Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute pain is an urgent condition for the patient. Pain should be rapidly assessed, treated, and frequently reassessed in tandem with diagnostic evaluations.
Therapy for acute pain is different than for chronic pain. Chronic pain treatment should be undertaken in consultation with the clinician(s) responsible for the patient’s long-term management. In general, opioid analgesic agents should not be administered in the ED or prescribed for outpatient therapy for chronic pain patients unless the plan is agreed to by the responsible outpatient clinician.
Titrated IV opioid analgesics are the primary therapeutic approach for the treatment of moderate and severe acute pain. When intravenous access is not indicated or attainable, SC administration is preferable to the IM route.
Opioid therapy should be reserved for pain that has not been or cannot adequately be treated with nonopioid therapies.
Oral oxycodone, with an onset of action similar to that of IM or SC opioids, can be used for moderate pain when the IV route is not otherwise needed. Oxycodone and other oral opioids should be administered and prescribed as a single-drug preparation, not as part of a combination with acetaminophen.
Ambulatory treatment with opioids should be confined to the period of acute pain. Most opioid prescriptions from the ED for acute injury should be for 3 to 5 days, after which the patient is transitioned to nonopioid analgesia or reevaluated by an outpatient clinician.
Acetaminophen and NSAIDs should be added to pain therapy when not contraindicated. Their analgesic effects are additive to those of opioids and each other.
There is no evidence to support the concept that diagnosis based on physical examination findings will be impaired by administering opioid pain medications to achieve reasonable patient comfort.
There is no evidence that morphine causes more smooth muscle spasm than other opioids. Morphine is safe and appropriate for patients with acute biliary or renal colic.
Patients who are known to be diverting or abusing opioids should not be prescribed opioids for use as outpatients. Patients with chronic pain syndromes, or those with chronic conditions that may cause acute pain (e.g., dental caries), should be offered alternative pain management options, and opioids should generally be avoided.
Topical and local anesthetics can treat pain associated with most ED procedures and should be considered for use in isolated painful conditions.
Low tissue pH (5 or 6) in infected tissue impairs the effectiveness of local anesthesia.
Pain-related complaints represent the primary concern in up to 70% of patients presenting to the emergency department (ED). , Uncontrolled pain should be considered a medical emergency, and the estimated degree of pain experienced by a patient should play a role in determining a patient’s overall acuity and urgency for therapy. Pain estimations, using both clinician- and patient-derived scales, should be obtained and recorded to determine the presence of pain and the response to pain therapy.
Although pain can be present in a wide variety of physical and psychosocial situations, it is typically present in the context of tissue injury. Pain can therefore be assumed to be present in patients with physically apparent disease or injury, even in those who cannot effectively communicate their condition. Important terms relating to analgesic practices are listed in Box 6.1 .
Allodynia—pain from a stimulus that does not normally provoke pain
Amnestic—an agent that suppresses the formation of memories
Local anesthesia—creates an area of insensibility to pain by injection of a local anesthetic agent
Analgesia—relief from pain
Hypnotic—agent that promotes the onset of sleep
Narcotic—term with legal implications describing opioid agents together with various central nervous system depressant drugs of abuse
Nociceptor—receptor that is sensitive and responsible for transmitting pain stimuli
Noxious stimulus—stimulus that is damaging or potentially damaging and results in sensation of pain
Opiate—naturally occurring derivative of opium alkaloid that binds opiate receptors and produces effects similar to those of the endogenous endorphins
Opioid—naturally occurring or semisynthetic derivative of opium alkaloid (includes all opiates) that binds opiate receptors and produces effects similar to those of endogenous endorphins
Pain—unpleasant sensory and emotional experience arising from actual or potential tissue damage or described in terms of such damage
Procedural sedation—pharmacologic induction of a state of sedation or dissociation with amnesia for pain control during a painful procedure
Sedative—agent that decreases a patient’s level of awareness
A wide variety of treatments are available for both acute and chronic pain. The treatment of pain can be difficult and is often one of the most challenging and frustrating aspects of the practice of emergency medicine.
Patients’ perceptions of their ED care are highly influenced by pain treatment. Satisfaction with emergency care often depends on the techniques and timeliness of analgesia, as well as the discharge plans for pain relief. In every interaction with a patient in pain, a balance must be achieved between relief of patient suffering and the diagnosis and treatment of the underlying medical condition.
A growing body of evidence has supported the importance of pain management as a central aspect of disease treatment. Unrelieved pain is associated with various potentially negative physiologic outcomes, including increases in sympathetic outflow, peripheral vascular resistance, myocardial oxygen consumption, and the production of carbon dioxide. Other adverse effects of unrelieved pain include hypercoagulability, decreases in gastric motility, and immune function impairment. Poorly treated acute pain can promote the development of chronic pain syndromes and vegetative symptoms, as well as increase the need for pain management during any recovery period. Similarly, pain during serial medical procedures may increase if successful analgesia was not provided during initial procedures. It is also likely that a patient’s experience of pain increases his or her ability to perceive pain from similar stimuli in the future.
As an affirmation of the recognized importance of pain management in health care, the Center for Medicare and Medicaid Services and The Joint Commission for accreditation of health care organizations require hospitals to develop quality improvement efforts related to acute pain management, in addition to comprehensive programs for the measurement, documentation, and treatment of pain.
Comprehensive approaches to pain management have become complicated by the widespread abuse of opioids. In the latter part of the twentieth century, American clinicians and institutions emphasized using opioid therapy as a cornerstone of acute and chronic pain management. This emphasis rested on the widely held belief that opioid therapy prescribed to patients in pain posed a relatively small risk for chronic addiction, abuse, or harm. A dramatic rise in addiction rates, diversion, and death directly attributed to this practice has been documented in the early part of the twenty-first century. This “opioid crisis” has largely been a phenomenon unique to the American health care system and culture. Additionally, chronic opioid therapy has not been demonstrated to confer patient-centric gains in life function, productivity or longevity.
As a result, pain management strategies have quickly shifted to support selective brief periods of opioid therapy in acute pain along with a concurrent reduction, or altogether removal, of opioids as a mainstay in patients with extended, chronic periods of pain.
Emergency medicine as a specialty accounts for 4% of opioid prescriptions in the US, with 17% of patients receiving a median of 15 pills per prescription, so while emergency departments represent a small part of this opioid crisis, there are significant opportunities for improvements in practice.
