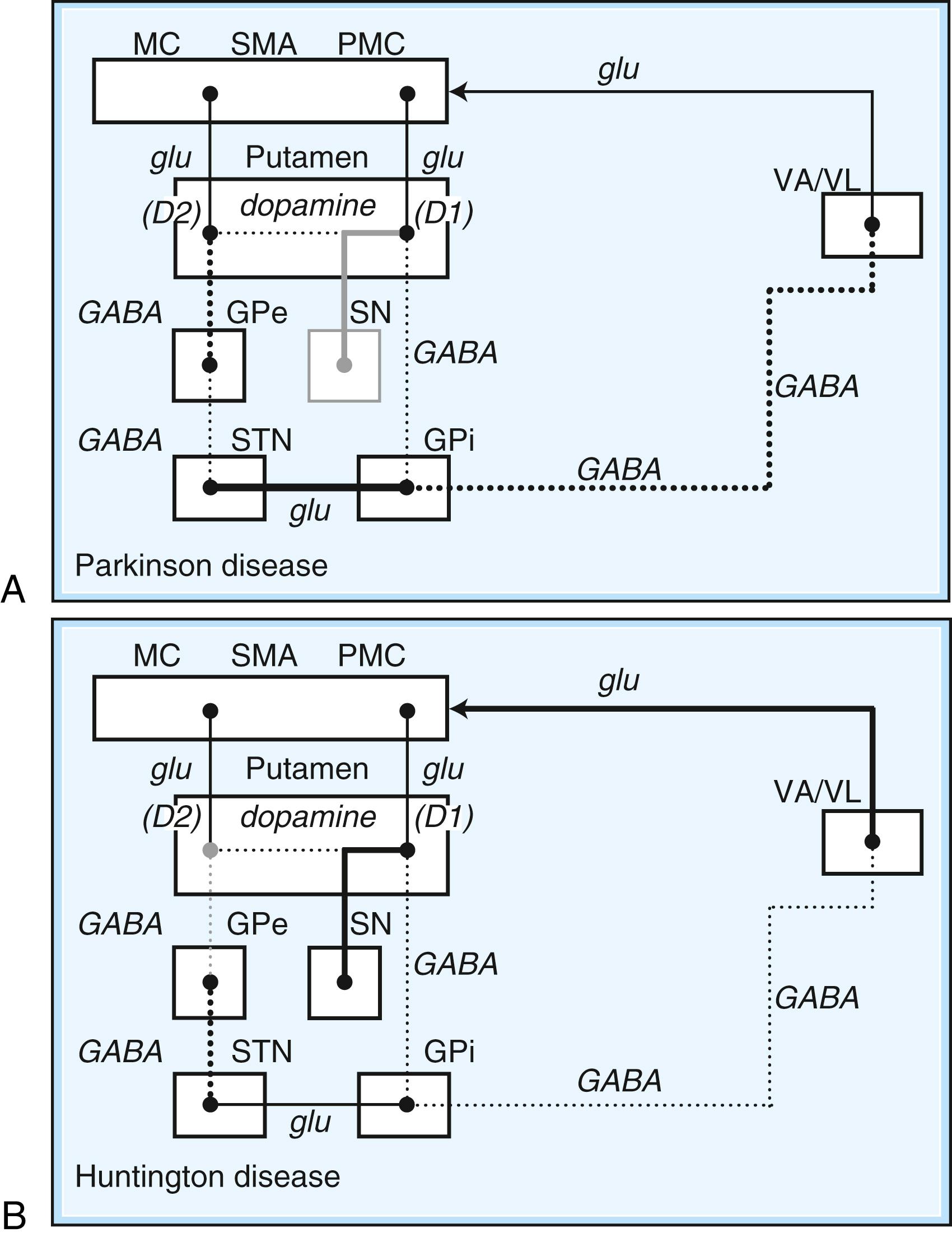
On the other hand, predominant loss of indirect pathway neurons, as in HD, interferes with suppression of involuntary movements. Choreic involuntary movements are the usual result (see Fig. 96.3 , B ). Direct electrophysiological recordings of the STN and GP during stereotactic functional neurosurgical procedures confirm that the GPi and STN are overly active in patients with PD. The activity of these nuclei returns toward normal with effective pharmacotherapy, and chorea is associated with lower firing rates of neurons in these nuclei. Unfortunately, this model does not completely explain some important features of movement disorders. For example, bradykinesia and chorea coexist in HD and in patients with PD treated with levodopa (LD). Thalamic lesions that might be expected to worsen parkinsonism by reducing excitatory thalamocortical activity do not do so. Pallidal lesions that might be expected to worsen chorea by decreasing inhibition of thalamocortical pathways instead are dramatically effective at reducing chorea. The model is even more problematic when applied to dystonia. It has been suggested that in dystonia there is overactivity of both the direct and indirect pathways. Yet, intraoperative recordings in dystonia have shown low rates and abnormal patterns of neuronal firing in the GPi. A simple change in firing rate of the STN or GPi is thus insufficient to explain the underlying physiology of dystonia. It is likely that disordered patterns and synchrony of pallidal firing, as well as changes in sensorimotor integration and the control of spinal and brainstem reflexes, are important. These factors are under investigation, but current models remain useful for understanding the rationale of pharmacological and ablative surgical procedures for certain movement disorders.
Although much of the emphasis has been on GPi and SNr efferents to the thalamocortical system, there is growing evidence that descending pathways, particularly to the zona incerta and PPN, are important in movement disorders. The PPN appears to play a role in locomotion, muscle tone, and akinesia. A number of other pathways also seem particularly relevant to myoclonus, including a corticolemniscal-thalamocortical circuit and a spinobulbar-spinal circuit that primarily involves the spinoreticular tracts, nucleus reticularis gigantocellularis of the medullary reticular formation, and the reticulospinal tracts. The Guillain-Mollaret triangle is a network connecting the red nucleus, dentate nucleus, and inferior olive, which has been implicated in palatal myoclonus (PM) (also known as palatal tremor ) and myorhythmia ( ). The propriospinal pathways and segmental spinospinal loops are important in the genesis of propriospinal and spinal segmental myoclonus, respectively.
It is beyond the scope of this chapter to review all the brain structures involved in motor control, but there has been considerable recent interest in the lateral habenula, located above the posterior thalamus. The lateral part of the habenula has an inhibitory influence on the SNc, but its exact role in various movement disorders is unknown, although it has been implicated in some mood disorders ( ). Another structure that has received some interest, particularly as it relates to ET, is zona incerta. This nucleus appears to be an extension of the reticular nucleus of the thalamus, situated between the thalamus and the fields of Forel, with its fiber tracts conveying the pallidal output to the thalamus. Chiefly inhibitory (GABAergic), the zona incerta may act to synchronize activity generated by the basal ganglia and cerebellum. Indeed, there is a growing body of evidence of communication between the cerebellum and basal ganglia involving γ-aminobutyric acid (GABA) and other neurotransmitters ( ).
Our understanding of basal ganglia neurotransmitters and pharmacology is growing rapidly. In addition to dopamine, there are many other neurotransmitters that play a role in motor and nonmotor functions ( ; Klein et al., 2014). Along with this growth is an expanding spectrum of practical applications for pathology, neuroimaging, and therapeutics. For example, catecholamine and amino acid neurotransmitters coexist with peptides. This co-localization may allow histopathological differentiation among medium spiny striatal projection neurons that secrete γ-aminobutyric acid (GABAergic neurons), further elucidating the specific nature and progress of striatal neurodegeneration. Neuroimaging technology has been aided by the development of radiopharmaceutical ligands with such discrete targets as the dopamine transporter on the presynaptic dopamine neuron and subpopulations of dopamine receptors on the postsynaptic neuron. The pharmaceutical industry is searching for ways to provide better-targeted and more physiological stimulation of neurotransmitter receptors and is expanding its investigations from the primary targets themselves to approaches that may modify responsiveness of the primary targets.
The major neurotransmitters of the basal ganglia are outlined in Table 96.2 (see also Fig. 96.2 ). Most excitatory synapses of the basal ganglia and its connections—including those from the cerebral cortex to the striatum, the STN to the GPi, and the thalamocortical projections—use glutamate. Projections from the striatum to the GPe and GPi, from the GPe to the STN, and from the GPi to the thalamus are inhibitory and employ GABA. Medium spiny GABAergic neurons in the direct pathway co-localize substance P and dynorphin. GABAergic neurons in the indirect pathway co-localize enkephalin. Dopamine is the major neurotransmitter in the nigrostriatal dopamine system; it has excitatory or inhibitory actions depending on the properties of the stimulated receptor. Acetylcholine is found in large aspiny striatal interneurons and the PPN. Norepinephrine, important in the autonomic nervous system, is most concentrated in the lateral tegmentum and locus coeruleus. Serotonin is found in the dorsal raphe nucleus of the brainstem, hippocampus, cerebellum, and spinal cord.
| Pathway | Transmitter |
|---|---|
| Striatal Afferents | |
| Cerebral cortex → striatum | Glutamate |
| Cerebral cortex → STN | Glutamate |
| Locus coeruleus → striatum | Norepinephrine |
| Locus coeruleus → SN | Norepinephrine |
| Raphe nuclei → striatum | Serotonin |
| Raphe nuclei → SN | Serotonin |
| Thalamus → striatum | Acetylcholine? |
| SNc → striatum | Glutamate? |
| Dopamine, cholecystokinin | |
| Intrinsic Connections | |
| Striatal interneurons | GABA, acetylcholine |
| Striatum → GPi | Somatostatin, neuropeptide Y |
| Striatum → SNr | Nitric acid, calretinin |
| Striatum → GPe | GABA, substance P |
| GPe → STN | GABA, dynorphin, substance P |
| STN → GPi, SNr, GPe | GABA, enkephalin, glutamate |
| Striatal Efferents | |
| GPi → thalamus | GABA |
| SNr → thalamus | GABA |
For each of these neurotransmitters, multiple types of receptors may exist. Glutamate is active at a number of types of ligand-gated ion channel receptors named for their selective agonists: N -methyl- d -aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and kainate. The NMDA receptor has been the focus of particular attention because of its potential role in excitotoxic neuronal injury. There are also metabotropic glutamate receptors ( ). Glutamate is not only an excitatory neurotransmitter by opening calcium channels, but it is also involved in many metabolic processes by creating short- and long-term changes in synaptic excitability that are thought to be fundamental in brain plasticity.
GABA, the main inhibitory neurotransmitter in the brain, is synthesized by glutamic acid decarboxylase (GAD) from glutamate. There are three classes of GABA receptors, GABA A , GABA B , and GABAc. The subclasses are largely differentiated by their relative sensitivity to benzodiazepines. For example, benzodiazepines can increase the inhibitory action of a GABA A synapse. GABA A receptors are ligand-gated chloride channels and have many subtypes. The GABA B receptor is a metabotropic receptor.
Five types (D 1 through D 5 ) and two families (D 1 and D 2 ) of dopamine receptors have been identified ( ). The D 1 family of receptor is adenylate cyclase dependent and contains subtypes D 1 and D 5 . D 1 receptors reside primarily in the direct pathway, cerebral cortex, and limbic system. D 2 receptors are located primarily in the indirect pathway, cerebral cortex, and limbic system, as well as in the pituitary gland. There are many types of cholinergic receptors, designated as M1–M5, which mediate both excitatory and inhibitory effects ( ). Most striatal cholinergic receptors are muscarinic. In the norepinephrine system, there are two primary receptor systems, α and β. There are many distinct receptor subtypes of serotonin receptors, including G protein–coupled receptors in the 5-HT1, 5-HT2, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 families and the 5-HT3-type ligand-gated ion channels. Adenosine A 2A receptors are co-localized with striatal dopamine D 2 receptors on GABAergic medium spiny neurons, which project via the indirect striatopallidal pathway to the GPe. Drugs targeting specific subpopulations of receptors are in use or under development for movement disorders, but there remains a knowledge deficit about the relative clinical utility of specific receptor agonists and antagonists.
There is a growing interest in the tetrahydrocannabinol and the cannabinoid system but its role in motor control or various movement disorders is still not well understood, although there is evidence that the cannabinoids modulate dopaminergic effects ( ; ; ). CB1 receptor is the principal receptor in the central nervous system (CNS), particularly abundant in the basal ganglia ( ). TRVP 1 (transient receptor potential vanilloid type1) receptors also respond to cannabinoids. The main endogenous ligands for the CB1 receptor are anandamide and 2-arachidonoylglycerol (2-AG).
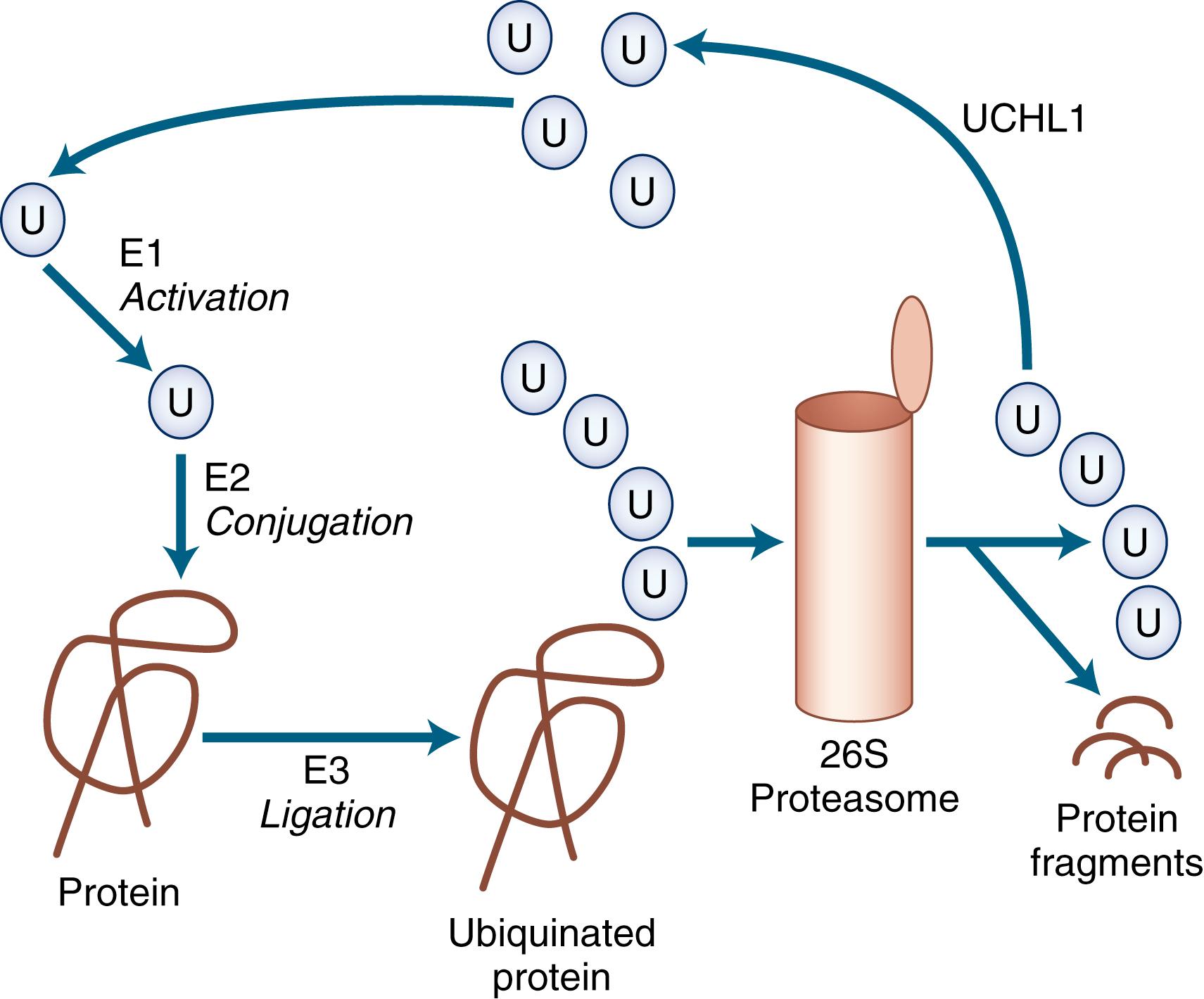
Many of the neurodegenerative movement disorders share the property of neuronal damage caused by the accumulation of aggregation-prone proteins that have toxic effects ( Table 96.3 ). For a protein to function normally, it must be properly synthesized and folded into its normal three-dimensional structure. Nascent proteins are aided in folding by molecular chaperones. Proteins that are not properly folded, are otherwise damaged, or are beyond their useful lives are degraded by the ubiquitin-dependent proteasome protein degradation system ( ). In the ubiquitin-dependent proteasome system, proteins are first labeled for degradation by attachment of a polyubiquitin chain ( Fig. 96.4 ). This three-step process involves activation, conjugation, and ligation steps catalyzed by three types of enzymes—E1, E2, and E3, respectively. Polyubiquitinated protein enters the 26S proteasome, a cylindrical complex of peptidases. The end products of proteasome action are protein fragments and polyubiquitin. The polyubiquitin is then degraded and recycled to the cellular ubiquitin pool, a process requiring enzymatic action by ubiquitin carboxy-terminal hydrolase 1.
| α-Synuclein | Tau | Polyglutamine Tract |
|---|---|---|
| Parkinson disease | Four-repeat tau | Huntington disease |
| Diffuse Lewy body disease | Progressive supranuclear palsy | Spinocerebellar ataxias |
| Multiple system atrophy | Corticobasal degeneration Frontotemporal dementia with parkinsonism (chromosome 17) Parkinsonism dementia complex of Guam Postencephalitic parkinsonism |
Dentatorubral-pallidoluysian atrophy |
The cascade of pathogenic events linking abnormal protein aggregation to cell death is the subject of intense investigation. Although aggregates are the most striking physical change in surviving cells, the actual role of the aggregate remains a mystery. Indeed, many now believe that the formation of aggregates may be a protective mechanism sequestering the wayward protein from vulnerable cell processes ( ). Nevertheless, there are now many clinical trials designed to suppress α-synuclein as a potential disease-modifying strategy ( ).
Misfolded proteins may produce the most mischief as they form protofibrils. A number of mechanisms have been described. In some cases, these are specifically related to the type of protein, but in many other cases, they are nonspecific mechanisms shared by all the misfolded protein diseases. There is growing evidence that preformed fibrils generated from full-length and truncated recombinant α-synuclein enter neurons, probably by endocytosis, and act as “seeds” that induce recruitment of soluble endogenous α-synuclein into insoluble Lewy body–like inclusions, resulting in progressive prion-like spread of neurodegeneration ( ). Some potential mechanisms of neurodegeneration related to misfolded protein stress are listed in Box 96.1 . The mutant protein may be unable to perform a vital function or may interfere with the function of the wild-type protein. Mutant protein, protofibrils, or aggregates might interfere with other proteins. Interference with transcription factors may be particularly important in this regard. Mutant proteins may activate caspases or in other ways activate the apoptotic cascade. They may interfere with intracellular transport or other vital processes. They may suppress activity of the proteasome, enhancing protein aggregation. They may interfere with mitochondrial function, making cells more vulnerable to excitotoxicity. In addition to the ubiquitin-proteasome system, lysosomes play an important role in degrading intracellular proteins by a process termed autophagy ( ). When the function of the ubiquitin proteasome system is not sufficient to clear the accumulating cellular proteins, the autophagy lysosome pathway becomes the other important route for degradation of aggregated/misfolded proteins as well as sick or abnormal mitochondria. Indeed, mitophagy is an increasingly recognized mechanism for removing sick mitochondria and maintaining cellular health ( ). Accumulation of iron, increased oxidative stress, and microglial activation have also been thought to play important roles in the pathogenesis of various neurodegenerative disorders ( ).
Loss of protein function
Interaction of the mutant protein with the wild-type protein
Interaction with other proteins, including transcription factors
Caspase activation
Apoptosis
Suppression of proteasome function
Interference with mitochondrial function
Oxidative stress
Microglial activation
Many neurodegenerative movement disorders can be linked to abnormal synthesis, folding, or degradation of specific proteins or protein families and the notion that progression of neurodegenerative disease is mediated via seeding of misfolded proteins has extended to a broad range of mutated proteins, including α-synuclein, tau, huntingtin, SOD-1, and TDP-43. The synucleinopathies include PD, Lewy body disease (LBD), and multiple system atrophy (MSA). The tauopathies include progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), familial frontotemporal dementia (FTD) with parkinsonism (FTDP), postencephalitic parkinsonism (PEP), post-traumatic parkinsonism, and amyotrophic lateral sclerosis (ALS)-PD of Guam. The polyQ disorders include HD, dentatorubral-pallidoluysian atrophy (DRPLA), and many spinocerebellar ataxias ( ; ).
Within the CNS, certain neuronal populations seem selectively vulnerable to the various pathogenic mechanisms of cell death. This preferential degeneration of specific neuronal populations ultimately determines the phenotype of the disorder. Better understanding of the various pathogenic cellular mechanisms and selective vulnerability may lead to neuroprotective therapeutic strategies that favorably modify the natural course of the neurodegenerative disease.
In his monograph, The Shaking Palsy (1817), James Parkinson identified the hallmark features of the illness through descriptions of cases observed in the streets of London as well as in his own patients ( ). Over time, Parkinson disease or idiopathic PD has replaced the original term paralysis agitans as the name for the clinical syndrome of asymmetrical parkinsonism, usually with rest tremor, in association with the specific pathological findings of depigmentation of the SN due to loss of melanin-laden dopaminergic neurons containing eosinophilic cytoplasmic inclusions (Lewy bodies) (see Chapter 24 ). Dopamine deficiency in parkinsonian brain was described by Hornykiewicz in 1959, a discovery that ultimately led to highly effective pharmacotherapy with LD and direct-acting dopamine agonists (DAs). Recently, genetic forms of parkinsonism that are clinically indistinguishable from PD have been linked to mutations in several genes ( ). The discovery of different genetic forms of parkinsonism with variable penetrance has led to the current concept of PD as a syndrome with genetic and environmental etiologies, but, overall, gene mutations are a rare cause of parkinsonism, particularly in those patients with late-onset disease ( ; ) ( Chapter 24 ).
In community-based series, PD accounted for more than 80% of all parkinsonism, with a prevalence of approximately 360 per 100,000 and an incidence of 18 per 100,000 per year ( ). PD is an age-related disease, showing a gradual increase in prevalence beginning after age 50, with a steep increase in prevalence after age 60. Disease before 30 years of age is rare and often suggests a hereditary form of parkinsonism. Prevalence rates in the United States are higher than those in Africa and China, but the role of race remains unclear. Within the United States, race-specific prevalence rates vary, with some studies suggesting a similar prevalence among Whites and Blacks. Unfortunately, Blacks make up only a small fraction of most specialty clinic populations and thus are underrepresented in clinic-based studies and clinical trials. One study showed the world’s highest prevalence of PD may be among the Amish in the US Northeast—nearly 6% of those 60 years of age or older, more than three times the reported 1%–2% prevalence for the rest of the country ( ).
Typically, the onset and progression of PD are gradual. We have developed a screening tool that can be used to detect early symptoms of PD ( ). The most common presentation is with rest tremor in one hand, often associated with decreased arm swing and shoulder pain ( ; ). Although 4–5 Hz rest tremor is considered the typical tremor of PD, the more troublesome tremor experienced by patients with PD is postural tremor, either re-emergent tremor occurring after a latency of a few seconds following the assumption of position of outstretched arms, or the postural tremor of PD ( ). In contrast to the rest and re-emergent tremor which may be related to dopamine deficiency, the PD action-postural tremor appears to correlate with serotonergic deficiency ( ). Bradykinesia and rigidity are often detectable on the symptomatic side ( ), and midline signs such as reduced facial expression or mild contralateral bradykinesia and rigidity may already be present. The presentation may be delayed if bradykinesia is the earliest symptom, particularly when the onset is on the nondominant side. The disorder usually remains asymmetrical throughout much of its course. With progression of the illness, generalized bradykinesia may cause difficulty arising from a chair or turning in bed. Patients typically develop stooped posture and in some cases the flexion of the trunk can become quite severe, the so-called camptocormia (see ![]() , ) ( ). Some patients may also develop deformities in the hands and feet which can resemble arthritis, the so-called “striatal deformities” (see ) ( ). The gait and balance are progressively affected, and falls may occur. Sudden arrests in movement, also called freezing or motor blocks , soon follow, first with gait initiation, turning and traversing narrow or crowded environments, and then during walking (see
, ) ( ). Some patients may also develop deformities in the hands and feet which can resemble arthritis, the so-called “striatal deformities” (see ) ( ). The gait and balance are progressively affected, and falls may occur. Sudden arrests in movement, also called freezing or motor blocks , soon follow, first with gait initiation, turning and traversing narrow or crowded environments, and then during walking (see ![]() ). Bulbar functions deteriorate, impairing communication and nutrition. The tremor-dominant form of PD generally has a more favorable clinical course than PD dominated by gait disorder and postural instability ( ).
). Bulbar functions deteriorate, impairing communication and nutrition. The tremor-dominant form of PD generally has a more favorable clinical course than PD dominated by gait disorder and postural instability ( ).
Parkinson Disease; Patient with Young-Onset Parkinson Disease and Gait Difficulty Due to Freezing (Motor Blocks)
Patient with Parkinson Disease and Anterocollis and Camptocormia
Patient with Parkinsonism and Striatal Hand Deformities
Parkinson Disease; Patient with Young-Onset Parkinson Disease and Gait Difficulty Due to Freezing (Motor Blocks)
The Unified Parkinson’s Disease Rating Scale (UPDRS) has been used to quantitate the various motor symptoms and signs of PD and to chart the course of the disease. This traditional scale, now known as the Movement Disorder Society (MDS)-UPDRS , has been revised to clarify some ambiguities in the original version and to capture early motor and also nonmotor symptoms associated with PD ( http://www.movementdisorders.org ). The Hoehn and Yahr staging, first described before effective dopaminergic treatment became available, outlines the milestones in progression of the illness from mild unilateral symptoms through the end-stage nonambulatory state. A modified version of the Hoehn and Yahr stage is commonly used in contemporary clinical trials ( Table 96.4 ).
| Stage | Disease state |
|---|---|
| Original Scale | |
| I | Unilateral involvement only, minimal or no functional impairment |
| II | Bilateral or midline involvement, without impairment of balance |
| III | First sign of impaired righting reflex, mild to moderate disability |
| IV | Fully developed, severely disabling disease; patient still able to walk and stand unassisted |
| V | Confinement to bed or wheelchair unless aided |
| Modified Scale | |
| 0 | No signs of disease |
| 1 | Unilateral disease |
| 1.5 | Unilateral plus axial involvement |
| 2.0 | Bilateral disease without impairment of balance |
| 2.5 | Mild bilateral disease with recovery on pull test |
| 3.0 | Mild to moderate bilateral disease, some postural instability, physically independent |
| 4.0 | Severe disability, still able to walk or stand unassisted |
| 5.0 | Wheelchair bound or bedridden unless aided |
Nonmotor symptoms are increasingly recognized as a major cause of disability in PD and contribute prominently to declining quality of life, particularly in the more advanced stages of the disease ( ). Autonomic symptoms include reduced gastrointestinal transit time with postprandial bloating and constipation, urinary frequency and urgency (sometimes with urge incontinence), impotence, disordered sweating, and orthostatic hypotension. Cognitive and behavioral changes are also very common. Attention and concentration wane. Executive dysfunction with diminished working memory, planning, and organization is common. Global dementia occurs in approximately 30% of patients, increasing in frequency with the age of the patient. Those with prominent early executive dysfunction and more severe motor signs seem particularly at risk. Anxiety, depression, and other mood disorders are common in PD. Sleep disturbance is nearly universal in PD and is multifactorial.
Disordered sleep onset and maintenance lead to fragmentation of nocturnal sleep. A variety of motor movements including RLS and periodic leg movements of sleep may be seen, and many patients have rapid eye movement (REM) sleep behavior disorder (RBD) with active motor movements during REM sleep. The following question was found to have 94% sensitivity and 87% specificity in detecting RBD: “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?” ( ). Some patients with PD have sleep apnea. Vivid dreams and nightmares are very common, particularly in treated patients. Sleep disorders in PD variably relate to the pathological changes of the disease itself, arousals due to immobility, comorbid primary sleep disorders, and side effects of antiparkinsonian medications. Many patients with PD are excessively sleepy during the day, sometimes with serious consequences such as unintended sleep episodes while driving. In most cases, this excessive daytime drowsiness is related to dopaminergic drugs. Fatigue is a common and complex symptom of PD. The differentiation of fatigue from excessive daytime sleepiness, depression, apathy, and other conditions can be difficult, and there is not yet a useful body of literature on its assessment and treatment.
Clinicopathological studies have found that the clinical variable that best predicts the typical pathological changes of PD, in the absence of other diagnoses known to cause parkinsonism, is an asymmetrical illness with rest tremor along with rigidity or bradykinesia and marked improvement with LD, motor fluctuations, dyskinesias, and hyposmia ( ). Misdiagnosed cases generally are found to have MSA, PSP, or subcortical vascular disease. When making the diagnosis of early PD, the clinician should be aware of a number of red flags ( Box 96.2 ) (see Chapter 24 ). Cognitive impairment within the first year should raise the possibility of Alzheimer disease (AD), dementia with Lewy bodies (DLB), corticobasal syndrome (CBS), PSP, or FTDP. Symmetrical or prominent midline or bulbar signs suggest MSA or PSP. Early gait disorder with falls points to the diagnosis of PSP or to subcortical vascular disease. Dependence on a wheelchair within 5 years of onset is suggestive of PSP or MSA. Early orthostatic hypotension or incontinence points to the autonomic dysfunction of MSA. Severe sleep apnea, inspiratory stridor, or involuntary sighing also suggests MSA. Apraxia, alien limb, or cortical sensory loss is typically seen in CBS.