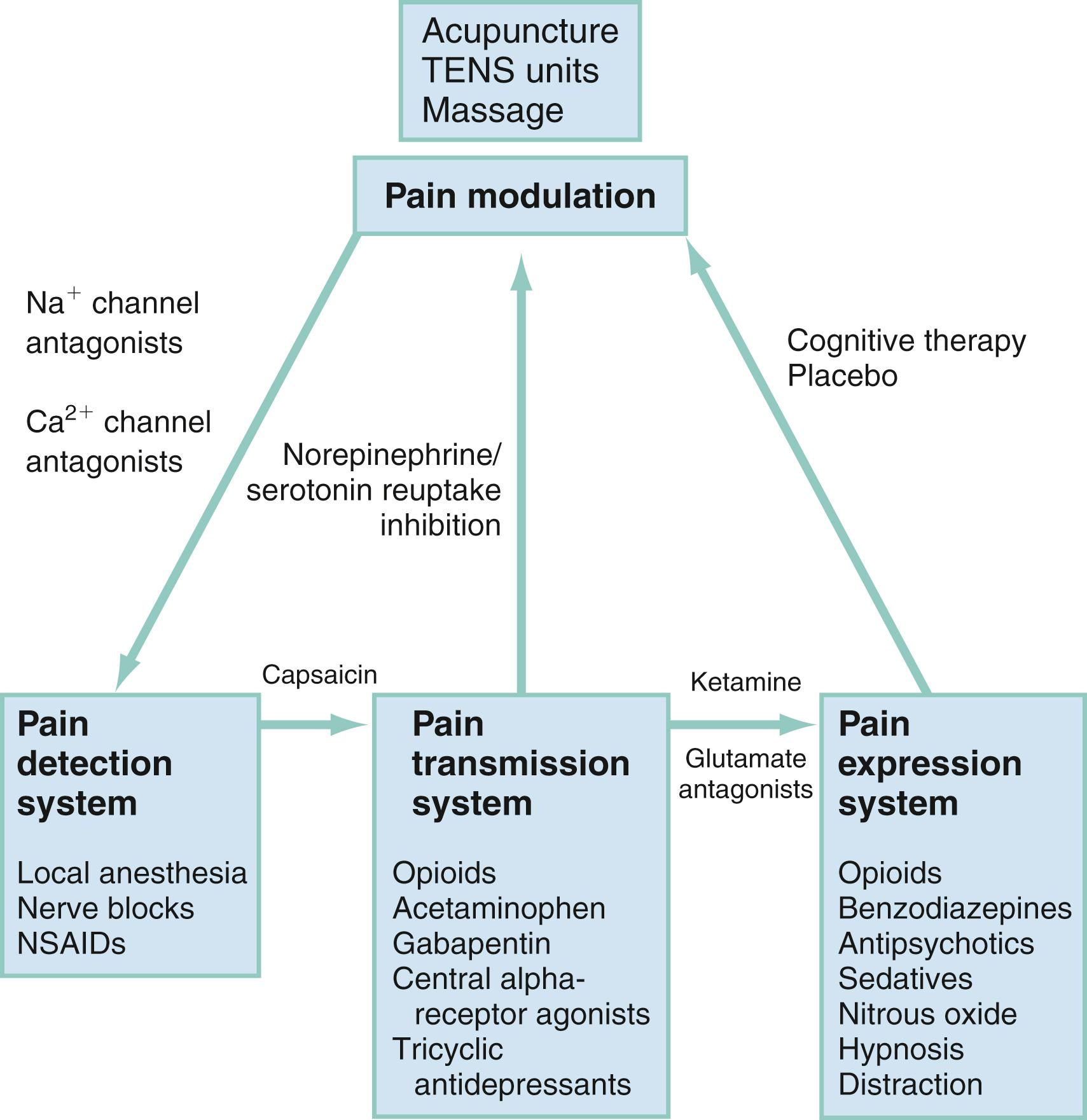
Pain can be generally described by the terms nociceptive and neuropathic . Nociceptive pain results from the activation of sensory neurons that signal pain (nociceptors) in response to noxious stimuli. Neuropathic pain results from signal-processing changes in the central nervous system (CNS). Neuropathic pain is usually described as burning, tingling, or shooting sensations and includes neuropathies and deafferentiation. Both nociceptive and neuropathic pains involve peripheral and central sensitization with a complex array of mediators to sensitize peripheral nociceptors and perpetuate thalamic signals ( Fig. 6.1 ). At each level in the physiologic process of pain production or transmission, there are interventions and therapeutic opportunities to consider to alter the process and improve the patient’s pain experience.