Early or prominent dementia
Symmetrical signs
Bulbar dysfunction
Early gait disorder
Falls within the first year
Wheelchair dependence within 5 years
Early autonomic failure
Sleep apnea
Inspiratory stridor
Apraxia
Alien limb
Cortical sensory loss
Routine laboratory studies are not helpful in the diagnosis of PD, and their use should be reserved for patients with atypical features. There is a growing interest in serums and cerebrospinal fluid (CSF) biomarkers that may differentiate between PD and atypical parkinsonism. In this regard, several studies have found that neurofilament light chain (NFL) protein levels have been found elevated in the serum and CSF of patients with atypical parkinsonism but not in PD ( ). When genetic causes (see below) are suspected, testing for specific PD-related monogenic mutations or by whole-exome or whole-genome sequencing may be indicated when coupled with appropriate genetic counseling ( ).
Neuroimaging studies such as computed tomography (CT) and magnetic resonance imaging (MRI) are usually not very helpful in making a diagnosis of PD, because they are generally normal or show only incidental abnormalities. Sometimes neuroimaging abnormalities can be useful in suggesting alternative diagnoses such as PSP or MSA (see below). The radiopharmaceutical 6-[ 18 F]-fluorodopa (F-dopa) is taken up by dopaminergic neurons in the SN and metabolized to 6-[ 18 F]-fluorodopamine. Positron emission tomography (PET) scans using this radiopharmaceutical agent show reduced F-dopa uptake in dopaminergic nerve terminals in the putamen and caudate proportional to the severity of degeneration in the ipsilateral SN and symptoms in the contralateral hemibody ( Fig. 96.5 ). Although these tests are used in PD research, they are not readily clinically available at this time. Single-photon emission CT (SPECT) with radioligand that labels the dopamine transporter on nerve terminals in the striatum (DaTscan) is a very helpful tool in differentiating PD from ET, drug-induced parkinsonism (DIP), or functional (psychogenic) parkinsonism ( ; ). Since most atypical parkinsonian disorders have striatal dopaminergic denervation, DaTscan is not helpful in differentiating these disorders from PD. Routine electrophysiological testing is not helpful in the diagnosis of PD.
![Fig. 96.5, Positron emission tomography scan with [ 11 C]RTI-32, which labels the presynaptic dopamine transporter in a normal control (A) and a subject with early Parkinson disease (PD) (B) . There is asymmetrically reduced uptake in PD, indicating asymmetrical loss of presynaptic dopaminergic neurons. Fig. 96.5, Positron emission tomography scan with [ 11 C]RTI-32, which labels the presynaptic dopamine transporter in a normal control (A) and a subject with early Parkinson disease (PD) (B) . There is asymmetrically reduced uptake in PD, indicating asymmetrical loss of presynaptic dopaminergic neurons.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ParkinsonDiseaseandOtherMovementDisorders/4_3s20B9780323642613000966.jpg)
The most striking pathological changes in PD occur in the SNc. The SN appears pale to the naked eye. Microscopic changes include neuronal loss, gliosis, and the presence of extracellular pigment. Surviving neurons may show characteristic cytoplasmic inclusions ( Fig. 96.6 ). These inclusions, called Lewy bodies , have a dense eosinophilic core and a pale halo (except for those located in the cortex) ( ). They contain hyperphosphorylated neurofilament proteins, lipids, iron, ubiquitin, and α-synuclein. Pigmented nuclei elsewhere in the brainstem, including the locus coeruleus, dorsal motor nucleus of the vagus, and others, may also show Lewy bodies and characteristic degenerative changes. The substantia innominata and intermediolateral cell column in the spinal cord also are affected. Patients with PD and dementia show more diffuse Lewy body pathology or comorbid AD. Even the myenteric intestinal and cardiac plexus of patients with PD may contain Lewy bodies, showing that PD is not just a CNS disease.
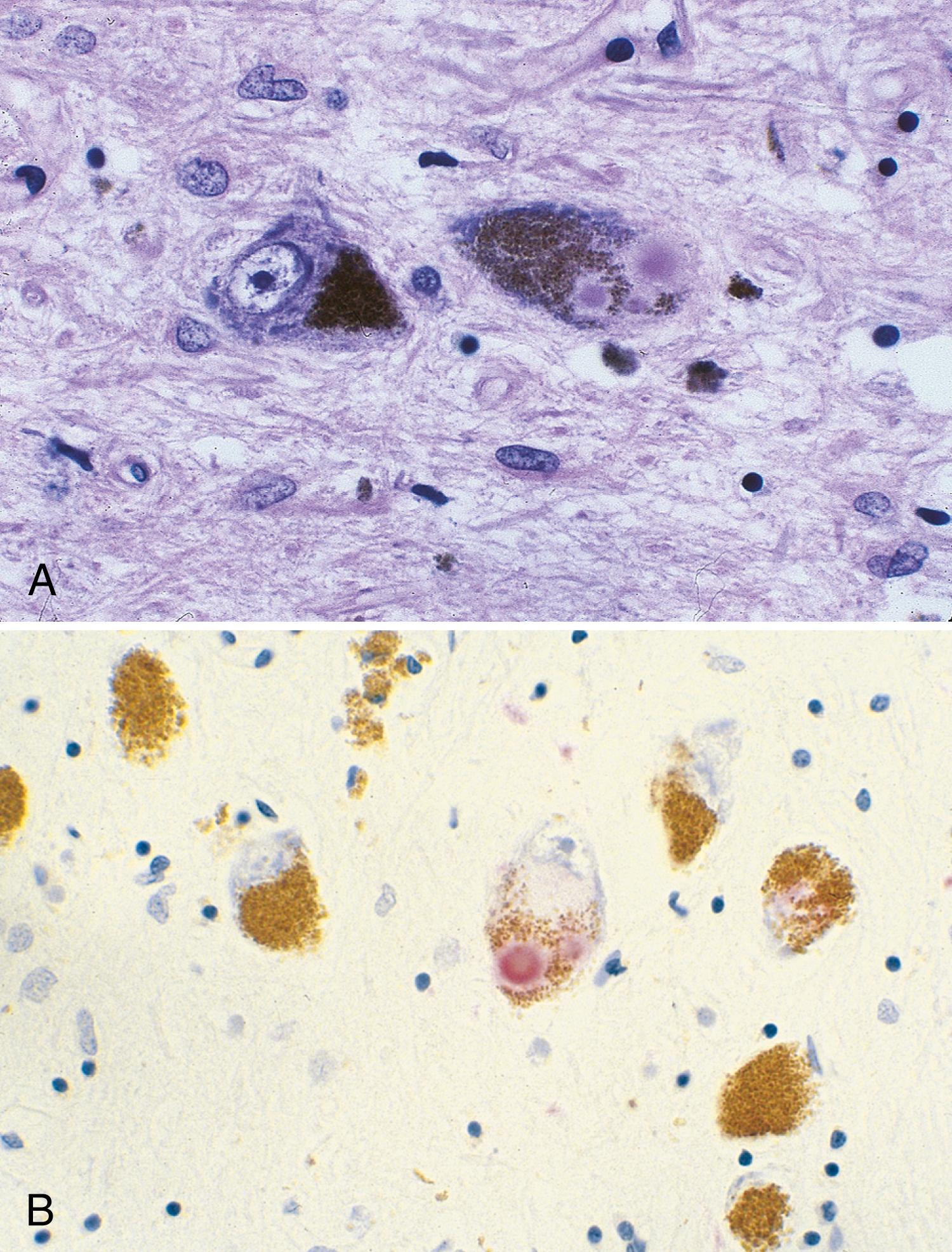
A staging system introduced by Braak ( ) has been developed to characterize the progression of neuropathological changes associated with PD. According to Braak staging, during the presymptomatic stages (1 and 2), the PD-related inclusion body pathology remains confined to the medulla oblongata and olfactory bulb. In stages 3 and 4, the SN and other nuclear grays of the midbrain and basal forebrain are the focus of initially subtle and then severe changes, and the illness reaches its symptomatic phase. In the end stages (5 and 6), the pathological process encroaches upon the telencephalic cortex. Although the Braak hypothesis is supported by early olfactory, sleep, and autonomic involvement in patients with PD, the staging proposal has been challenged for many reasons and inconsistencies, such as absence of cell counts to correlate with the described synuclein pathology, absence of immunohistochemistry to identify neuronal types, absence of observed asymmetry in the pathological findings that would correlate with the well-recognized asymmetry of clinical findings, absence of bulbar symptoms as early features of PD, and the observation that brain synucleinopathy consistent with Braak stages 4 and 6 has been found in individuals without any neurological signs. Although the Braak hypothesis and the central role of α-synuclein in the pathogenesis of PD have been challenged ( ) these concepts provide a useful framework for understanding the progression of neurodegeneration in PD.
Studies of large numbers of patients with PD have suggested that PD is a multifactorial illness with likely genetic and environmental determinants ( ; ). Twin studies suggest that heredity plays a relatively small role in the population at large, but the hereditary component is greater if one twin has disease onset at younger than age 50. Moreover, PET studies of twins suggest that most monozygotic twins of patients with PD show subclinical declines in dopamine innervation, strengthening the evidence for a significant hereditary contribution irrespective of age at onset.
Although the majority of cases of PD appear to be sporadic, it is becoming increasingly evident that genetic factors play an important role in the pathogenesis of PD, particularly if onset is earlier than age 50 (see Chapter 24 ). Some 20%–25% of patients have at least one first-degree relative with PD, and first-degree relatives are two to three times as likely as relatives of controls to develop PD. The most cogent evidence for genetic contribution to the pathogenesis of PD has been provided by reports of large multicase kindreds with dominantly inherited autopsy-proven PD. A genome scan in the Contursi kindred of Greek-Italian origin found a genetic marker on chromosome 4q21-q23 linked to the PD phenotype. Subsequent studies identified at least three different mutations in the α-synuclein gene (SNCA), the first monogenetic form of PD, designated PARK1 (see Table 24.1 ). In addition to the typical PD features, this family exhibited dementia, severe central hypoventilation, orthostatic hypotension, prominent myoclonus, urinary incontinence, and pathological involvement of the brainstem pigmented nuclei, hippocampus, and temporal neocortex. Later, the application of quantitative real-time PCR amplification of the SNCA gene showed that some families with a PD phenotype, originally designated as PARK4, had duplication and triplication of the gene, with marked increase in the amount of α-synuclein protein. Thus, an overexpression of α-synuclein may lead to neurodegenerative disease, with features overlapping with PD, DLB, and MSA. Based on screening, the entire coding region of the gene in a large number of PD patients shows that mutation in the SNCA gene is a rare cause of PD.
Discovery of a linkage between an autosomal recessive, young-onset,LD-responsive form of PD to a locus on chromosome 6q25.2-27 led to subsequent identification of numerous mutations in the gene called parkin (PARK2). This 500-kb, 12-exon gene encodes a 465-amino acid protein with E3 ubiquitin-ligase activity through interaction with the ubiquitin-conjugating enzyme UbcH7 (E2). Associated with the Golgi complex, the parkin protein has also been thought to be involved in vesicular transport. Parkin strongly binds to a variety of proteins and microtubules, a disruption of which in patients with parkin mutations affects vesicular transport and may contribute to the nigrostriatal degeneration. Whereas normal parkin is involved in ubiquitination and subsequent degradation of certain proteins by proteasomes, mutated parkin protein loses this activity and thus may lead to an accumulation of proteins, causing a selective neural cell death without formation of Lewy bodies. In addition to typical PD features, patients with PARK2 exhibit a variety of atypical features such as hyperreflexia, dystonia, leg tremor, autonomic dysfunction, sensory axonal peripheral neuropathy, marked sleep benefit, LD-induced dyskinesias, psychosis, and other behavioral and psychiatric problems. Although PARK2 has been identified in patients with late age at onset, up to half of patients with onset of PD before age 40 years have parkin mutations.
A growing number of novel genes have been implicated in the pathogenesis of PD ( ; ) (see Chapter 24 ). In addition to α-synuclein ( SNCA gene), there are many other monogenetic causes of PD (see Table 24.1 ) ( ) Mutations in the PTEN-induced putative kinase 1 (PINK1) gene on chromosome 1p36 were identified in autosomal recessive families with early-onset parkinsonism (PARK6). The PINK1 gene codes for a putative serine-threonine kinase located in the mitochondria, thus providing further support for the role of oxidative stress in the pathogenesis of PD. The mean age at onset is in the fourth decade, and the course is quite benign, associated with LD-induced dyskinesias. These clinical features are similar to those of another autosomal recessive form of PD (PARK7) localized to the same chromosomal region in the DJ-1 gene. Besides slow progression and good, prolonged response to LD, patients with a DJ-1 mutation may exhibit blepharospasm, leg dystonia, anxiety, and parkinsonism-dementia-ALS complex. In contrast to parkin mutations that may account for up to 50% of young-onset PD, the DJ-1 mutations account for approximately 1% of all young-onset PD cases.
Another locus mapped to 12p11.23-q13.11 (PARK8) was initially identified in a Japanese family with typical PD inherited in an autosomal dominant pattern with incomplete penetrance and has been subsequently found to be the most common form of familial adult-onset PD ( ). The course of the disease is relatively benign, usually presenting with unilateral hand or leg tremor without cognitive deficit; the patients respond well to LD. Other clinical phenotypes have included parkinsonism with dementia, hallucinations, dysautonomia, amyotrophy, or both and otherwise typical ET. Autopsy studies demonstrate variable pathology, ranging from Lewy body and tau neurofibrillary tangle pathology to no pathological changes. The gene responsible for PARK8 on 12p11.2-q13.1, called LRRK2 (leucine-rich repeat kinase 2), belongs to the ROCO protein family and includes a protein kinase domain of the MAPKKK class and several other major functional domains. The gene product, a protein called dardarin (from Basque word dardara , meaning “tremor”), is a novel protein that probably functions as a cytoplasmic kinase involved in phosphorylation of proteins such as α-synuclein and tau. LRRK2 is closely associated with a variety of membrane and vesicular structures, membrane-bound organelles, and microtubules, suggesting its role in vesicular transport and membrane and protein turnover, including the lysosomal degradation pathway. This mutation has been found to be particularly frequent in PD patients of North African origin and in Ashkenazi Jewish patients ( ). The penetrance is quite variable, and many elderly individuals have the mutation but no signs of PD have been reported.
It is beyond the scope of this chapter to discuss all the various genetic causes of PD but the reader is referred to Chapter 24 and other reviews on this topic ( ). The variable penetrance and growing number of causative and susceptibility genes, coupled with a growing number of commercially available DNA tests, has obvious implications for genetic counseling ( ).
Evidence for environmental causes of PD comes primarily from two sources: the fortuitous discovery of parkinsonism in parenteral drug users exposed to the contaminant MPTP and epidemiological associations of sporadic PD or other parkinsonisms with certain lifestyle or occupational exposures. The discovery that a handful of drug addicts had developed a severe LD-responsive form of parkinsonism following parenteral administration of a meperidine analog contaminated with the mitochondrial protoxin MPTP suggested that environmental toxins might cause PD. The discovery of MPTP-induced parkinsonism in humans was a sentinel event in our understanding of the disease because it pointed to a class of environmental toxins that might be important in sporadic disease. Although MPTP spawned the development of reproducible models of disease in many kinds of animals, its role in human disease is limited to the cluster of cases in drug addicts and a few others. Intriguing studies have confirmed that certain pesticides (e.g., paraquat, rotenone) can reproduce the pathology of PD in animals, but their role in human disease remains undefined.
Epidemiological studies suggest that exposure to environmental metals or organic toxins may be associated with an increased risk of PD or an earlier age at onset. Case-controlled studies have suggested that the risk of PD is increased in persons who have worked in the agricultural industry, have been exposed to pesticides, or have sustained significant head injury. Whether exposure to welding predisposes to earlier onset of PD, possibly as a result of manganese poisoning, is controversial ( ). On the other hand, the risk of PD seems lower in those with a high dietary intake of antioxidant-rich foods, as well as caffeine drinkers and those who have smoked cigarettes. Although PD and cancer are two distinct diseases that result from either degeneration or over-proliferation, respectively, several recent studies have provided evidence that while PD provides some type of biological protection against most types of cancers, the disease confers increased risk for other cancers such as melanoma. The relationship between PD and melanoma is being explored, but the higher frequency of melanoma does not appear to be due to LD. It is possible that high concentrations of α-synuclein in the skin of patients with PD may increase their risk of melanoma by inhibiting tyrosine hydroxylase, an enzyme involved in dopamine and melanin biosynthesis or by some other mechanism ( ).
Before discussing specific treatment strategies for PD, it is important to recognize that the quantitative assessment of clinical symptoms and progression of the course is an essential component of any therapeutic trial ( ; Jankovic and Tan, 2020). In addition to sensitive clinical rating scales (e.g., MDS-UPDRS), reliable diagnostic, presymptomatic, and progression biomarkers are needed ( ; ).
A preclinical period lasting years, its slow progression rate, and our increasing understanding of disease etiopathogenesis make PD an ideal candidate for neuroprotective therapeutic strategies. However, double-blind placebo-controlled trials designed to explore therapies that may have favorable disease-modifying effects and slow disease progression have been thus far disappointing. The first “neuroprotective” trial, DATATOP (Deprenyl and Tocopherol Antioxidative Therapy of Parkinsonism), randomized patients with early PD to treatment with placebo, tocopherol, selegiline (deprenyl), or both, using the time until the patients needed potent symptomatic dopaminergic therapy, LD, as a proxy endpoint for disease progression. The selective monoamine oxidase (MAO-B) inhibitor, selegiline, successfully delayed this endpoint, but interpretation of the study was contaminated by the drug’s mild symptomatic antiparkinsonian and antidepressant properties, as well as the potential effects of its amphetamine metabolites. Although disease-modifying effects of selegiline have been suggested by some clinical trials, further studies are needed before it can be concluded that selegiline is neuroprotective in PD. Another MAO-B inhibitor, rasagiline, has been shown to have modest symptomatic benefit ( ), but its effects on disease progression are also still being debated. In a randomized multicenter, double-blind, placebo-controlled, parallel-group study prospectively examining rasagiline’s potential disease-modifying effects (ADAGIO [Attenuation of Disease Progression with Azilect Given Once-Daily]), delayed-start design was used to assess the potential disease-modifying effects of rasagiline ( ). A total of 1176 patients with early untreated PD (mean time from diagnosis, 4.5 months) from 129 centers in 14 countries were randomized into 4 treatment groups (either 1 or 2 mg/day, early-start versus delayed-start treatment, 9 months each). Early-start treatment consisted of 72 weeks of rasagiline (either 1 or 2 mg once daily), and delayed-start treatment consisted of 36 weeks of placebo followed by 36 weeks of rasagiline (either 1 or 2 mg once daily [active treatment phase]). The primary analyses of the trial were based on change in total UPDRS score and included slope superiority of rasagiline over placebo in the placebo-controlled phase, change from baseline to week 72, and noninferiority of early-start versus delayed-start slopes during weeks 48 through 72 of the active phase. The 1-mg dose group met all three endpoints, but there was no observable benefit with the higher 2-mg dose, although when analyzing the upper quartile group, the 2-mg dose group met all the primary endpoints. Some possible explanations for the seemingly confusing outcome include early symptomatic treatment helping some compensatory mechanism, cumulative symptomatic effect, and other possibilities.
Development of neuroprotective strategies has been challenging, partly because of lack of reliable and sensitive biomarkers of progression ( ; ). Animal models are essential in preclinical testing of potential symptomatic and neuroprotective therapies ( ). One of the most exciting developments of potential neuroprotective or disease-modifying therapies is the use of α-synuclein monoclonal antibodies to reduce α-synuclein formation and rescue dying neurons ( ; ).
Many types of medications are available for symptomatic treatment of PD: anticholinergics, amantadine, LD, MAO inhibitors (MAOIs), catechol-O-methyltransferase inhibitors (COMTIs), and DAs ( ; ). Anticholinergics such as trihexyphenidyl and benztropine antagonize the effects of acetylcholine at muscarinic receptors postsynaptic to striatal interneurons. They reduce tremor and rigidity but have no effects on bradykinesia. Toxicity relates to antagonism of acetylcholine at central receptors, causing confusion, and peripheral receptors, causing blurred vision, dry mouth, constipation, and urine retention. Although amantadine has been available for nearly 4 decades (it was originally marketed as an anti-influenza, antiviral agent), its antiparkinsonian mechanisms have been poorly understood. It has been thought to stimulate release of endogenous dopamine stores, block reuptake of dopamine from the synaptic cleft, and have anticholinergic properties. However, amantadine has been found to have antiglutamatergic properties and as such is the only antiparkinsonian drug that improves LD-induced dyskinesia. Extended release formulation of amantadine has been found to improve not only dyskinesia but also motor fluctuations ( ).
Combining LD with carbidopa, an aromatic acid decarboxylase inhibitor that prevents its peripheral metabolism, markedly reduces its peripheral adverse effects, particularly nausea. The global antiparkinsonian efficacy of LD is so dramatic and predictable that a positive therapeutic response is used to define the disease itself. Adverse effects of LD include nausea and vomiting, orthostatic hypotension, sedation, confusion, sleep disturbance, alterations of dream phenomena, hallucinations, and dyskinesias (see ![]() ). Many studies have concluded that DAs such as pramipexole, ropinirole, and rotigotine, when introduced early in the course of PD treatment, may delay LD-related complications such as motor fluctuations and dyskinesias. But evidence is lacking to support the hypothesis that early introduction of DAs slows progression of the disease or even improves long-term quality of life ( ). The PROUD study (Pramipexole on Underlying Disease), which assessed early versus delayed pramipexole treatment in early PD, involved 535 untreated PD patients who were randomized to double-blind placebo or pramipexole (1.5 mg/day) for 6–9 months and continued with pramipexole for up to 15 months. The researchers found no difference in UPDRS (−0.4 UPDRS units) or PDQ-39 scores in the 411 patients who completed the 15-month study, but at the end of the placebo-controlled phase, the difference in adjusted means was −4.8 UPDRS units (95% confidence interval [CT], −6.3, −3.2; P < .0001), and there was a significant difference in PDQ-39 ( P = .0001), both in favor of pramipexole ( ). Furthermore, many studies have shown that LD is more effective than DAs in reducing motor symptoms in early as well as advanced stages of PD ( ). In a pragmatic, open-label, randomized trial involving 1620 patients with a newly diagnosed PD randomized to receive a DA ( N = 632), a monoamine oxidase inhibitor ( N = 460), or LD ( N = 528), after median follow-up of 3 years there was a slightly better (1.8 points) PDQ-39 mobility score with LD than with the other two treatments ( ). Although the study suggested small but persistent benefits when PD patients were initially treated with LD compared with LD-sparing therapy, the study did not adequately address whether patients with young-onset PD should be treated differently than those with late-onset PD. Despite the findings from the PD MED study, most parkinsonologists would probably still employ LD-sparing strategy in patients with young-onset PD.
). Many studies have concluded that DAs such as pramipexole, ropinirole, and rotigotine, when introduced early in the course of PD treatment, may delay LD-related complications such as motor fluctuations and dyskinesias. But evidence is lacking to support the hypothesis that early introduction of DAs slows progression of the disease or even improves long-term quality of life ( ). The PROUD study (Pramipexole on Underlying Disease), which assessed early versus delayed pramipexole treatment in early PD, involved 535 untreated PD patients who were randomized to double-blind placebo or pramipexole (1.5 mg/day) for 6–9 months and continued with pramipexole for up to 15 months. The researchers found no difference in UPDRS (−0.4 UPDRS units) or PDQ-39 scores in the 411 patients who completed the 15-month study, but at the end of the placebo-controlled phase, the difference in adjusted means was −4.8 UPDRS units (95% confidence interval [CT], −6.3, −3.2; P < .0001), and there was a significant difference in PDQ-39 ( P = .0001), both in favor of pramipexole ( ). Furthermore, many studies have shown that LD is more effective than DAs in reducing motor symptoms in early as well as advanced stages of PD ( ). In a pragmatic, open-label, randomized trial involving 1620 patients with a newly diagnosed PD randomized to receive a DA ( N = 632), a monoamine oxidase inhibitor ( N = 460), or LD ( N = 528), after median follow-up of 3 years there was a slightly better (1.8 points) PDQ-39 mobility score with LD than with the other two treatments ( ). Although the study suggested small but persistent benefits when PD patients were initially treated with LD compared with LD-sparing therapy, the study did not adequately address whether patients with young-onset PD should be treated differently than those with late-onset PD. Despite the findings from the PD MED study, most parkinsonologists would probably still employ LD-sparing strategy in patients with young-onset PD.
Parkinson Disease; Levodopa-Induced Dyskinesia
Because of various adverse effects related to their ergot structure, particularly fibroproliferative lesions of heart valves, lung, and other tissues, bromocriptine, pergolide, and cabergoline have been discontinued from clinical use. Apomorphine, a nonergoline DA, is water soluble and lipophilic and is therefore suitable for intravenous, subcutaneous, sublingual, intranasal, or transdermal administration. Apomorphine is available as an acute intermittent subcutaneous injection, as a rapid rescue from hypomobility off episodes (end-of-dose wearing off and unpredictable on/off episodes) associated with advanced PD. DAs cause side effects similar to those of LD, although orthostatic hypotension, sleepiness, and hallucinations are more common or severe. Continuous apomorphine infusion has been found to meaningfully reduce off time in PD patients who experience troublesome motor fluctuations ( ).
One major concern with DAs is the relatively high frequency of a variety of behavioral problems that include pathological gambling, compulsive shopping and eating, hypersexuality, and other impulse-control disorders (ICD) ( ). Patients with PD who experience ICD seem to have a variety of associated psychiatric symptoms, such as psychoticism, interpersonal sensitivity, obsessive-compulsive symptoms, and depression ( ) and seem to be prone to dopamine dysregulation syndrome, an addictive behavior and excessive use of dopaminergic medication ( ).
Selegiline and rasagiline block MAO-B-dependent dopamine degradation and have modest effects in potentiating the action of LD. These drugs are now used very infrequently, but some clinicians prescribe these MAO-B inhibitors as the initial pharmacological agents in newly diagnosed patients in an attempt to delay LD therapy. This approach is in part supported by the ADAGIO study ( ). COMTIs (entacapone and tolcapone) block peripheral degradation of peripheral LD and central degradation of LD and dopamine (tolcapone), increasing central LD and dopamine levels. Hepatotoxicity associated with tolcapone has limited its use. Triple-combination therapy containing LD, carbidopa, and entacapone is available for patients with moderately advanced PD. The primary role of COMTIs is to prolong the effects of LD, so they are useful as adjunctive drugs for patients who experience LD-related motor fluctuations. Besides increasing LD-related dyskinesias, COMTIs may cause nausea, postural hypotension, diarrhea, and orange discoloration of urine, but they are generally well tolerated. There is no evidence that COMTIs prevent or delay the onset of LD-related motor complications. Symptomatic pharmacological treatment should begin when the patient is noticing functional, occupational, or social disability related to PD symptoms. Prospective studies have suggested that approximately 70% of patients with PD will require symptomatic therapy within 2 years of disease onset. Less potent therapies such as selegiline, rasagiline, amantadine, and DAs may be useful for initial therapy, particularly in patients with young-onset PD, but LD should be used when more potent therapy is indicated or in patients with late-onset disease. The argument that LD might be toxic to dopaminergic neurons is based on (1) the recognition that dopamine metabolites increase oxidative stress and (2) the observation that LD is toxic to cultures of mesencephalic neurons in vivo. There is, however, no in vivo evidence from animal or human studies that LD accelerates disease progression, and it is difficult to reconcile the potential of dopamine toxicity with the obvious fact that the drug prolongs life in patients with PD. A 9-month study called the Earlier versus Later L-DOPA (ELLDOPA) trial compared different doses of LD with placebo and found no evidence of LD toxicity ( ). Nevertheless, as a result of “LD phobia,” many patients and physicians still unnecessarily delay LD therapy in patients who would clearly benefit from symptomatic relief ( ).
Clinical experience with LD treatment of PD indicates that there is a progressive increase in the prevalence of drug-related motor fluctuations (wearing off, dyskinesia) over time, and that about half of patients experience wearing off, and a third experience dyskinesias within 2 years after initiation of LD therapy. Wearing off results from loss of DA storage in the striatum as a result of loss of nigrostriatal terminals. Experiments in animal models relate the development of dyskinesia to changes in striatal glutamate receptor sensitivity consequent to pulsatile stimulation of striatal dopamine receptors. Continuous dopamine receptor stimulation with LD or with long-acting DAs prevents or reverses this phenomenon.
In eliciting a description of the patient’s response to medication, it is important to understand the severity of symptoms in the morning on arising and the latency, magnitude, and duration of benefit from each dose of LD. Information about the onset of motor and nonmotor symptoms during wearing off as well as the phenomenology, timing, and distribution of dyskinesia. The usefulness of historical information may be augmented by careful patient education on symptom recognition and the development of a shared vocabulary. Completing motor diaries ( Fig. 96.7 ) helps both the patient and the treating physician recognize patterns of motor response and adjust the medications accordingly.