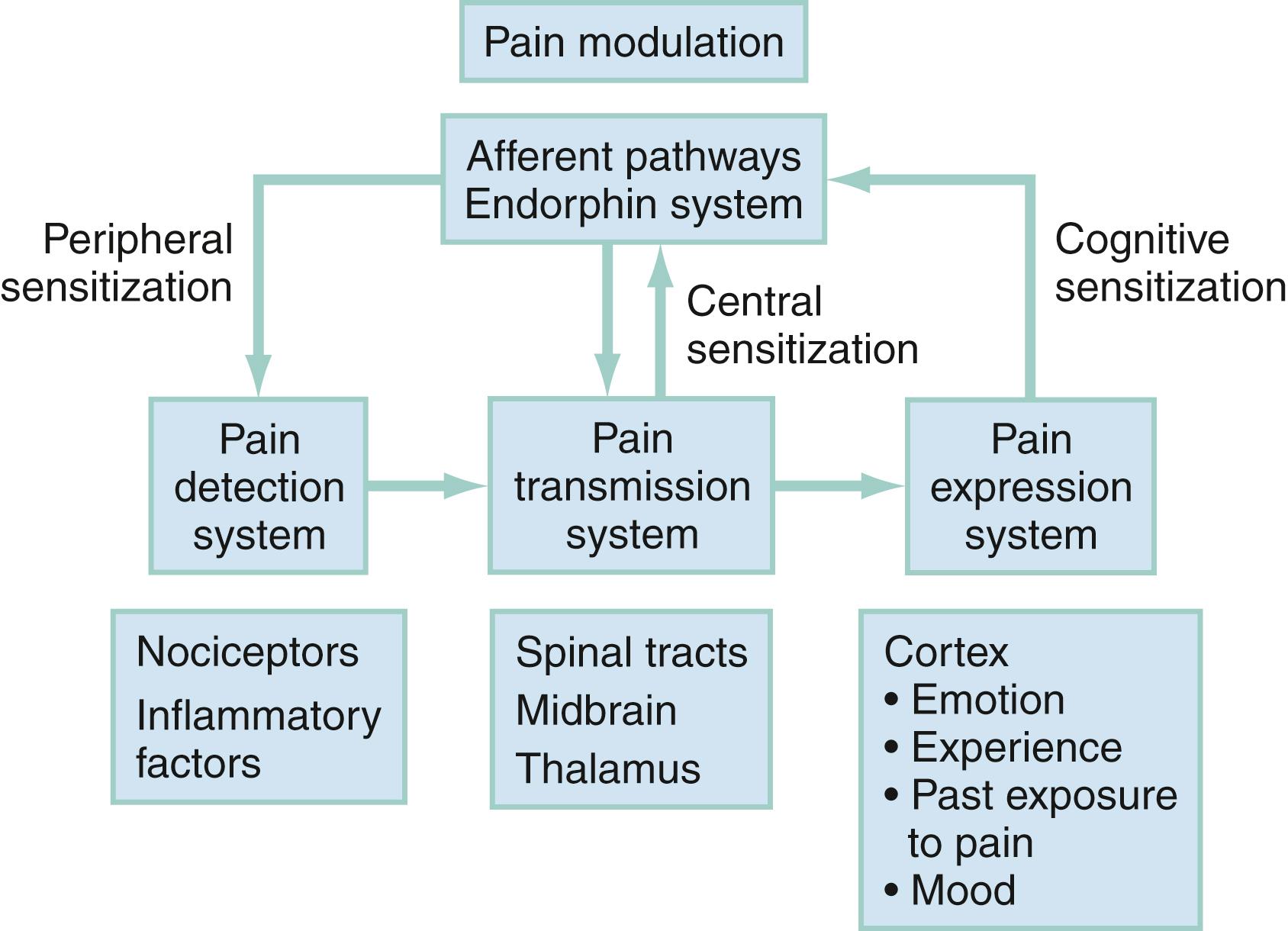
Pain can be divided into four separate processes (see Fig. 6.1 ): pain detection, pain transmission, pain modulation, and pain expression (perception).
The somatosensory system is responsible for detecting pain as well as tactile, proprioceptive, and thermal sensations. Receptors responsible for the detection of pain are termed nociceptors . Nociceptors include sensory nerves that are capable of detecting mechanical, thermal, or chemical stimulation. Several different subtypes of nociceptors are present in cutaneous tissues, including mechanoreceptors, polymodal nociceptors (PMNs), and a variety of thermoreceptors.
The threshold of activation of a nociceptor can be modulated—increased or decreased—by various chemical mediators, including prostaglandin, cyclic adenosine monophosphate, leukotrienes, bradykinins, serotonin, substance P, thromboxanes, platelet-activating factor, and endorphins. This change in nociceptor activation thresholds is termed peripheral sensitization . Trigger points, for example, are areas of frequent or constant low-level sensory stimulation (e.g., scar tissue or a degenerative joint) that have developed peripheral sensitized nociceptors that perceive pain from otherwise innocuous stimuli (allodynia).
All sensory neurons are composed of a cell body located in the dorsal root ganglia. The dorsal root ganglia are connected by nerve axon fibers with sensory receptors located in a number of body sites, including dermatomes (cutaneous input), sclerotomes (input from bones), and myotomes (input from muscle). The discrete areas covered by each nerve provide a sensory map of the body surface.