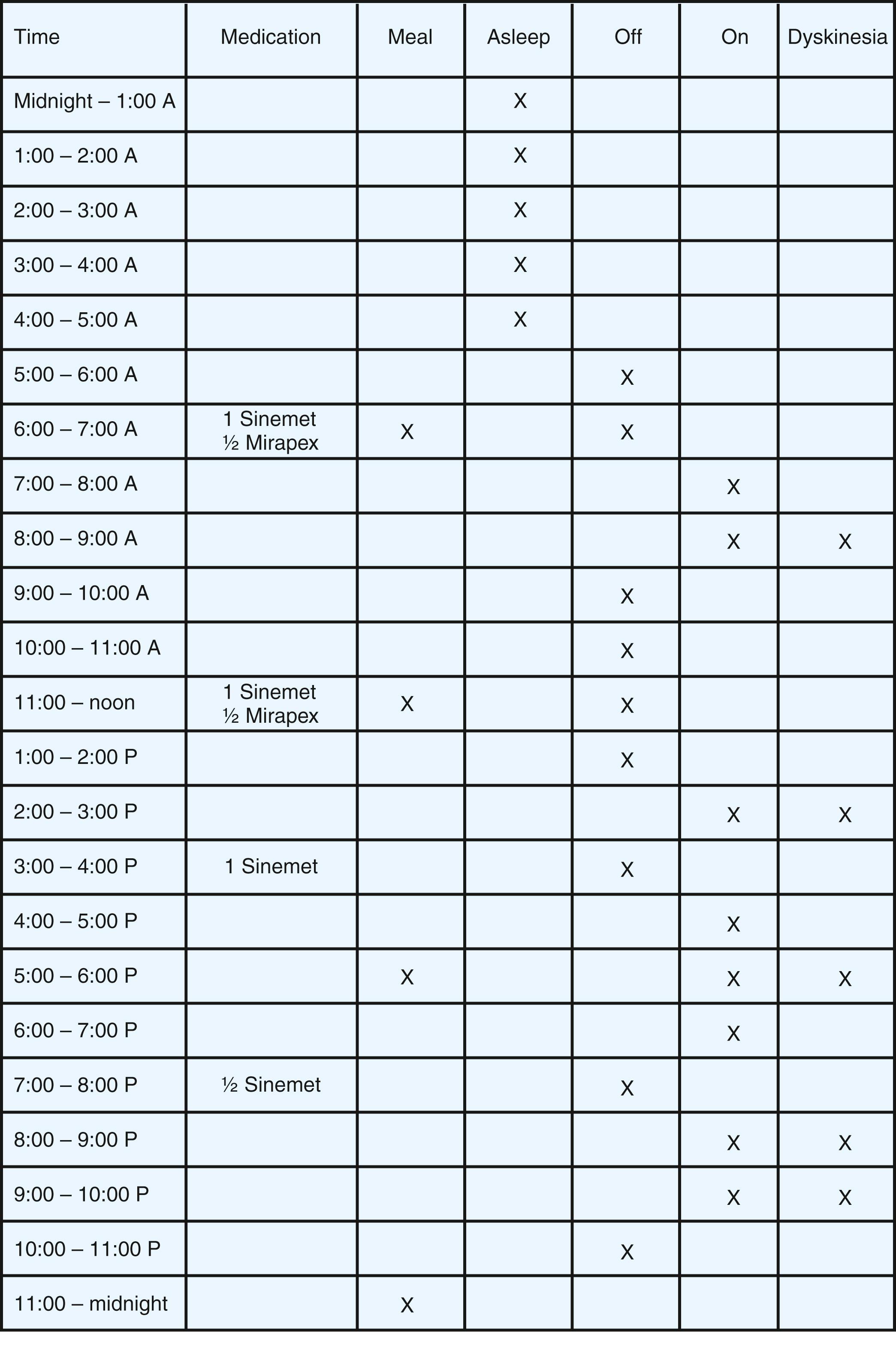
Wearing off is the most common type of motor fluctuation. It refers to the return of parkinsonian symptoms following the previous dose in advance of the next scheduled antiparkinsonian dose. On/off is the unpredictable reappearance of parkinsonism at a time when central levels of antiparkinsonian drugs are expected to be within the target therapeutic range. Delayed on is a prolongation of the time required for the central antiparkinsonian drug effect to appear. Dose failure is a complete failure to develop a favorable response to an incremental dopaminergic dose. This may be related to protein intake which interferes with the transport of LD across the intestinal wall as a result of competition for facilitated transport by large amounts of neutral amino acids. A variety of dyskinesias can further complicate the response to LD. Peak-dose dyskinesias are usually choreiform or stereotypical movements, such as head bobbing movement of the head or choreic movements of limbs and trunk, present at the peak of the therapeutic response. Off-period dystonia usually appears in the more severely affected foot in the morning before the first daily doses, sometimes reappearing during wearing off. Diphasic dyskinesias are usually large-amplitude dyskinetic movements of the lower body during the time of increasing and decreasing LD levels.
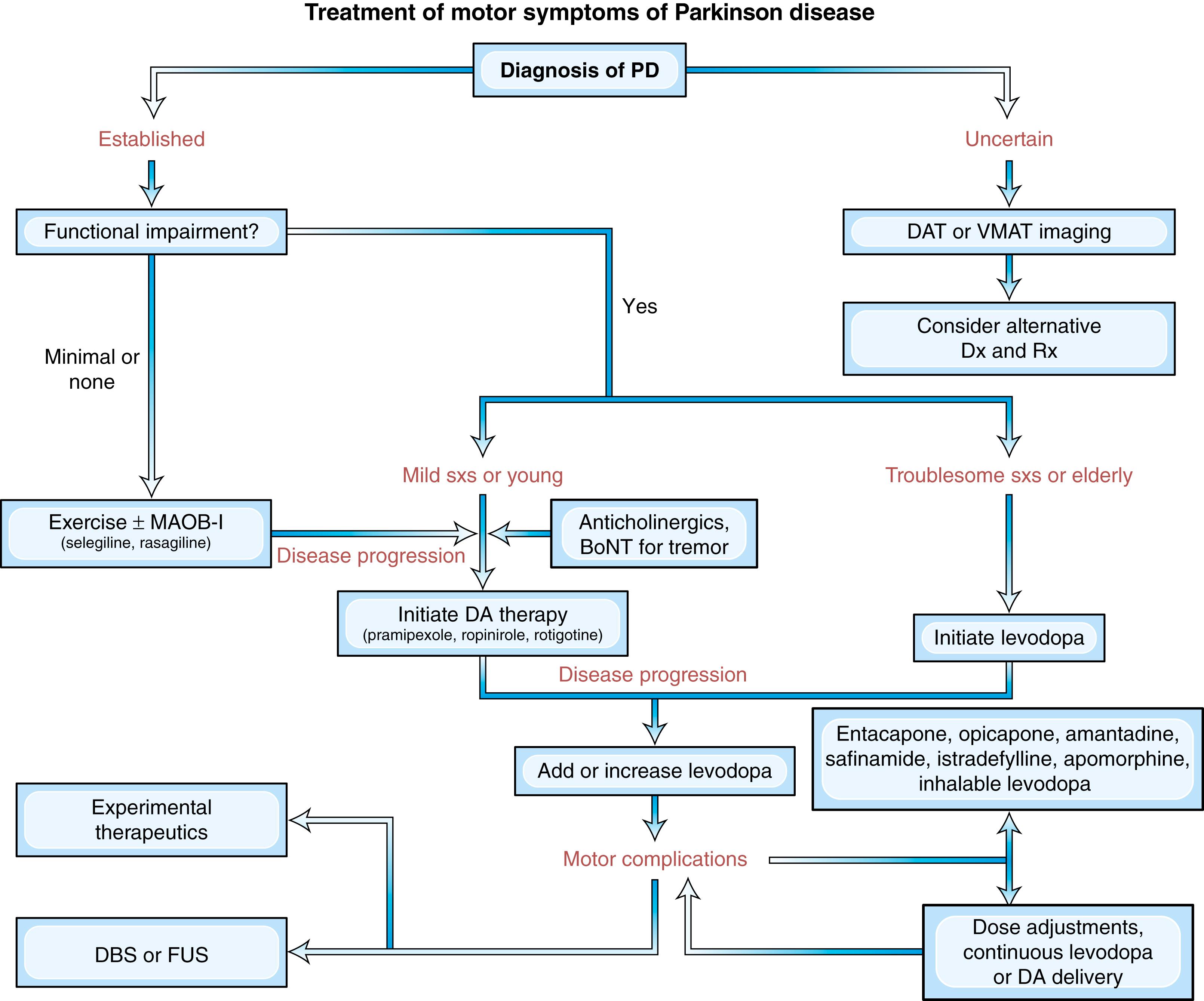
Armed with a few basic principles and a commonsense approach, the clinician can usually smooth out fluctuations for most patients with appropriate selection of drugs and dose ( ) ( Figs. 96.8 and 96.9 ). Delay to onset of therapeutic benefit can be hastened by taking the medication on an empty stomach (if tolerated without nausea), avoiding or reducing protein intake, or by crushing the LD tablet and mixing it with a carbonated beverage. The duration of benefit increases when the individual dose is increased or dopamine metabolism is blocked with an MAO-B or COMTIs. This, however, may increase the risk of dyskinesia and the patient may do better on smaller, more frequent LD doses. Thus fractionation of LD dose is usually the initial strategy in an attempt to smooth out fluctuations and prevent wearing off symptoms.
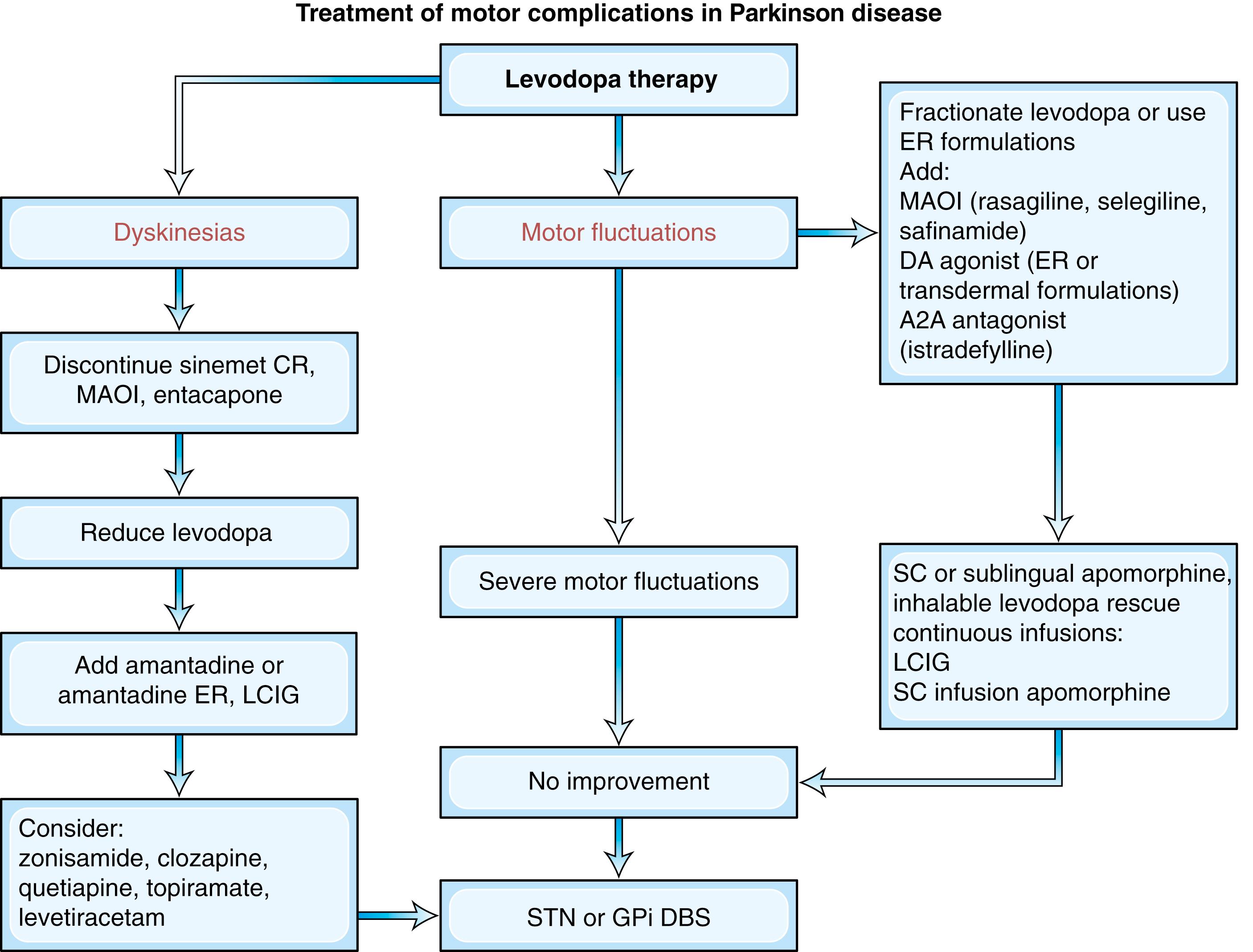
In addition to the fluctuating response, some patients, particularly those with advanced disease, may acquire LD-resistant motor symptoms such as freezing, progressive gait dysfunction, dysarthria and dysphagia, and recurrent falling due to loss of balance and postural instability. Other features of advanced illness (cognitive impairment, autonomic dysfunction, psychiatric complications) may limit the types and dosage of tolerated medications. Freezing , sudden immobility of the feet while walking, often with falls, may be seen in either the off or the on period. Although off-period freezing may improve with optimization of medications, on-period freezing is usually resistant to pharmacological treatment. Physical therapy, including strategies that utilize sensory cues, such as stepping over a horizontal laser beam, may be helpful. Dysarthria and dysphagia are often treated by speech therapists, although documentation of improvement from these techniques is scant.
Cognitive impairment increases mainly with the age of the patient and with disease severity. Preliminary reports suggest that cholinesterase inhibitors might be useful in PD-associated dementia, but these studies require confirmation in carefully controlled trials. Orthostatic hypotension can be managed conservatively with salt supplementation, fludrocortisone, midodrine, and droxidopa for orthostatic hypotension ( ). Urological medications may improve bladder dysfunction, and dietary changes along with medications such as linaclotide and lubiprostone may improve constipation. Hallucinations occur in approximately 30% of treated patients; a loss of insight that the visions are not real or the appearance of psychotic thinking signals a particularly disabling complication. Hallucinations often improve with atypical antipsychotics such as quetiapine and clozapine. Also, pimavanserin, a non-dopaminergic and selective serotonin inverse agonist with high affinity at the 5-HT2A receptor has been found to be effective in the treatment of psychosis and hallucinations related to dopaminergic therapy ( ).
Cholinesterase inhibitors, in addition to improving cognitive function, may reduce hallucinations in some patients. Sleep disorders may respond to hypnosedatives, tricyclic antidepressants, mirtazapine, trazodone, quetiapine, or nighttime dopaminergic therapy. Excessive daytime sleepiness may respond to methylphenidate, modafinil, or armodafinil.
Despite optimal medical therapy, many patients with moderate to advanced disease have a poor quality of life because of fluctuating response, troublesome dyskinesia, or LD-unresponsive symptoms. Palliative surgical approaches such as stereotactic destruction of physiologically defined overactive brain nuclei (thalamotomy, pallidotomy) have been replaced by deep brain stimulation (DBS) using implanted pulse generators. The chief advantage of DBS over ablative lesioning is that the stimulation parameters can be customized to the needs of the patient to optimize the benefits. With improvements in technology the outcomes of DBS will likely continue to improve ( ).