Peripheral nerve fibers can be classified by the roles of each fiber group ( Table 6.1 ). A-δ and C fibers are responsible for the transmission of pain. A-δ fibers transmit sharp, initial pain; C fibers, in contrast, transmit dull, aching, or burning pain. The pain transmitted by A-δ fibers persists only as long as the initial stimulus is in effect, whereas C fiber pain persists longer than the initial stimuli, rendering a prolonged pain sensory experience. The relative concentration of nerve fiber types, both C and A-δ, varies by body tissue.
| Fiber | Function | Myelin | Mean Diameter (mm) | Ascending Tract | Conduction Velocity (m/s) |
|---|---|---|---|---|---|
| A-α | Skeletal muscle motor | Deep | 12–20 | Ipsilateral dorsal column | 70–120 |
| A-β | Light touch and pressure | Superficial | 5–15 | Contralateral spinothalamic tract | 30–70 |
| A-γ | Motor | Superficial | 6–8 | Ipsilateral dorsal column | 15–30 |
| A-δ | Sharp pain (mechanoreceptors, thermoreceptors, PMNs) | Superficial | 1–4 | Contralateral spinothalamic tract | 12–30 |
| B | Sympathetic | 1–3 | Preganglionic | 3–15 | |
| C | Long-lasting burning pain | Superficial | 0.5–1.5 | Contralateral spinothalamic tract | 0.5–2 |
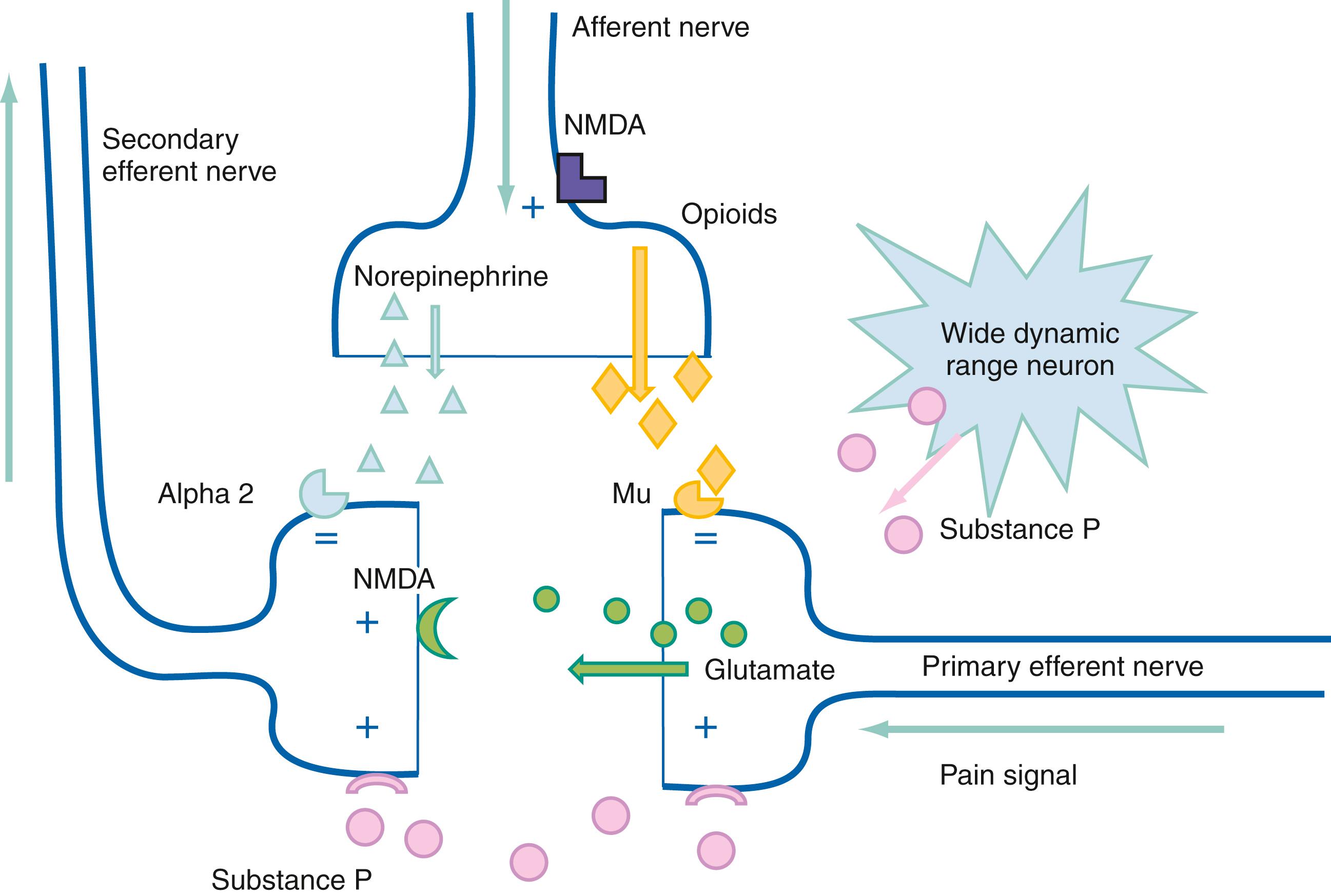
The dorsal horn is the gray matter of the posterior aspect of the spinal cord ( Fig. 6.2 ). The dorsal horn acts as an integration system in which sensory input is filtered, attenuated, or amplified before being relayed to other spinal segments or the cortex.
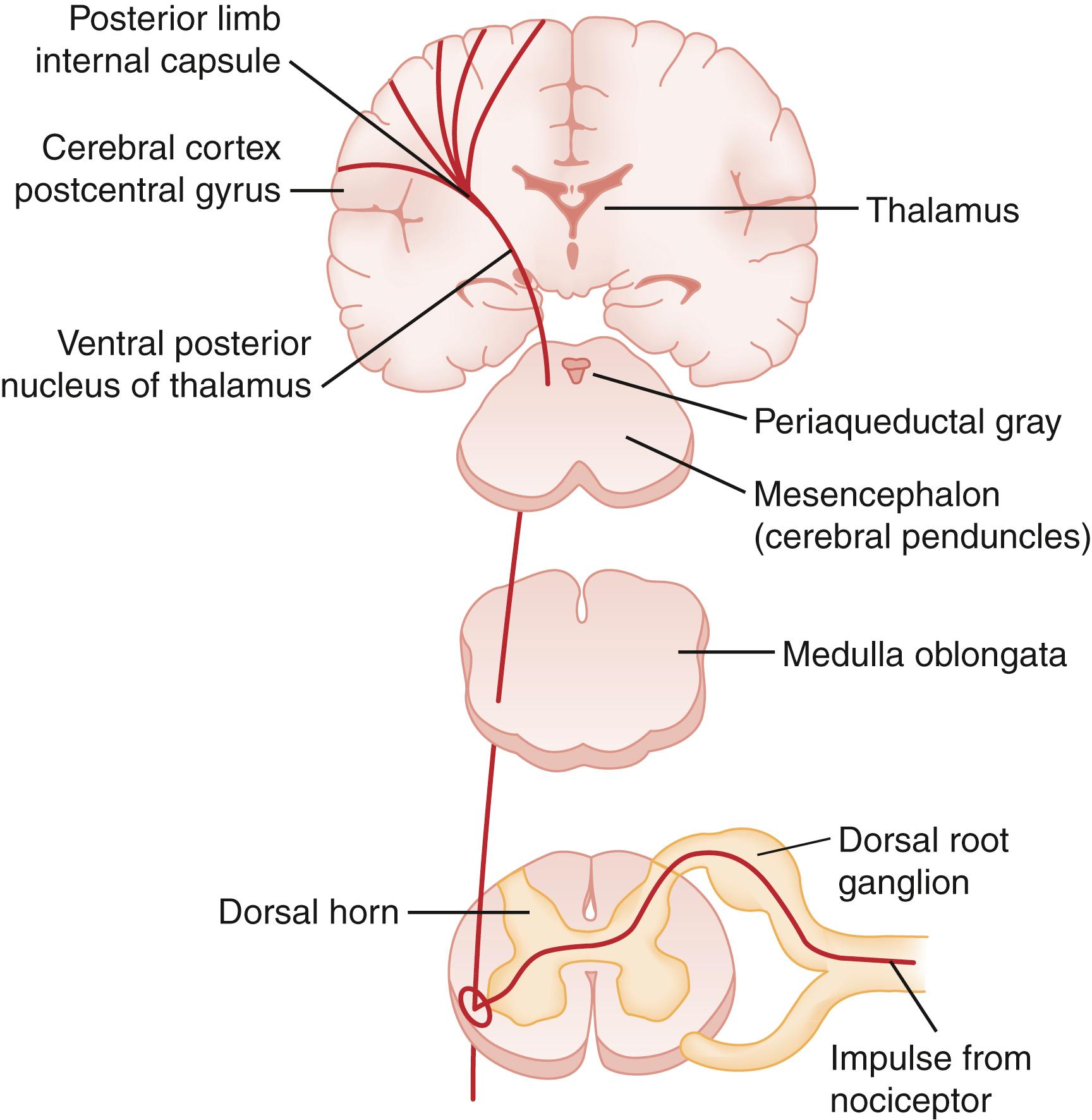
The dorsal horn is a processing center for incoming information and is extensively involved in modulating nociceptive input. Afferents from visceral, muscle, bone, and cutaneous areas converge in the dorsal horn and likely account for the cutaneous referred pain associated with noxious visceral, muscular, or bony stimuli from the same spinal level.
Differentiation between innocuous stimuli and nociceptor input occurs in the dorsal horn by stimuli received in cells referred to as wide dynamic range neurons (WDRNs). WDRNs receive modulating input from a variety of chemical pathways, such as opioids, substance P, or inflammatory factors. These cells also receive modulating input from efferent and afferent neuronal pathways.
The quantity and type of stimuli that produce pain vary among visceral structures. The myocardium, for example, is sensitive to ischemia but not mechanical stimulation. Tissues in the intestine may be severed, crushed, or burned without pain; however, traction or distention produces pain.
The quality of visceral pain is unique from somatic pain. Somatic pain is initially sharp, later becoming burning or throbbing in nature as the response is modulated. In contrast, visceral pain tends to start as poorly localized, burning or throbbing pain, with pronounced autonomic activation. These sensations may then develop into sharp, localized, referred pain as modulation progresses and the associated dermatome is activated. Visceral pain often produces referred pain. For example, periumbilical pain is often associated with appendicitis. This referred pain sensation occurs due to visceral afferents supplying the small bowel and traveling through the celiac ganglia and splanchnic nerves to enter the spinal cord at T10. This input sensitizes the dorsal horn at T10, leading to sensitization of all the dorsal horn nociceptive neurons and ultimately leading to the perception of pain in the T10 dermatome. As appendicitis progresses, the pain localizes to the right lower quadrant as inflammation extends to the parietal peritoneum with the same nerve supply as the overlying dermatome.
Fibers carrying pain impulses exit the dorsal horn and ascend the spinal cord to the brain. The predominant pathways for pain conduction through the spinal cord are the spinothalamic, spinomesencephalic, and spinoreticular tracts, located in the anterolateral aspect of the spinal cord ( Fig. 6.3 ; see Fig. 6.2 ).
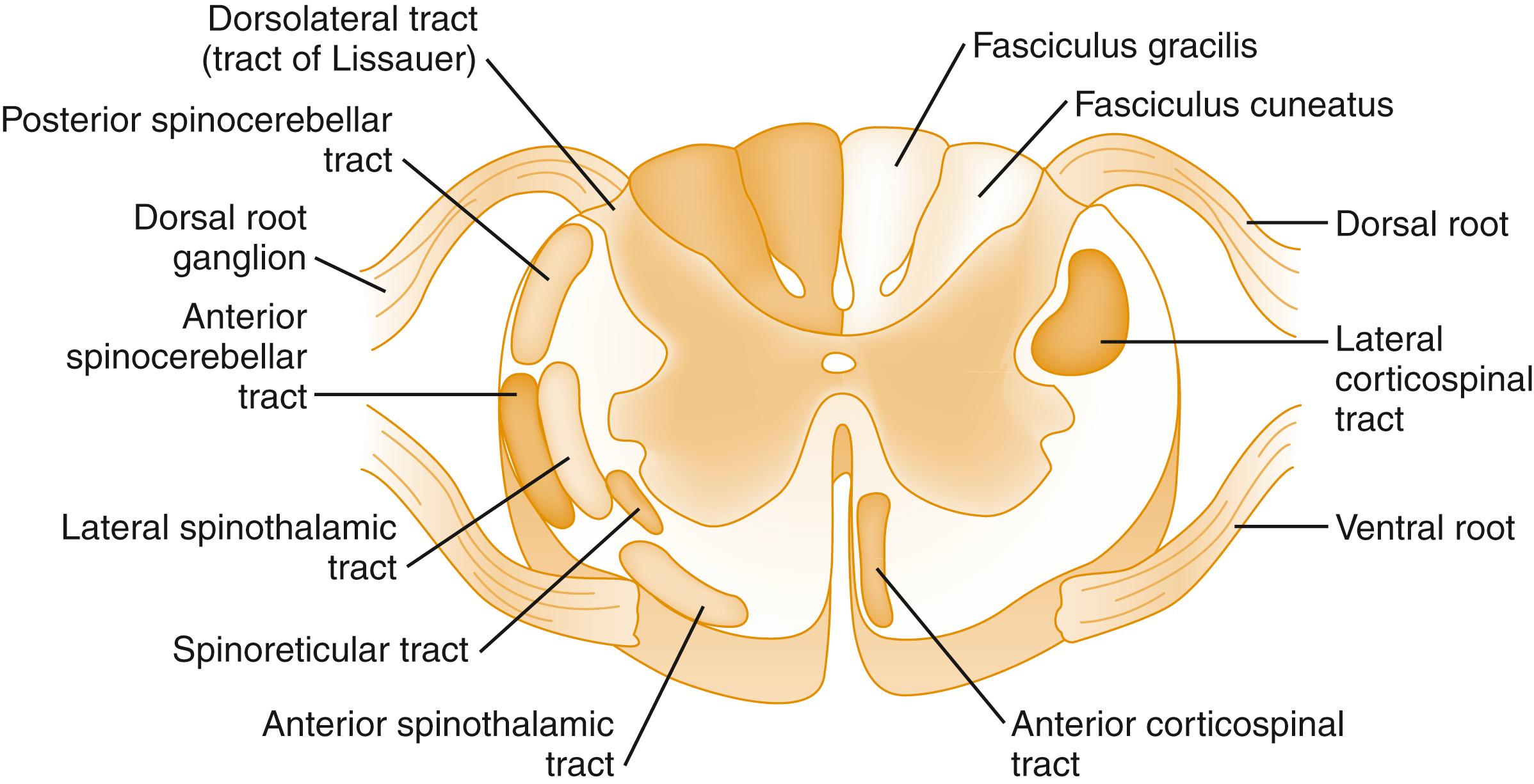
Impulses from nociceptors are modulated by descending tracts in the spinal cord. The two primary descending pathways appear to be serotonergic and noradrenergic. These pathways originate in the midbrain (periaqueductal gray matter and locus ceruleus), and medulla (nucleus raphe magnus and nucleus reticularis gigantocellularis) and are transmitted to the spinal cord via the dorsolateral funiculus.