Thalamic DBS is most frequently used to control high-amplitude tremor (either PD or ET), but STN or GPi are the most frequent targets for DBS treatment of patients with PD with disabling LD-related complications. To address the question whether optimal medical therapy or DBS provides more robust improvement, 255 patients at seven Veterans Affairs and six university hospitals were enrolled in a randomized controlled trial designed to compare the effects of DBS (STN, n = 60; or GPi, n = 61) and “best medical therapy” ( n = 134) after 6 months of treatment ( ). Patients treated with DBS gained a mean of 4.6 hours/day of on time without troubling dyskinesia, compared to 0 hours/day for patients who received best medical therapy ( P < .001). Furthermore, motor function improved by 5 or more points on the motor UPDRS in 71% of DBS and 32% of medical therapy patients. This was accompanied by improvements in the majority of PD-related HRQOL (health-related quality of life) measures and only minimal decrement in neurocognitive testing. The overall risk of experiencing a serious adverse event, however, was 3.8 times higher in the DBS than in the medical therapy group (40% vs. 11%). In a follow-up analysis of the Veterans Affairs Cooperative Studies Program outcomes, STN and GPi DBS were analyzed after 24 months in 299 patients, and there were no differences in mean changes in the motor (Part III) UPDRS between the two targets ( ). Patients undergoing STN required a lower dose of DAs than those undergoing pallidal stimulation ( P = .02), and visuomotor processing speed declined more after STN than after GPi stimulation ( P = .03). On the other hand, there was worsening of depression after STN DBS, but mood improved after GPi DBS ( P = .02). Slightly more than half of the patients experienced serious adverse events, but there was no difference in the frequency of these events between the two groups. Based on these and other studies, there is emerging evidence that GPi DBS may be particularly suitable for patients who may have troublesome dyskinesias as well as mild cognitive or behavioral impairment, whereas bilateral STN DBS may be the surgical choice for patients who are cognitively intact but in whom reduction in LD dosage is the primary goal. While DBS is a proven effective therapeutic strategy, its success depends on the appropriate selection of patients and the experience and skill of the stereotactic surgeon in order to optimize the results and minimize complications. Advances in DBS technology, such as the use of adaptive stimulation, improving connectivity, directional stimulation ( ), and searching for new targets, will undoubtedly provide additional benefits from this procedure and reduce complications ( ). It should be noted that there is a trend toward recommending DBS earlier in the course of PD ( ).
Unilateral focused ultrasound lesioning of the STN or thalamus (in tremor-dominant forms of PD) has been found to be beneficial in some patients, particularly if the symptoms are markedly asymmetric ( ; ). Finally, spinal cord stimulation is increasingly being explored in patients with PD who are most troubled by their gait disorder ( ).
MSA is a neurodegenerative disorder manifested by dysautonomia and various combinations of parkinsonism and ataxia ( ; ). Originally referred to as Shy-Drager syndrome , MSA is subdivided into two major categories according to the predominant clinical manifestation. Predominantly parkinsonian MSA (MSA-P) replaces the term striatonigral degeneration . Cerebellar MSA (MSA-C) replaces the now obsolete term olivopontocerebellar atrophy .
MSA is considerably less common than PD, with a prevalence of 4–5 per 100,000, compared to 360 per 100,000 for PD. This sporadic neurodegenerative disorder with a mean age at onset of 54 years may be difficult to differentiate from PD, particularly in the early stages. The most common signs of dysautonomia in pathologically confirmed cases are bladder dysfunction (89%), particularly urinary incontinence (44%) and urinary retention (26%), bowel dysfunction (77%), particularly constipation (46%) and fecal incontinence (27%), orthostatic hypotension (75%), sexual dysfunction (64%), RBD (54%), sweating dysfunction (40%), sleep apnea (37%), and nocturnal stridor (30%) ( ). In contrast to PD, MSA-P usually presents with symmetrical parkinsonism, often without tremor, with early instability and falls. Most patients become wheelchair bound within 5 years after onset (see ![]() ).
).
Multiple System Atrophy; Patient Describes Symptoms of Dysautonomia, Demonstrates Flexion of the Neck and Apraxia of Eyelid Opening, Typical of Multiple System Atrophy
Several clinical studies have addressed differentiating between MSA-P and parkinsonism, and a collection of “red flags” has been generated and recently validated as having high diagnostic specificity ( ). The red flags were grouped into six categories: (1) early instability, (2) rapid progression, (3) abnormal postures (includes Pisa syndrome, disproportionate anterocollis, and/or contractures of hands or feet) ( Fig. 96.10 ), (4) bulbar dysfunction (includes severe dysphonia, dysarthria, and/or dysphagia), (5) respiratory dysfunction (includes diurnal or nocturnal inspiratory stridor and/or inspiratory sighs) ( ), and (6) emotional incontinence (includes inappropriate crying and/or laughing). They proposed that a combination of two out of these six red-flag categories be used as additional criteria for the diagnosis of probable MSA-P. Other characteristic features of MSA include early hypokinetic dysarthria, distal myoclonus, and cold hands and feet with bluish discoloration of the distal extremities. MSA patients also have more autonomic symptoms at baseline and more progression to global anhidrosis than patients with PD ( ). The autonomic symptoms (particularly sexual dysfunction) and RBD may precede the onset of motor symptoms by years or even decades. About 10% of patients originally diagnosed with pure autonomic failure eventually transition to MSA ( ). Patients with MSA-C have parkinsonism with prominent cerebellar signs, especially wide-based ataxic gait. Although there may be a positive response to LD, this is generally relatively short lived and often associated with facial and oromandibular dyskinesia. In a prospective study of 141 patients with moderately severe MSA (mean age at symptom onset 56.2± 8.4 years) who had a median survival of 9.8 years (95% CI 8.1–11.4), shorter survival was suggested by the parkinsonian variant of MSA and incomplete bladder emptying, and shorter symptom duration at baseline and absent LD response predicted rapid progression ( ).

Clinical tests of autonomic dysfunction may be helpful in diagnosis or treatment (Mostile and Jankovic, 2010). Testing of cardiovascular reflexes such as heart rate variability at rest and during forced respiration, as well as blood pressure changes during head-up tilt, may help establish a clinical diagnosis of MSA. A lack of responsiveness of growth hormone to clonidine challenges and denervation on rectal sphincter electromyography (EMG) are also characteristic findings. T2-weighted MRI brain scans may show a hyperintense rim at the lateral edge of the dorsolateral putamen, with decreased signal within the putamen. Cruciform hyperintensity within the pons, the so-called hot-cross-bun sign, may also be a helpful marker ( ). PET scan with [11C]PMP that images subcortical acetyl cholinesterase (AChE) activity was significantly more decreased in MSA-P and PSP than in PD, possibly reflecting greater impairment in the pontine cholinergic group (PPN); this may account for the greater gait disturbances in the early stages of these two disorders compared to PD ( ). There is a need for development of highly specific and sensitive biomarkers that support the diagnosis and track the progression of the disease. In this regard, NFL protein levels have been found elevated in the serum and CSF of patients with MSA but not in PD but this does not differentiate between MSA and other forms of atypical parkinsonism ( ).
At autopsy, MSA brains show neuronal loss and gliosis in the striatum, SN, locus coeruleus, inferior olive, pontine nuclei, Purkinje cells, intermediolateral cell column, and the Onuf nucleus in the sacral spinal cord. Glial cytoplasmic inclusions containing α-synuclein ( Fig. 96.11 ) are the most characteristic histological features linking the different types of MSA. Neuron-to-oligodendrocyte transfer of α-synuclein by prion-like spread, leading to oligodendroglial and myelin dysfunction associated with chronic neuroinflammation, has been suggested to lead to the MSA pattern of neurodegeneration ( ). There is a severe depletion of cholinergic neurons in the PPN and laterodorsal tegmental nucleus. The etiology of MSA remains unknown, but genetic factors do not seem to play an important role.
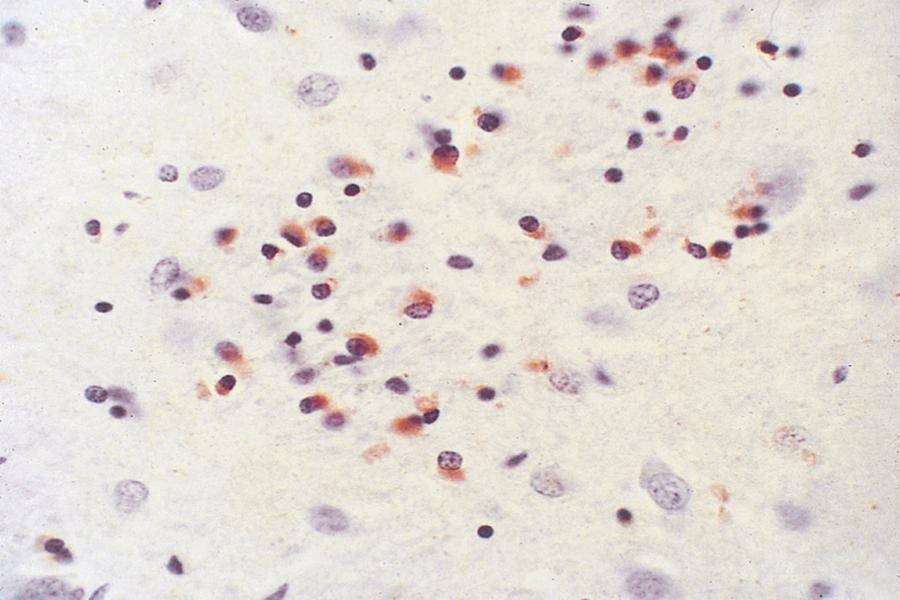
Treatment of MSA is difficult ( ; ). There are no specific interventions, and symptomatic therapies provide only partial relief of disability. Parkinsonism may respond to LD, particularly early in the disease course, but the results are not dramatic or sustained. DAs are not helpful and may be poorly tolerated because of orthostatic hypotension. There is no effective treatment for the cerebellar signs. Orthostatic hypotension may improve with nonpharmacological measures such as liberal salt and water intake, compression stockings, and sleeping with the head up, but most patients require pharmacotherapy with fludrocortisone, midodrine, droxidopa, or other agents ( ). Treatment of orthostatic hypotension often worsens supine hypertension. Even in the best hands, MSA has a poor prognosis, with a mean survival of 7–9 years.
First described in 1964 by Steele, Richardson, and Olszewski as a progressive illness characterized by vertical supranuclear ophthalmoplegia, axial rigidity, pseudobulbar palsy, and mild dementia, PSP has evolved into a broad spectrum of syndromes with different pathological substrates ( ; ; ). In addition to the classic Richardson syndrome, other subtypes include PSP-parkinsonism (with features suggestive of PD), pure akinesia with gait freezing, CBS, non-fluent variant primary progressive aphasia, behavioral variant FTD, and PSP presenting with cerebellar ataxia ( ). In addition, based on four core clinical features of PSP (oculomotor dysfunction, postural instability, akinesia, and cognitive dysfunction) other variants of PSP have been described. These include PSP with predominant ocular motor dysfunction (PSP-OM), with predominant postural instability (PSP-PI), with predominant parkinsonism (PSP-P), with predominant frontal presentation (PSP-F), with progressive gait freezing (PSP-PGF), with predominant corticobasal syndrome (PSP-CBS), with predominant speech and language disorder (PSP-SL), with predominant ataxia (PSP-C), and with predominant primary lateral sclerosis (PSP-PLS) ( ; ).
After vascular parkinsonism ( ), which can also have PSP-like features, PSP represents the third most common cause of parkinsonism, but it is still a relatively rare disease, with prevalence estimates ranging from 1.39 to 6.4 per 100,000. Men are affected more often than women.
The diagnosis of PSP is made based on clinical criteria ( ; ). PSP typically begins with a gait disorder and falling in the sixth to seventh decades of life. Patients develop an akinetic rigid state with symmetrical signs and prominent axial rigidity. In contrast to the flexed posture of patients with PD, those with PSP may have an extended trunk or retrocolic neck posture. A characteristic facial appearance features a wide-eyed stare, furrowing of the forehead with frowning expression (“procerus sign”), and deepening of other facial creases, allowing experienced clinicians to make an instant diagnosis ( Fig. 96.12 ; see also ![]() , , , , ). Pseudobulbar palsy with dysarthria and dysphagia lend the patient a characteristic dysarthria with spasticity, hypokinesia, and ataxia and often “silent” aspiration. Frontal lobe features are common. There is striking executive dysfunction early in the disease course; concrete thought, difficulty shifting set, decreased verbal fluency, and personality changes such as impulsivity and poor judgment are nearly universal. One of the characteristic, although not specific signs of PSP is the applause sign, which is manifested by persistence of applauding by the patient beyond the number of claps performed by the examiner. This is highly correlated with impairments in executive, visuospatial, and language function as well as measures of disease severity ( ). A progressive apathetic state ensues, but true dementia may not be prominent until the advanced stages of the disease. The presence of square wave jerks should suggest the diagnosis of PSP, although this neuro-ophthalmological sign may also be observed, but much less frequently, in other parkinsonian disorders ( ). Abnormal vertical saccades, best demonstrated by examination for opticokinetic nystagmus, compared to horizontal saccades, is one of the earliest ophthalmological signs of PSP. Typically, the vertical saccades are more impaired when the opticokinetic tape moves in an upward rather than downward direction. Electro-oculographic recordings in PSP show decreased amplitude and normal latency of horizontal saccadic eye movements. Although considered a clinical hallmark of PSP, supranuclear vertical gaze palsy may not appear until later in the disease course, and some patients may never develop gaze palsy. Another neuro-ophthalmological sign in PSP is blepharospasm with or without apraxia of eyelid opening ( ).
, , , , ). Pseudobulbar palsy with dysarthria and dysphagia lend the patient a characteristic dysarthria with spasticity, hypokinesia, and ataxia and often “silent” aspiration. Frontal lobe features are common. There is striking executive dysfunction early in the disease course; concrete thought, difficulty shifting set, decreased verbal fluency, and personality changes such as impulsivity and poor judgment are nearly universal. One of the characteristic, although not specific signs of PSP is the applause sign, which is manifested by persistence of applauding by the patient beyond the number of claps performed by the examiner. This is highly correlated with impairments in executive, visuospatial, and language function as well as measures of disease severity ( ). A progressive apathetic state ensues, but true dementia may not be prominent until the advanced stages of the disease. The presence of square wave jerks should suggest the diagnosis of PSP, although this neuro-ophthalmological sign may also be observed, but much less frequently, in other parkinsonian disorders ( ). Abnormal vertical saccades, best demonstrated by examination for opticokinetic nystagmus, compared to horizontal saccades, is one of the earliest ophthalmological signs of PSP. Typically, the vertical saccades are more impaired when the opticokinetic tape moves in an upward rather than downward direction. Electro-oculographic recordings in PSP show decreased amplitude and normal latency of horizontal saccadic eye movements. Although considered a clinical hallmark of PSP, supranuclear vertical gaze palsy may not appear until later in the disease course, and some patients may never develop gaze palsy. Another neuro-ophthalmological sign in PSP is blepharospasm with or without apraxia of eyelid opening ( ).