Electrical stimulation of descending pathways produces analgesia comparable to that produced with opioids. Stimulation of the thalamus can also produce analgesia. Inputs to this system come from the frontal cortex, limbic system, hypothalamus, reticular system, locus ceruleus, and spinal cord. Multiple neurotransmitters are involved in these pathways, including serotonin, norepinephrine, and substance P. It is believed that the activation of this system is responsible for effects such as placebo, acupuncture, and transcutaneous electrical nerve stimulation (TENS) units, as well as stress-associated pain tolerance.
Central sensitization involves the amplification of nociceptive signals. Central sensitization is mediated by several substances, including nitric oxide, glutamate, substance P, aspartate, prostaglandins, leukotrienes, norepinephrine, and serotonin. It plays a major role in the development of chronic pain and can be the result of damage at any point along the pain transmission system, or more commonly a feedback loop of propagation and sensitization. Central sensitization has been best described in the setting of traumatic and degenerative conditions of the spinal cord and brainstem and can be associated with thalamic strokes, multiple sclerosis, Parkinson’s disease, Arnold-Chiari formation, and cervical stenosis.
The transduction, transmission, and modulation of pain stimuli develop the perception of the subjective emotional experience of pain. Many factors other than the simple stimulation of nociceptors influence the final perception of pain. The discrete cognitive processes and pathways involved in the interpretation and experience of painful stimuli remain a mystery and are affected by cultural expectations, personality, experiences, and the underlying emotional state. Many of these factors, and therefore the subsequent perception of pain, can be greatly influenced by both pharmacologic and nonpharmacologic interventions.
Much of the analgesic effect for drugs such as nitrous oxide and low-dose opioids is due to the cognitive interpretation and emotional reaction to pain rather than an effect on transmission of the pain stimulus. Similarly, noninvasive techniques such as distraction and hypnosis can limit pain perceptions and increase pain tolerance. Changes in how a person experiences pain, based on previous experiences and learned behaviors, are referred to as cognitive (de)sensitization.
There are two types of reflex responses to nociceptor input, spinal segmental (or suprasegmental) and cortical. Spinal reflexes are generated by the transmission of nociceptive impulses from the dorsal horn to motor and autonomic neurons in the spinal cord, provoking a range of responses, including tachycardia, vasoconstriction, paralytic ileus, and muscle spasm ( Box 6.2 ). Suprasegmental reflexes are transmitted through ascending tracts to the brainstem, hypothalamus, and cortex, where withdrawal reflexes and autonomic responses occur in connection with conscious responses. The autonomic reflex responses to pain are variable and cannot be used to quantify pain in an individual.
Vasoconstriction producing increased peripheral resistance
Increased cardiac output from increased stroke volume and heart rate
Increased blood pressure
Increased metabolic rate and oxygen consumption
Decreased gastric tone and gastric emptying (may progress to ileus)
Decreased urinary tract tone (may lead to urinary retention)
Decreased insulin production
Increased cortisol levels
Increased antidiuretic hormone levels
Increased growth hormone levels
Increased renin, angiotensin II, aldosterone levels
Increased glucagon levels
Increased catecholamine levels
Hyperventilation
Anxiety and fear
The endorphin system is a neuroendocrine system that serves to modulate responses to pain and stress. The endorphin system consists of widely scattered neurons that produce three types of opioids—beta-endorphin, met- and leu-enkephalins, and dynorphins. These opioids act as neurotransmitters and neuromodulators at three major classes of receptors—mu, delta, and kappa—and produce analgesia as well as counter the stress response ( Table 6.2 ).
| Opioid Receptor Class | Effects | Associated Endogenous Endorphin |
|---|---|---|
| Mu 1 | Euphoria, supraspinal analgesia, confusion, dizziness, nausea | Beta-endorphin |
| Mu 2 | Respiratory depression, CV and GI effects, miosis, urinary retention | Beta-endorphin |
| Delta | Spinal analgesia, CV depression, decreased brain and myocardial oxygen demand | Enkephalin |
| Kappa | Spinal analgesia, dysphoria, psychomimetic effects, feedback inhibition of endorphin system | Dynorphin, beta-endorphin |
| Epsilon | Hormone | Beta-endorphin |
| Gamma | Dysphoria, psychomimetic effects | Beta-endorphin |
Under normal circumstances, the endorphin system decreases pain and stress after a person has adequately dealt with the inciting noxious stimuli. The endorphin system is typically a responsive system that can increase or decrease effect to produce the appropriate response to a painful event. Like other neuroendocrine systems, increasing stimulation of the endorphin system produces feedback inhibition of endorphin production. During prolonged periods of pain with high stimulation levels, the system can become less responsive and less effective at modulating the pain response.
Like their endogenous counterparts, opiates act at chemical receptors to produce analgesia and undesirable side effects. As opioid drugs are given over prolonged periods, they inhibit a person’s endogenous endorphin system, blunting its ability to modulate pain and stress, and decreasing the endorphin system’s adaptive effects. As these drugs are withdrawn, the normal effects of the endorphin system can resume over time.
Acute pain is usually associated with an identifiable pathologic condition and serves an adaptive function by warning the individual that an illness or injury exists. This sequence will motivate the person to cease activity that is causing the pain, look for a cause, seek help, and avoid the future stimulus.
Acute pain becomes chronic pain when the pain pattern persists, in changed or unchanged form, after the original physiologic insult has resolved. All chronic pain starts as acute pain, but only small subsets of patients with acute pain develop chronic pain ( Table 6.3 ). The physiologic transition from acute to chronic pain is a complex process, with physiologic and psychosocial components. In many cases, the development of chronic pain is likely related to the treatment of acute pain.
| Parameter | Acute Pain | Chronic Pain |
|---|---|---|
| Inciting factor | Associated pathology present and recovery is expected | Associated pathology not identifiable or not expected to improve; recovery unpredictable or not expected |
| Relation to healing | Pain improves as the injury heals; limitation of activity due to pain assists healing | Neither pain nor injury expected to improve; pain may limit activities that could improve condition |
| Psychosocial effects | Limited to acute stress reaction | Negative effects a prominent feature of disease |
| Treatment | Analgesics, immobilization | Psychosocial aspects must be addressed; analgesics play a smaller role |
Acute pain serves an important purpose in that it stimulates a person to protect the injured area and seek help. Also, the neurochemical factors that contribute to acute pain acknowledgment generally initiate and support recruitment of tissue repair mechanisms. As an injury heals, these adaptive responses may become maladaptive if the pain persists, with the pain leading to a decreased range of motion, decreased function of the area and, ultimately, increased susceptibility to injury and pain. Pain also causes a stress response that is initially adaptive in the face of injury. A prolonged stress response, however, causes an impaired immune system, hypercoagulabilty, sleep disturbances, anxiety, and depression.