Progressive Supranuclear Palsy; Typical Worried, Frowning Facial Expression (Procerus Sign), Apraxia of Eyelid Opening, Although Vertical (Downward) Gaze is Preserved, Vertical Optokinetic Nystagmus is Absent, When Walking Patient Pivots on Turning (in Contrast to Patients with Parkinson Disease Who Turn En Bloc)
Progressive Supranuclear Palsy; Marked Vertical Ophthalmoparesis, Perseveration of Gaze to Left even though the Body Faces Forward
Progressive Supranuclear Palsy; Typical Facial Expression with Deep Facial Folds, Square Wave Jerks on Primary Gaze, Slow Saccades, Inappropriate Laughter (Pseudobulbar Palsy), Right Arm Levitation
Progressive Supranuclear Palsy; Deep Facial Folds, Vertical Ophthalmoplegia, Marked Postural Instability, Slumps into a Chair
Progressive Supranuclear Palsy; Deep Facial Folds, Apraxia of Eyelid Opening, in Addition to Vertical Ophthalmopareses, Patient Demonstrates Evidence of Internuclear Ophthalmoplegia, the Presence of Right Arm Tremor (Atypical for Progressive Supranuclear Palsy) Suggests the Co-Existence of Parkinson Disease
In contrast to PD, patients with PSP tend to have a more broad-based gait with knee extension, and instead of turning en bloc they tend to pivot on their toes and sometimes even cross their legs, which contributes to frequent falls ( ). Atypical presentations are often seen, especially pure akinesia manifested by severe motor blocks while walking (freezing). PSP is rapidly progressive; by the fourth year of illness, half of patients need assistance for walking and have troublesome dysarthria and visual symptoms. Dysphagia becomes prominent shortly thereafter.
There are no diagnostic tests for PSP, but elevated serum and CSF levels of NFL have been found in patients with PSP compared to those with PD ( ). Although not diagnostic, NFL can serve as a possible biomarker. Typical MRI signs of PSP include midbrain atrophy, increased signal in the midbrain and GP, atrophy or increased signal in the red nucleus, third ventricle dilation, and atrophy of the frontal or temporal lobes. On the midsagittal view of the MRI, as a result of atrophy of the rostral midbrain tegmentum, the most rostral midbrain, the midbrain tegmentum, the pontine base, and the cerebellum appear to correspond to the bill, head, body, and wing, respectively, of a hummingbird or a penguin.
At autopsy, the midbrain in PSP is atrophied, and the sylvian aqueduct is dilated. The SN is depigmented and appears orange and shrunken. The locus coeruleus may also show some depigmentation, but this is less prominent than in idiopathic PD. Other structures may also show atrophy, most notably the frontal lobe, STN, and superior cerebellar peduncle. Histopathologically, the degenerative process involves mainly the basal ganglia, diencephalons, and brainstem. Pathological findings include neuronal loss, gliosis, neurofibrillary tangles, and granulovacuolar degeneration in neurons of the brainstem. There are tufted astrocytes in the motor cortex and the striatum, and the typical neuronal lesion is the globose neurofibrillary tangle, made up of hyperphosphorylated four-repeat tau protein filaments ( Fig. 96.13 ).
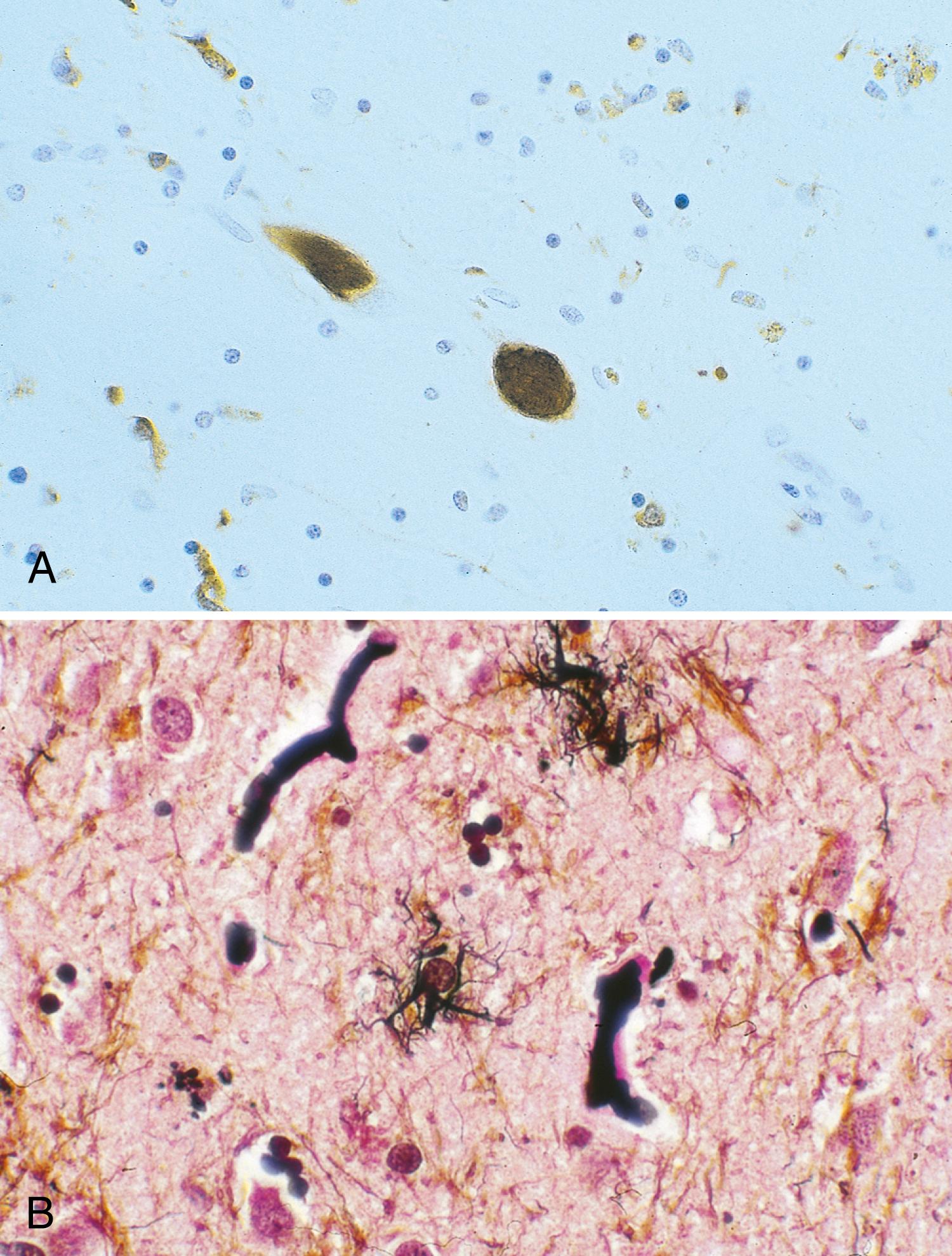
On the basis of an analysis of 103 pathologically confirmed consecutive cases of PSP, PSP was divided into two categories: Richardson syndrome , characterized by the typical features described in the original report, and PSP-P , in which the clinical features overlap with PD and the course is more benign ( ). The latter group, representing about a quarter of all patients with PSP, has less tau pathology than the classic Richardson syndrome. The mean 4R-tau/3R-tau ratio of the isoform composition of insoluble tangle-tau isolated from the pons was significantly higher in Richardson syndrome (2.84) than in PSP-P syndrome (1.63).
PSP almost always occurs sporadically, yet an increasing number of familial cases suggests a genetic etiology in some cases. Pedigrees with apparent dominant and recessive inheritance have been described. Affected families may show phenotypical heterogeneity, with some affected persons showing dementia, dystonia, gait disorder, or tics. Mutations in the tau gene have been reported in patients with a familial PSP-like illness, but these have been quite rare, and mutations are not believed responsible for most PSP cases. However, patients with PSP are homozygous for a common haplotype that contains a normally occurring polymorphism in the tau intron immediately preceding exon 10. There is growing support for the notion of altered regulation of tau gene expression in PSP. Genetic polymorphisms are increasingly being identified, some of which might increase risk for PSP via effects on tau. No toxic, viral, or other environmental risk factors have been described.
Dopaminergic agents, particularly LD, may provide temporary improvement in bradykinesia in approximately 40% of patients, but LD usually does not improve dysarthria, gait, or balance problems. No other drug has been shown to provide any meaningful improvement in symptoms of PSP. A randomized placebo-controlled trial of donepezil showed modest cognitive improvements but poor tolerability. Amitriptyline may be helpful in improving pseudobulbar affect and emotional incontinence. Botulinum toxin injections may be useful to treat blepharospasm or retrocolic neck posture in PSP. The prognosis of PSP is poor, with serious impact on quality of life and a median duration of survival of approximately 8 years. It is possible that future strategies targeting toxic tau, currently investigated in other tauopathies, may also exert disease-modifying effects in PSP.
In 1967, Rebeiz and colleagues described three patients with akinetic rigidity, apraxia, dystonia, tremor, and aphasia, who at autopsy had pale achromatic ballooned neurons similar to those seen in Pick disease. The condition was named corticodentatonigral degeneration with neuronal achromasia in 1989 but has since become known simply as corticobasal degeneration . Although CBD generally brings to mind a particular motor syndrome of asymmetrical rigidity, apraxia, and cortical sensory dysfunction, its underlying pathological features may be seen in other clinical syndromes including PSP, progressive aphasia, and FTDP. One study based on 35 cases from the Queen Square Brain Bank, in which there were 21 clinically diagnosed cases of CBS and 19 pathologically diagnosed with CBD, was designed to address the clinical and pathological overlap between CBD and PSP ( ). Of 19 pathologically confirmed CBD cases, only five had been diagnosed correctly in life (sensitivity =26.3%). All had a unilateral presentation, a clumsy useless limb, limb apraxia, and myoclonus; four had cortical sensory impairment and focal limb dystonia, and three had an alien limb. Eight cases of CBD had been clinically diagnosed as PSP, all of whom had vertical supranuclear palsy, and seven had falls within the first 2 years. Of 21 cases with CBS, only five had CBD (positive predictive value = 23.8%); six others had PSP pathology, five had AD, and the remaining five had other non-tau pathologies. Forty-two percent of CBD cases presented clinically with a PSP phenotype, and 29% of CBS cases had underlying PSP pathology. The authors suggested the CBD-Richardson syndrome for the overlap cases and concluded that CBD “is a discrete clinico-pathological entity but with a broader clinical spectrum than was originally proposed” ( ).
CBD is one of the least common and most asymmetrical forms of atypical parkinsonism ( ). The mean age at onset is 60–64 years. In its most recognizable form, it is predominantly a motor disease, but its presentation is clinically heterogeneous. In addition to parkinsonism with strikingly asymmetrical rigidity, CBD patients often exhibit asymmetrical dystonia, myoclonus, apraxia, alien limb, and cortical sensory loss. They may also present with primary progressive aphasia and may evolve into global dementia (see ![]() , , , ) ( ).
, , , ) ( ).
Corticobasal Degeneration; Patient Describes Apraxia of Left Leg, Demonstrates Ideomotor Apraxia in Left More than Right Hand and Marked Left Leg and Foot Apraxia
Corticobasal Degeneration; Patient Describes Alien Hand Phenomenon in the Right Arm, Demonstrates Marked Apraxia in the Right More than Left Hand, Spontaneous and Evoked Myoclonus in the Right Hand, Markedly Impaired Graphesthesia
Corticobasal Degeneration; Evoked Hand and Arm Myoclonus
Corticobasal Degeneration; Patient Describes Right Alien Hand Phenomenon, Right Hand Myoclonus, Marked Ideomotor Apraxia in the Right More than Left Hand
Patients with CBS have asymmetrical and often focal cortical atrophy on MRI, with widening of the sylvian and interhemispheric fissures and dilation of frontal, parietal, and temporal sulci ( ). Fluorodeoxyglucose PET scans show asymmetrical hypometabolism in the thalamus and motor cortex. SPECT scans show marked asymmetry of cortical blood flow.
At autopsy, patients with a clinical syndrome consistent with CBD have gross brain atrophy. Typical microscopic changes are tau-positive neuronal and glial lesions, especially gray and white matter astrocytic plaques and threadlike lesions, and neuronal loss in the cortex and SN. The inclusions are formed of hyperphosphorylated four-repeat tau. Overlap with other conditions including AD, PSP, PD, FTDP, and hippocampal sclerosis is common ( ).
As with other tauopathies, the etiology of CBD is unknown. There are no familial forms of the illness, and no mutations in the tau gene have been identified. There is clinical and pathological overlap with other tauopathies, and patients with CBD share a similar tau haplotype with patients with PSP.
There is no treatment for the degenerative process. Parkinsonian features do not seem to respond to LD or other dopaminergic drugs. Benzodiazepines, particularly clonazepam, may help myoclonus. Botulinum toxin injections may improve focal dystonia early in the disease and help relieve pain and facilitate care in advanced disease. The prognosis is poor, with a reported median survival after onset of about 7 years.
DLB is the second most prevalent degenerative dementia after AD. In one study, among 542 incident cases of parkinsonism, 64 had DLB and 46 had PD dementia (PDD); the pathology was consistent with the clinical diagnosis in 24 of 31 patients (77.4%) who underwent autopsy ( ). DLB is a progressive dementia characterized especially by fluctuating cognitive impairment, prominent disruption of attention and visuospatial abilities, visual hallucinations, and parkinsonism (see ![]() ). RBD and depression are also very common. These behavioral symptoms are typically present at least 1 year prior to the onset of motor (parkinsonian) features ( ). Patients with DLB are extremely sensitive to dopamine receptor antagonists and experience severe parkinsonism when treated with neuroleptics.
). RBD and depression are also very common. These behavioral symptoms are typically present at least 1 year prior to the onset of motor (parkinsonian) features ( ). Patients with DLB are extremely sensitive to dopamine receptor antagonists and experience severe parkinsonism when treated with neuroleptics.
Parkinson Disease; Patient Describes Levodopa-Induced Visual Hallucinations (e.g., Seeing and Picking Worms)
Characteristic pathological changes include cortical and brainstem (SN) Lewy bodies. Spongiform changes, neurofibrillary tangles, and dystrophic Lewy neuritis may also be seen, and overlap with AD is considerable. Treatment of DLB is difficult but medications used in the treatment of both PD and dementia are often employed here. Although antiparkinsonian agents are used to treat parkinsonian signs, the degree of sensitivity of parkinsonian signs to dopaminergic therapy has not been well defined. Psychiatric and behavioral symptoms may improve with atypical antipsychotics, and cholinesterase inhibitors such as rivastigmine may improve delusions and hallucinations.
PD dementia is defined as cognitive impairment that includes cognitive and motor slowing, executive dysfunction, and impaired memory retrieval. The relationship of PDD to AD and other dementing disorders such as DLB has not yet been well defined. Although some investigators suggest that clear clinicopathological separation is possible between the three disorders, the differences in neuropathological and neurochemical characteristics suggest that there is a continuum.