The early accurate recognition and assessment of a patent’s pain are the most important aspects of effective acute pain management. When pain is inadequately treated, inaccurate assessment is often the root cause of the problem.
The degree to which a person experiences pain is a complex and subjective interaction between the physical stimulus and patient’s cognitive and emotional state. However, it is clear that the degree of pain a patient perceives is not directly determined by the degree of physiologic injury. Patients in the ED with relatively identical injuries may experience and display completely different amounts of pain. Therefore, pain treatments, analgesic requirements, and how a patient describes pain cannot be uniformly described based on the nature of a patient’s injury.
The assessment of pain depends on both the patient’s ability to communicate the nature of the painful experience and the clinician’s ability to accurately obtain this information. Unfortunately, there is no objective test or physiologic index to measure pain reliably. Objective observations, such as hypertension, diaphoresis, or tachycardia, do not correlate well with the degree of pain. Because pain cannot be objectively measured, a clinician’s assessment depends on communication with the patient—verbal and nonverbal. Barriers to communication between patients and clinicians, including linguistic, socioeconomic, and cultural differences, limit the ability to assess pain effectively.
Because effective treatment is based on the assessment of pain, patients who have difficulty communicating are at risk of undertreatment of their pain (oligoanalgesia). These groups include infants and children, patients whose cultural and linguistic background differs significantly from the treating clinician, or are developmentally delayed, cognitively impaired, under severe emotional stress, or mentally ill. Racial and ethnic disparities have been identified in the treatment of acute pain in the ED. ,
There are a variety of reasons why some patients receive inadequate analgesia. These include ineffective pain assessment, misconceptions about the safety and efficacy of various treatments, and concerns about the effect of analgesic interventions on a patient’s evaluation. There may be a significant delay to providing adequate pain control, especially during high-volume periods. Additionally, when opioids are used, they are often given in subtherapeutic doses.
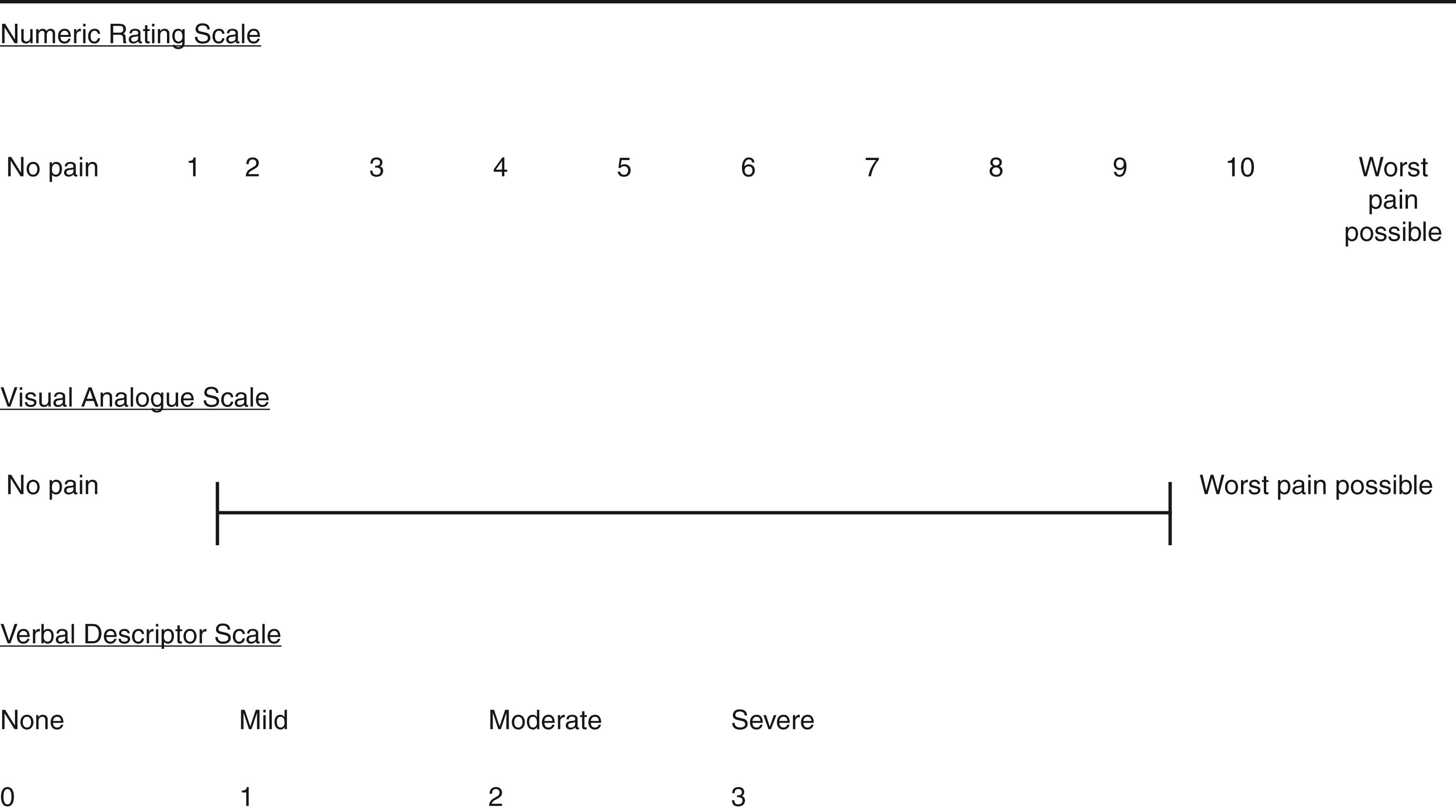
The use of numeric rating scales, such as a verbal 0 to 10 score (none to worst imaginable), are ubiquitous ( Fig. 6.4 ). Visual analogue scales, usually consisting of a 10-cm straight line with anchors at either extreme, are frequently used in research to provide continuous data for analysis. These scales offer little practical advantage over verbal reports in the clinical setting. Routine verbal or visual pain scale assessment encourages emergency clinicians to communicate with patients to assess their pain and to evaluate responses to analgesic intervention attempts.
Children require alternative communication techniques to acknowledge and relate pain. The FACES pain scales are designed for children younger than 7 years. This scale has a series of cartoon faces expressing a range of emotions, from happiness to severe distress. The child is asked to point to the face that corresponds to how he or she feels. The FACES pain scale, and others like them, require less of an abstract reference than numeric and verbal scales and are useful in pain assessment for toddlers and cognitively impaired adults.
In preverbal children, observer-derived scales need to be used. These include the Modified Pre-Verbal, Early Verbal Pediatric Pain Scale (M-PEPPS), Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS), and CRIES scale for neonates. These scales use a scoring system for observed criteria that is reproducible among trained observers, making them useful for research, but they have little clinical utility compared to the emergency clinician’s or parents’ overall impression of a child’s pain.
Pain scores are accepted as the most accurate and reliable measure of assessing a patient’s pain and response to pain treatment. Pain treatment should be targeted to a goal of reducing the pain score (e.g., by 50%, below 4/10, or referred to as mild/moderate or severe) rather than a specific (maximum) analgesic dose.
The approach to patients in pain begins with a characterization of pain into four unique treatment groups: (1) acute pain; (2) recurrent pain; (3) chronic pain of malignancy and neuropathic pain; and (4) chronic pain. Other than acute pain, therapy should focus on a long-term, multidisciplinary approach ( Box 6.3 ). Acute and chronic pain patients have different physiologic causes and thus require different treatment approaches based on their specific site and mechanism of affect ( Fig. 6.5 ).
Acetaminophen
Nonsteroidal antiinflammatory drugs (NSAIDs)
Opioids used in combination with NSAIDs and acetaminophen
Oxycodone
Hydrocodone
NSAIDs
Tramadol
Opioids used in combination with NSAIDs and acetaminophen a
a A variety of opioid and acetaminophen combination agents are available.
, b
b Chronic opioid management should be managed by the primary outpatient physician.
Oxycodone, long-acting preparation, or for breakthrough pain
Tricyclic antidepressants
Gabapentin
Tricyclic antidepressants
Carbamazepine
Symptomatic treatment of pain should be initiated promptly, titrated to an acceptable level of relief, and continued while the investigation for a cause is proceeding. Fig. 6.6 presents a common approach to acute pain in the ED setting, beginning at triage to assess pain type and severity and then progressing to clinical assessment, with therapeutic suggestions.
When the cause of acute pain is uncertain, immediate relief of pain occurs in parallel with initial efforts to establish the diagnosis. It is inappropriate to delay analgesic use until a diagnosis has been made. There is no evidence that the administration of adequate doses of opioid analgesia to establish patient comfort impairs the emergency clinician’s ability to diagnose the cause of an acutely painful condition. On the contrary, analgesia administration may enhance the accuracy of the physical examination and patient assessment.
The assessment of pain in the absence of acute or obvious physical injury requires a great deal of communication skill on the part of the clinician and patient. Many patients with chronic pain develop experiences, some adaptive and others maladaptive, in describing their pain and interacting with clinicians to receive pain treatment. Many behaviors such as exaggerating symptoms or attempting to manipulate clinicians are developed around the patient’s expectations for obtaining pain therapy or pain relief. These behaviors, combined with the negative psychosocial effects and sense of futility associated with chronic pain, can complicate the evaluation process and care of chronic pain patients.
The assessment of chronic pain represents some of the most challenging situations to obtain an accurate clinical history. Patients who are having difficulty describing their pain should be encouraged with detailed questions about the pain, combined with multiple examples, comparisons, and summarizing statements, to facilitate accurate communication. Assuring the patient that the questions are intended to aid understanding and enable the effective treatment of symptoms can facilitate developing a common goal and help establish the trust necessary to develop an effective treatment strategy.
Patients with chronic pain can exacerbate their chronic pain in the setting of ongoing therapy or with untreated chronic pain due to a gap or lack of ongoing care. These scenarios require different treatment approaches. For chronic pain patients with an exacerbation in their pain exceeding the pain control of their usual treatment strategy, treatment can be approached in a fashion similar to that for acute pain. The goal for these patients should be to control the exacerbation and return the patient to their baseline function and treatment strategy.
Many patients with chronic pain are in comprehensive treatment programs, most of which involve a so-called contract concerning the provision of their pain management. For such patients, a review of the pain management plan in the medical records, or contact with the health care clinician who manages the patient’s treatment plan, is a critical element in determining the best short-term strategy in the ED.
Patients with chronic pain who have a gap in their baseline treatment or who have never established appropriate treatment for chronic pain require an approach that addresses the need to establish a consistent treatment plan. Patients with no ongoing treatment plan should have a basic chronic pain treatment plan implemented during their ED visit. This should consist of acetaminophen, if not contraindicated, and a nonsteroidal antiinflammatory drug (NSAID), if tolerated. Adjuvants appropriate for neuropathic or central pain may be added, if appropriate.
Opioids for chronic pain, whether for a patient already on a treatment plan or without a treatment plan, are rarely indicated in the ED, with the exception of patients who experience pain related to advanced malignancy (see below.) Opioids should neither be administered nor prescribed unless their need is verified with the clinician responsible for the patient’s chronic pain management plan. In general, opioids for chronic pain management are the domain of clinicians in ambulatory pain centers or primary care physicians who will follow the patient’s therapy, response, and compliance. There is often difficulty communicating this with patients who may have received opioids at many EDs, including the one currently being visited. The establishment of a departmental policy empowers emergency clinicians to administer or prescribe opioids for chronic pain syndromes without judging the patient.
Chronic pain care in the ED should emphasize the use of multimodal treatments and reduce the use of opioid medications. Nonpharmacologic treatments in multimodal chronic pain care include physical rehabilitative therapies, interventional pain procedures, psychological therapy, and integrative approaches. An important aspect of caring for chronic pain is recommending a nonpharmacological treatment, or at least determining if a patient is willing to consider such therapies. The factors associated with a patient’s willingness to accept such therapies have not been well defined, and therefore nonpharmacological approaches should be discussed with chronic pain patients presenting to the ED.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here