Frontotemporal degeneration is a group of disorders characterized by behavioral changes and neuropsychological evidence of frontal lobe dysfunction. They include PSP, CBD, Pick disease, pallidopontonigral degeneration, disinhibition-dementia-parkinsonism-amyotrophy, familial multiple system tauopathy with presenile dementia, familial subcortical gliosis, FTD, FTD with ALS, FTD with inclusion body myopathy, and FTDP-17. In up to 60% of patients with FTD, there is a positive family history. Genetic loci on chromosomes 17 (FTDP-17), 9 (FTD with ALS; FTD with inclusion body myositis), and 3 (FTD) have been described. The prototype of FTDP is an inherited parkinsonism-dementia disorder, initially described as Wilhelmsen-Lynch disease (disinhibition-dementia-parkinsonism-amyotrophy complex) and subsequently found to be due to mutations in the tau gene on chromosome 17q21. Although tau mutations account for many of these diseases, similar phenotypes have been attributed to mutations in other genes such as p97 (also known as valosin-containing protein ) on chromosome 9p21-p12, CHMP2B (charged multivesicular body protein 2B) on the pericentromeric region of chromosome 3, and progranulin (PGRN) on chromosome 17q21 (1.5 Mb centromeric of tau). Plasma and CSF levels of progranulin have been found to be reduced nearly fourfold in affected and unaffected subjects with PGRN mutations, and low (75% reduction) plasma progranulin levels may be used as a screening tool for PGRN mutations.
There is considerable phenotypical, genotypical, and pathological heterogeneity in FTDP ( ). The disorder most often begins in the 50s or 60s with personality and behavioral changes that include disinhibition and aggressiveness as well as frontal executive dysfunction. Other common signs include social misconduct, stereotyped verbalizations, impaired recent memory, and parkinsonism. Some families present with early parkinsonism. Many mutations have been reported in the tau gene. They comprise mainly three groups: mutations in the coding region for a microtubule-binding domain, resulting in a dysfunctional protein; mutations outside the microtubule-binding domains; and mutations that alter the ratio of three- to four-repeat tau isoforms. Pathological findings include tau-positive neuronal and glial inclusions distributed variably throughout the brain. In patients with prominent parkinsonism, there is severe neuronal loss in the SN. The response of parkinsonism to symptomatic treatment is not known. The prognosis is poor, with death occurring within 10 years.
A high incidence of an ALS-like illness among the Chamorros, indigenous people of Guam, was noticed more than 50 years ago. In the same population, a smaller number of people had a syndrome of parkinsonism with dementia, the parkinsonism-dementia complex (PDC). Some had both motor neuron disease and PDC. Early in its course, PDC appears variably like PD, atypical parkinsonism, or PSP; however, in the end stages, it most resembles PSP. Familial aggregation of cases has been noted, but prior attempts to elucidate a hereditary basis to the illness proved fruitless. A similar constellation of ALS and PDC has been reported on the Kii peninsula of Japan.
Pathologically, the disorder is characterized by neuronal degeneration and abundant neurofibrillary tangles in the brain and spinal cord. A recent reanalysis of a patient registry suggests that both the spouses and the offspring of persons with PDC have a significantly higher risk of themselves developing ALS-PDC, suggesting both environmental and genetic risk factors. The critical age for exposure to the environmental factor was adolescence or early adulthood. Despite extensive analysis of the diet and other environmental factors, the etiology of PDC of Guam remains unknown, although neurotoxic damage from the cycad nut has been implicated.
A form of atypical parkinsonism has been described in the French West Indies. The so-called Guadeloupean parkinsonism shows clinical features of LD-unresponsive parkinsonism, postural instability with early falls, and pseudobulbar palsy. More than 25% of these patients have a phenotype like that of PSP. The etiology of this form of parkinsonism is unknown, but exposure to dietary or other environmental toxins is suspected. The disease may be associated with the use of indigenous plants ( Annona muricata [synonyms: soursop, corossol, guanabana, graviola, and sweetsop]) that contain the mitochondrial complex I and dopaminergic neuronal toxins, reticuline and coreximine.
After PD, vascular parkinsonism is the second most common form of parkinsonism encountered in movement disorders clinics, accounting for 8% of all parkinsonian patients ( ). Vascular changes on imaging studies are common, but the cause and effect are not always clearly established. Among stroke patients, parkinsonism is more common in patients with lacunar stroke. Adult-onset diabetes, chronic hypertension, and hyperlipidemia seem to be the most common risk factors associated with vascular parkinsonism ( ). Vascular parkinsonism usually presents as “lower body parkinsonism” with a broad-based shuffling gait and prominent start and terminal hesitation, as well as freezing (see ![]() , ). Postural instability and a history of falls are common. Many patients have dementia and corticospinal findings of incontinence. In a systematic review of 25 articles, patients with vascular parkinsonism were older, had a shorter duration of illness, presented with symmetrical gait difficulties, were less responsive to LD, and were more prone to postural instability, falls, and dementia ( ). Pyramidal signs, pseudobulbar palsy, and incontinence were more common in vascular parkinsonism, but tremor was not a main feature. Structural neuroimaging was abnormal in 90%–100% of vascular cases, compared to 12%–43% of PD cases. In contrast to PD, there is usually no abnormality in presynaptic striatal dopamine transporters as measured by SPECT in vascular parkinsonism.
, ). Postural instability and a history of falls are common. Many patients have dementia and corticospinal findings of incontinence. In a systematic review of 25 articles, patients with vascular parkinsonism were older, had a shorter duration of illness, presented with symmetrical gait difficulties, were less responsive to LD, and were more prone to postural instability, falls, and dementia ( ). Pyramidal signs, pseudobulbar palsy, and incontinence were more common in vascular parkinsonism, but tremor was not a main feature. Structural neuroimaging was abnormal in 90%–100% of vascular cases, compared to 12%–43% of PD cases. In contrast to PD, there is usually no abnormality in presynaptic striatal dopamine transporters as measured by SPECT in vascular parkinsonism.
Vascular Parkinsonism; Broad-Based Gait, Freezing on Turning (Lower Body Parkinsonism) Associated with Binswanger’s Disease
Vascular Parkinsonism; Gait Initiation Failure (Pure Freezing)
The pathology includes subcortical vascular disease with preservation of dopaminergic cells in the SN. There is a growing body of evidence that microstructural changes of normal-appearing white matter are common in the brains of patients with vascular parkinsonism ( ; ).
The symptoms of vascular parkinsonism are unlikely to show a significant response to LD, but a therapeutic trial is worth pursuing because as many as half of patients improve. Physical therapy may also be useful.
Calcification of the basal ganglia has many causes. It is an incidental finding in up to 1% of all CT brain scans. Basal ganglia calcifications can also be seen in infectious, metabolic, and genetic disorders affecting this brain region. There are familial and sporadic forms. When symptoms occur, they usually begin in adulthood between age 30 and 60 years. Cognitive dysfunction, seizures, cerebellar signs, dysarthria, pyramidal signs, psychiatric illness, gait disorder, and sensory impairment are common. About half of symptomatic patients have movement disorders. Among these, parkinsonism and chorea are most common. Fewer than 10% of patients have tremor, dystonia, athetosis, or orofacial dyskinesia. The presence of symptoms correlates with the amount of calcification. Calcification is most often seen in the GP but may also occur in the caudate, putamen, dentate, thalamus, and cerebral white matter, as well as internal capsules. Calcium is deposited in the perivascular extracellular space. Dominant and recessive inheritance patterns with many different gene mutations have been described ( ). There is no specific treatment other than symptomatic management.
Between 1916 and 1927, a worldwide epidemic of encephalitis lethargica killed approximately 250,000 persons and left an additional 250,000 with chronic disability. These survivors of the acute illness developed parkinsonism, usually within 10 years of the infection. PEP resembles PD, although more prominent behavioral and sleep abnormalities occur early in the disease course, extraocular movements are often abnormal, and oculogyric crises are common. Other common movement disorders include chorea, dystonia, tics, and myoclonus. Pyramidal tract signs are common. The pathological appearance of PEP includes degeneration of SN neurons, with neurofibrillary tangles in surviving neurons. Although the etiology is presumed to be a virus, none has ever been identified. There have been no subsequent epidemics of encephalitis lethargica, although sporadic cases of PEP are occasionally reported. The symptoms of PEP tend to be responsive to LD, but behavioral complications such as hallucinations and delusions are common, limiting therapy.
Dopamine receptor-blocking drugs reproduce the major clinical features of PD, although signs are usually symmetrical, and the tremor is more often present during posture holding than at rest ( ; ). The most common causes of DIP are the typical neuroleptic antipsychotic drugs, antidopaminergic antiemetics, and drugs that deplete presynaptic nerve terminals of dopamine, such as reserpine, tetrabenazine, deutetrabenazine, and valbenazine. Despite the marketing efforts by the drug manufacturers to minimize the risk of tardive dyskinesia (TD) with the atypical (third-generation) neuroleptics, all these drugs have been reported to cause TD. Among the newer, or atypical, antipsychotics, the relative propensity to cause DIP is as follows: risperidone = ziprasidone > olanzapine > quetiapine > clozapine. This ranking reflects their respective affinity for the D2 receptor. A number of other drugs have been associated with DIP: aripiprazole and other new atypical neuroleptics ( ), selective serotonin reuptake inhibitors, lithium, phenytoin, methyldopa, valproic acid, and the calcium channel antagonists flunarizine and cinnarizine, which are not marketed in the United States. DIP generally appears subacutely after weeks to months of therapy. Although it is reversible, DIP may resolve very slowly over a period of up to 6 months, and symptomatic treatment with anticholinergics, amantadine, or LD may be required. Occasionally, parkinsonism does not resolve, suggesting that the offending drug likely has unmasked an underlying parkinsonism. The use of antipsychotic medications is a strong predictor of subsequent PD and patients taking neuroleptics may be five times more likely to begin antiparkinsonian medications than nonusers. In some cases of DIP when the offending dopamine receptor blocking drug is discontinued TD may emerge ( ).
In 1982, a number of young California drug addicts developed acute and severe parkinsonism after intravenous injection of a synthetic heroin contaminated by MPTP. Subsequent study showed that the offending toxin was the metabolic product of MPTP produced by monoamine oxidase, 1-methyl-4-phenyl-propionoxypiperidine (MPP+). Postmortem examination in patients 10 years after the original exposure showed severe loss of SN neurons without Lewy body formation. Interestingly, despite the 10-year interval between exposure to the toxin and death, there was evidence of an active neurodegenerative process that included extracellular melanin and active neuronophagia. This suggests that intracellular mechanisms may promote neurodegeneration after a distant environmental insult. MPTP-induced parkinsonism is responsive to LD, but the response is complicated by early development of motor fluctuations and dyskinesias, which may become severe, and psychiatric complications such as hallucinations. Cognitive function usually remains intact.
Acute carbon monoxide poisoning is associated with parkinsonism. MRI scans show high-intensity white-matter lesions and necrosis of the GP bilaterally. Cognitive signs including decreased short-term memory, attention, and concentration are common. Patients with neurological sequelae of carbon monoxide intoxication may experience gradual clinical and radiological improvement over months to years.
Manganese toxicity is associated with LD-unresponsive symmetrical parkinsonism with dystonic features such as oculogyric crisis. The disorder may progress for years after cessation of exposure ( ). Striatal MRI T2-weighted hyperintensity may be present during the acute phase of poisoning. F-dopa PET scans in subjects with manganism show normal presynaptic dopamine function, suggesting postsynaptic pathology. The fungicide maneb (manganese ethylene-bis-dithiocarbamate) has also been shown to induce a toxic parkinsonism.
A fine tremor of the outstretched limbs is a universal finding. Physiological tremor appears to originate in the heartbeat, mechanical properties of the limbs, firing of motoneurons, and synchronization of spindle feedback. Its frequency ranges from 7 to 12 Hz. It is usually not noticeable except with electrophysiological recording, but its amplitude is accentuated by fatigue, anxiety, fear, excitement, stimulant use, and medical conditions such as hyperthyroidism ( Box 96.3 ).
Physiological tremor
Neuropathic tremor
Palatal tremor
Essential tremor
Orthostatic tremor
Parkinsonian rest tremor
Holmes tremor
Cerebellar tremor
Holmes tremor
ET is one of the most common movement disorders. In population-based studies, the prevalence increases steadily with age, occurring in up to 10% of patients older than 60 years of age. Meta-analysis of epidemiological studies has found the prevalence of ET to range between 0.01% and 20.5%, but the pooled prevalence is 0.9% ( ). In its purest form, ET is a monosymptomatic illness characterized by gradually increasing-amplitude postural and kinetic tremor of the forearms and hands (with or without involvement of other body parts) in the absence of endogenous or exogenous triggers or other neurological signs. In clinic-based series, as many as 50% of patients exhibiting ET do not conform to this clinical picture, suggesting substantial heterogeneity and an overlap in some cases with dystonia and parkinsonism ( ). The clinical definition of ET is problematic because there are no pathological, biochemical, genetic, or other established and validated diagnostic criteria. The Movement Disorders Society issued a “consensus statement” on classification of tremors ( ). It defined ET as isolated tremor syndrome of bilateral upper limb action tremor at least 3 years’ duration with or without tremor in other locations (e.g., head, voice, or lower limbs), absence of other neurological signs, such as dystonia, ataxia, or parkinsonism. They acknowledged that patients frequently have a family history, of tremors and small doses of alcohol may improve the tremor, but they felt that these clinical features are not consistent enough to be included in the definition of ET. They also introduced the term “ET-Plus,” a new tentatively and uncertainly defined entity characterized by the presence of additional neurological signs other than action tremor. This has engendered much controversy, and many believe that ET-Plus is more common than ET.
There seems to be a bimodal distribution for age at onset, peaking in the 2nd and 6th decade of life. The typical patient becomes aware of a barely perceptible postural or action tremor, usually in the distal arms and hands. The head and lower limbs are less commonly affected. Head tremor (titubation) is milder than limb tremor and is predominantly of a side-to-side, “no-no” type. Head tremor is often associated with cervical dystonia and some patients with head tremor merely have dystonic tremor as a manifestation of their cervical dystonia without associated ET ( ). Tremor of the face, trunk, and voice may also be present in patients with ET. The kinetic tremor is typically higher in amplitude than the postural tremor ( Fig. 96.14 ). In contrast to PD where the handwriting is small, the handwriting in patients with ET is tremulous.
A striking improvement after ingestion of a small amount of ethanol is seen in 50% of patients and may be helpful in diagnosis ( ). Over time, the tremor worsens, causing increasing functional disability. Only a fraction of affected persons seek medical attention, and there is often a long latency from onset to presentation for care. At the time of diagnosis, nearly all patients with ET have significant social, functional, or occupational disability, and as many as 25% must make occupational adjustments as a result of tremor-related disability. ET is thought to be a monosymptomatic illness without changes in cognition, strength, coordination, or muscle tone, and the results of the neurological examination are usually normal. However, detailed studies of patients with ET have demonstrated frontostriatal cognitive deficits, changes in tandem gait, and other (albeit subtle) evidence of cerebellar dysfunction. The worsening of ET over time likely relates to two phenomena. First, the frequency of tremor in ET decreases over time, and its amplitude increases. This results from decreased attenuation of lower-frequency tremor secondary to age-related changes in the mechanical properties of limbs and muscle. A second possible contributor is true progression of the underlying disorder. According to recent studies, the severity of ET relates to disease duration independent of aging and age-related changes in mechanical properties of the muscles and limbs.
The diagnosis of ET is made by history and physical examination. A tremor rating scale known as The Essential Tremor Rating Assessment Scale (TETRAS) has been developed by the Tremor Research Group to assess ET and has been found to correlate well with quantitative assessments using the kinesia system ( ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here